Simulation of the Scale-up Process of a Venturi Jet Pyrolysis Reactor
Abstract
:1. Introduction
2. Modeling
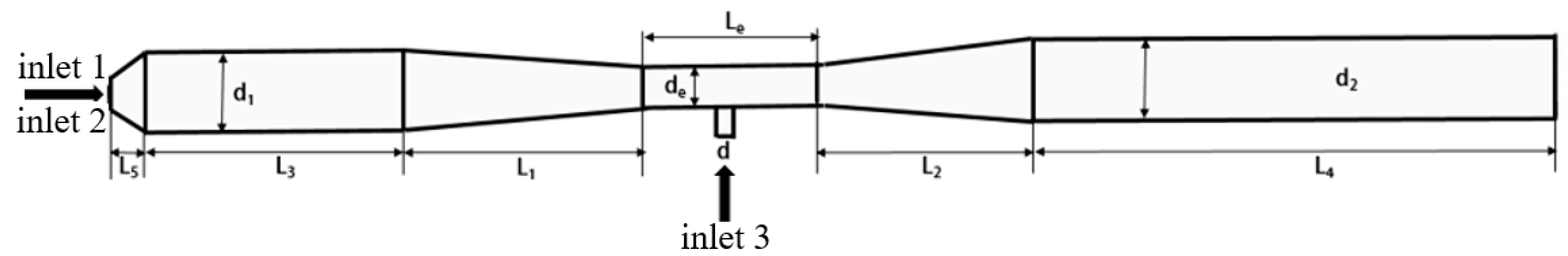
2.1. Venturi Jet Pyrolysis Reactor Model
2.2. Model Selection
2.3. Validation and Optimization
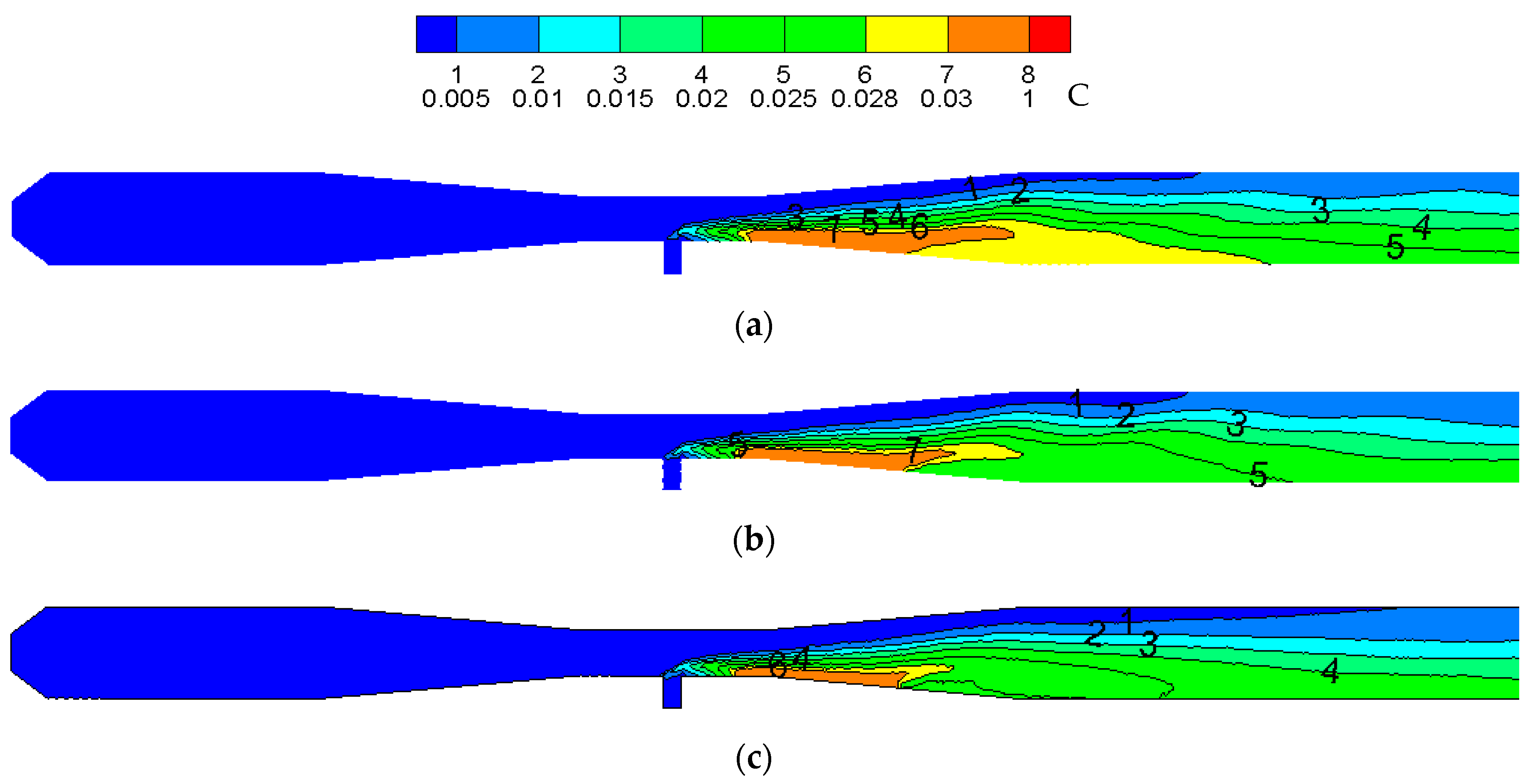
3. Results and Discussion
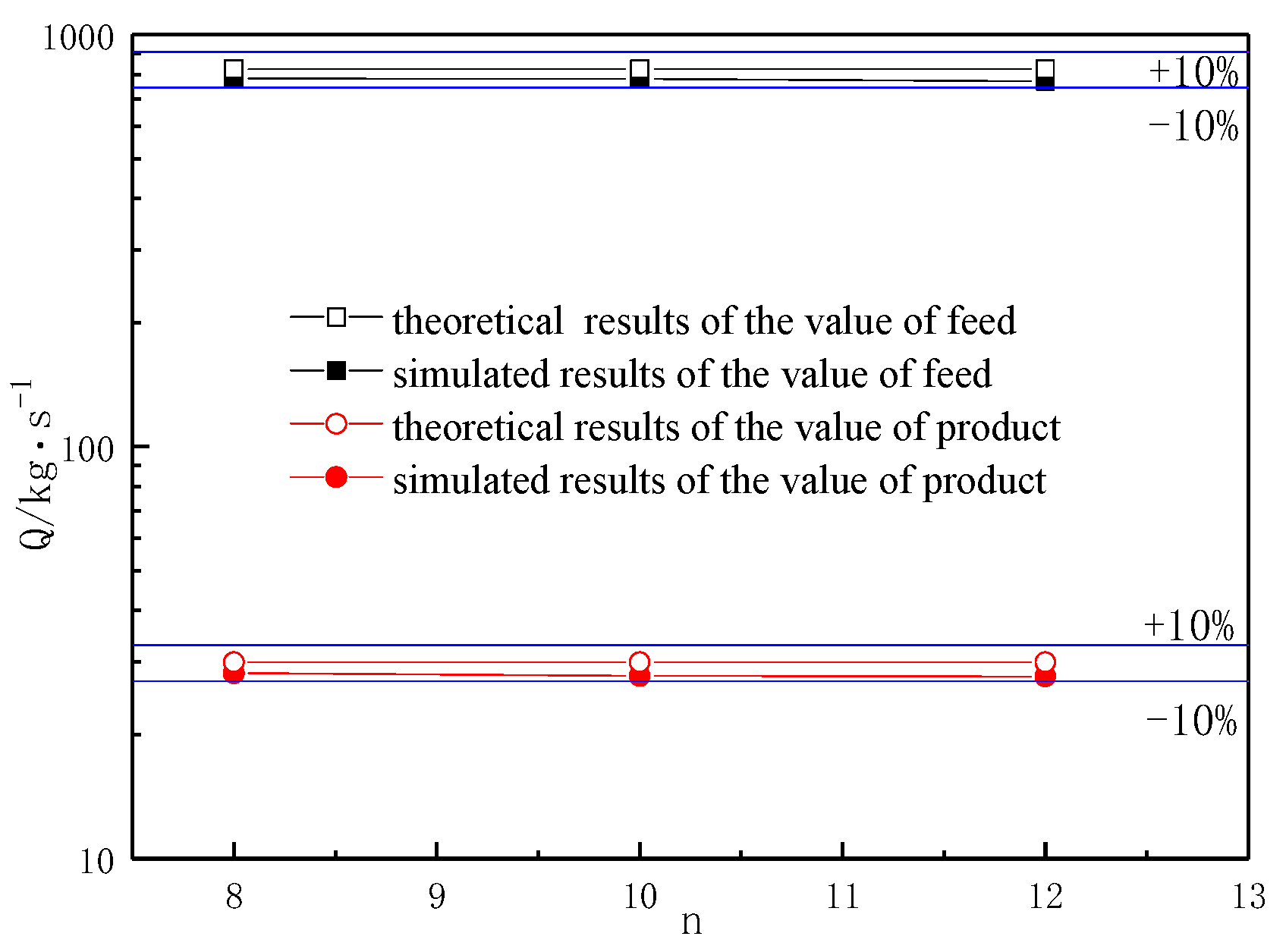
3.1. Dimension Analysis
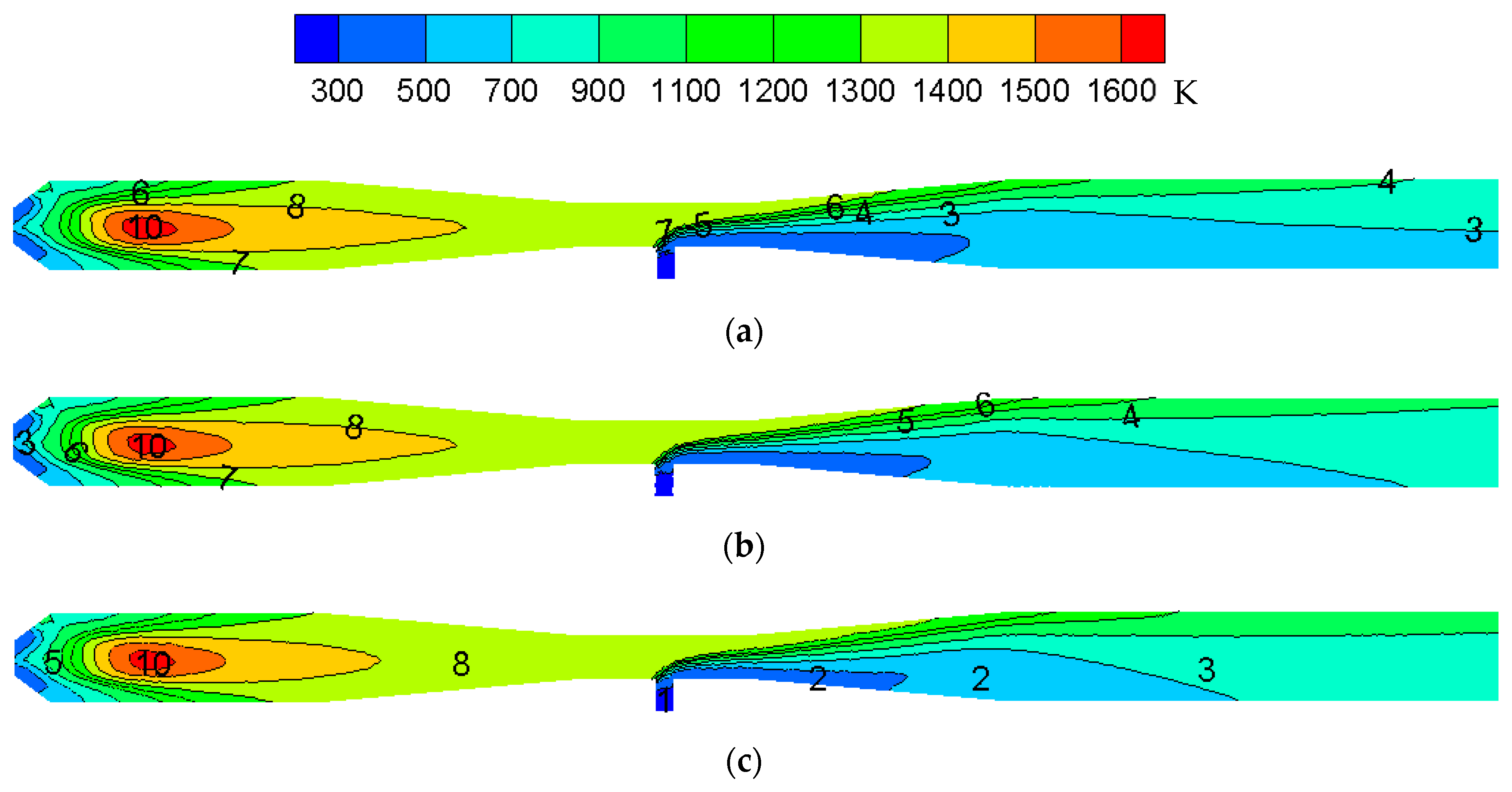
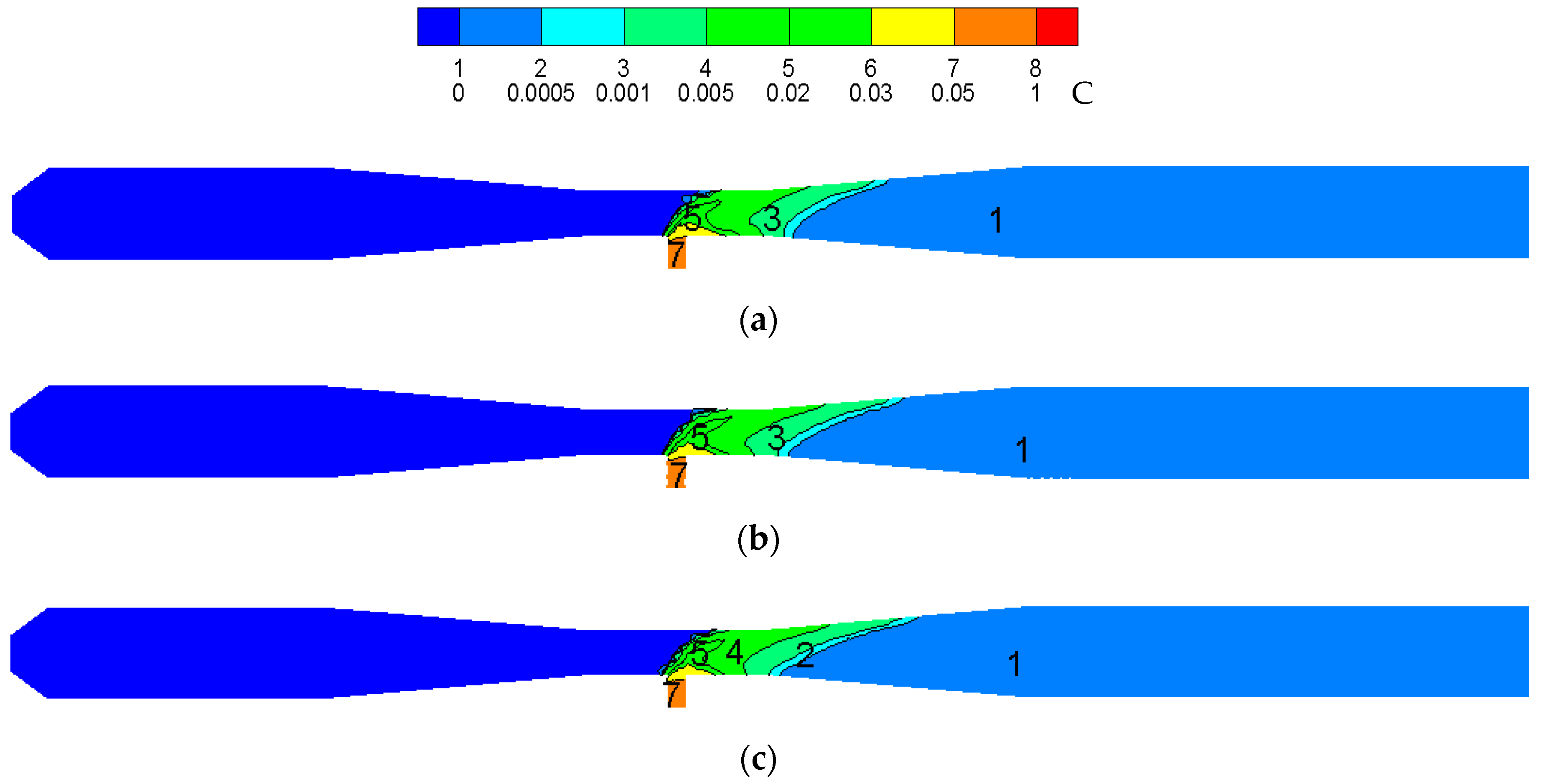
3.2. Reactor Ampification
4. Conclusions
- (1)
- The empirical formula obtained from dimensional analysis based on numerical simulation data is Q = 2.240727 × 10−4P0.004568ρ0.26223d−0.24801V1.25714n0.076479μ−0.26628. The reactor can be geometrically scaled up. The relationship between the gas-phase inlet velocity V and the reactor pipe diameter d in the pyrolysis of cerium chloride follows the formula: V = 0.0209d0.196.
- (2)
- The temperature distribution trend in the reactor and the maximum temperature in the reactor remains unchanged after amplification. With an increase in the amplification magnitude, CeCl3 has a smaller ratio in the high concentration region of the reactor, a narrower distribution at the throat region and a smaller proportion of CeO2 in the high concentration region.
- (3)
- The reactor amplification experiment will be carried out next to verify the accuracy of the amplification conditions obtained by the simulation, and to summarize the rules to be followed in the reactor amplification process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, Z.Y.; He, Y. General Situation and Application Prospect of Rare Earth Resources in China. Inner Mong. Sci. Technol. Econ. 2017, 7, 28–33. [Google Scholar]
- Wang, Z.F.; Fei, P.Y.; Xiong, H.Q.; Qin, C.L. CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Zhu, W.J.; Huang, H.; Zhang, W.K.; Tao, X.Y.; Gan, Y.P.; Xia, Y.; Yang, H.; Guo, X.Z. Synthesis of MnO/C composites derived from pollen template for advanced lithium-ion batteries. Electrochim. Acta 2015, 152, 286–293. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.A.; Dou, Z.H.; Zhao, Q.Y. Simulation of Process and Reactor Structure Optimization for CeO2 Preparation from Jet-Flow Pyrolysis. JOM 2019, 71, 1660–1666. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.A.; Dou, Z.H.; Zhao, Q.Y. Numerical Simulations of Irregular CeO2 Particle Size Distributions. JOM 2019, 71, 34–39. [Google Scholar] [CrossRef]
- Vijayaraghavan, V.; Wong, C.H. Transport characteristics of water molecules in carbon nanotubes investigated by using molecular dynamics simulation. Comput. Mater. Sci. 2014, 89, 36–44. [Google Scholar] [CrossRef]
- Vijayaraghavan, V.; Garg, A.; Gao, L.; Vijayaraghavan, R. Finite Element Based Physical Chemical Modeling of Corrosion in Magnesium Alloys. Metals 2017, 7, 83. [Google Scholar] [CrossRef]
- Coppens, M.O. A nature-inspired approach to reactor and catalysis engineering. Curr. Opin. Chem. Eng. 2012, 3, 281–289. [Google Scholar] [CrossRef]
- Smith, K.B.; Mackley, M.R. An Experimental Investigation into the Scale-up of Oscillatory Flow Mixing in Baffled Tubes. Chem. Eng. Res. Des. 2006, 84, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Coppens, M.O. Scaling-up and -down in a Nature-Inspired Way. Ind. Eng. Chem. Res. 2005, 44, 5011–5019. [Google Scholar] [CrossRef]
- Underwood, N.; Inouye, P.H.D. Large-Scale Questions and Small-Scale Data: Empirical and Theoretical Methods for Scaling up in Ecology. Oecologia 2005, 145, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Labík, L.; Vostal, R.; Moucha, T.; Rejl, F.; Kordac, M. Volumetric mass transfer coefficient in multiple-impeller gas–liquid contactors. Scaling-up study for various impeller types. Chem. Eng. J. 2014, 240, 55–61. [Google Scholar] [CrossRef]
- Geem, K.M.V.; Robert, Z.; Reyniers, M.F.; Marin, G.B. Dimensional analysis for scaling up and down steam cracking coils. Chem. Eng. J. 2007, 134, 3–10. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Z. Biochemical Reaction Engineering. Prog. Chem. Ind. 1991, 3, 33–39. [Google Scholar]
- Safoniuk, M.; Grace, J.R.; Hackman, L.; Mcknight, C.A. Use of dimensional similitude for scale-up of hydrodynamics in three-phase fluidized beds. Chem. Eng. Sci. 1999, 54, 4961–4966. [Google Scholar] [CrossRef]
- Krishna, R.; Baten, J.M.V.; Urseanu, M.I.; Ellenberger, J. A scale up strategy for bubble column slurry reactors. Catal. Today 2001, 66, 99–207. [Google Scholar] [CrossRef]
- Šoltys, M.; Balouch, M.; Kašpar, O.; Lhotka, M.; Ulbrich, P.; Zadrazil, A.; Kovacik, P.; Stepanek, F. Evaluation of scale-up strategies for the batch synthesis of dense and hollow mesoporous silica microspheres. Chem. Eng. J. 2017, 334, 1135–1147. [Google Scholar] [CrossRef]
- Gong, J.B.; Wei, H.Y.; Wang, J.K.; Garside, J. Simulation and Scale-up of Barium Sulphate Precipitation Process Using CFD Modeling. Chin. J. Chem. Eng. 2005, 13, 167–172. [Google Scholar]
- Nauha, E.K.; Kálal, Z.; Ali, J.M.; Alopaeus, V. Compartmental modeling of large stirred tank bioreactors with high gas volume fractions. Chem. Eng. J. 2018, 334, 2319–2334. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.D.; Lu, Y.C.; Wang, J.W.; Luo, G.S. Mixing characterization and scaling-up analysis of asymmetrical T-shaped micromixer: Experiment and CFD simulation. Chem. Eng. J. 2012, 181, 597–606. [Google Scholar]
- Lv, C.; Zhang, T.A.; Dou, Z.H.; Zhao, Q.Y. Numerical simulation of preparing ultrafine cerium oxides from jet-flow pyrolysis. Rare Met. 2019, 38, 12. [Google Scholar]


| Types | CH4 Inlet | O2 Inlet | CeCl3 Inlet | Outlet |
|---|---|---|---|---|
| Boundary conditions | Velocity-inlet | Velocity-inlet | Velocity-inlet | Outflow |
| Value (m/s) | 1.44–14.4 | 10.575–105.75 | 0.03 |
| Variables | Ρ (kg/m3) | Q (m3/s) | D (m) | V (m3/s) | P (Pa) | µ (Pa·s) |
|---|---|---|---|---|---|---|
| M (kg) | 1 | 0 | 0 | 0 | 1 | 1 |
| L (m) | −3 | 3 | 1 | 3 | −1 | −1 |
| T (s) | 0 | −1 | 0 | −1 | −2 | −1 |
| Types | P (Pa) | ρ (kg/m3) | d (m) | V (m3/s) | n |
|---|---|---|---|---|---|
| Model 1 | 42,413.25 | 1038.449 | 0.08 | 0.0127 | 10/5 |
| Model 2 | 46,302.17 | 0.1 | 0.0133 | ||
| Model 3 | 49,742.49 | 0.12 | 0.0138 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Zhang, T.; Hao, B. Simulation of the Scale-up Process of a Venturi Jet Pyrolysis Reactor. Metals 2019, 9, 979. https://doi.org/10.3390/met9090979
Lv C, Zhang T, Hao B. Simulation of the Scale-up Process of a Venturi Jet Pyrolysis Reactor. Metals. 2019; 9(9):979. https://doi.org/10.3390/met9090979
Chicago/Turabian StyleLv, Chao, Tingan Zhang, and Bo Hao. 2019. "Simulation of the Scale-up Process of a Venturi Jet Pyrolysis Reactor" Metals 9, no. 9: 979. https://doi.org/10.3390/met9090979




