Enhanced Strength, Durability, and Microstructural Attributes of Graphene Oxide-Modified Ultrafine Slag Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
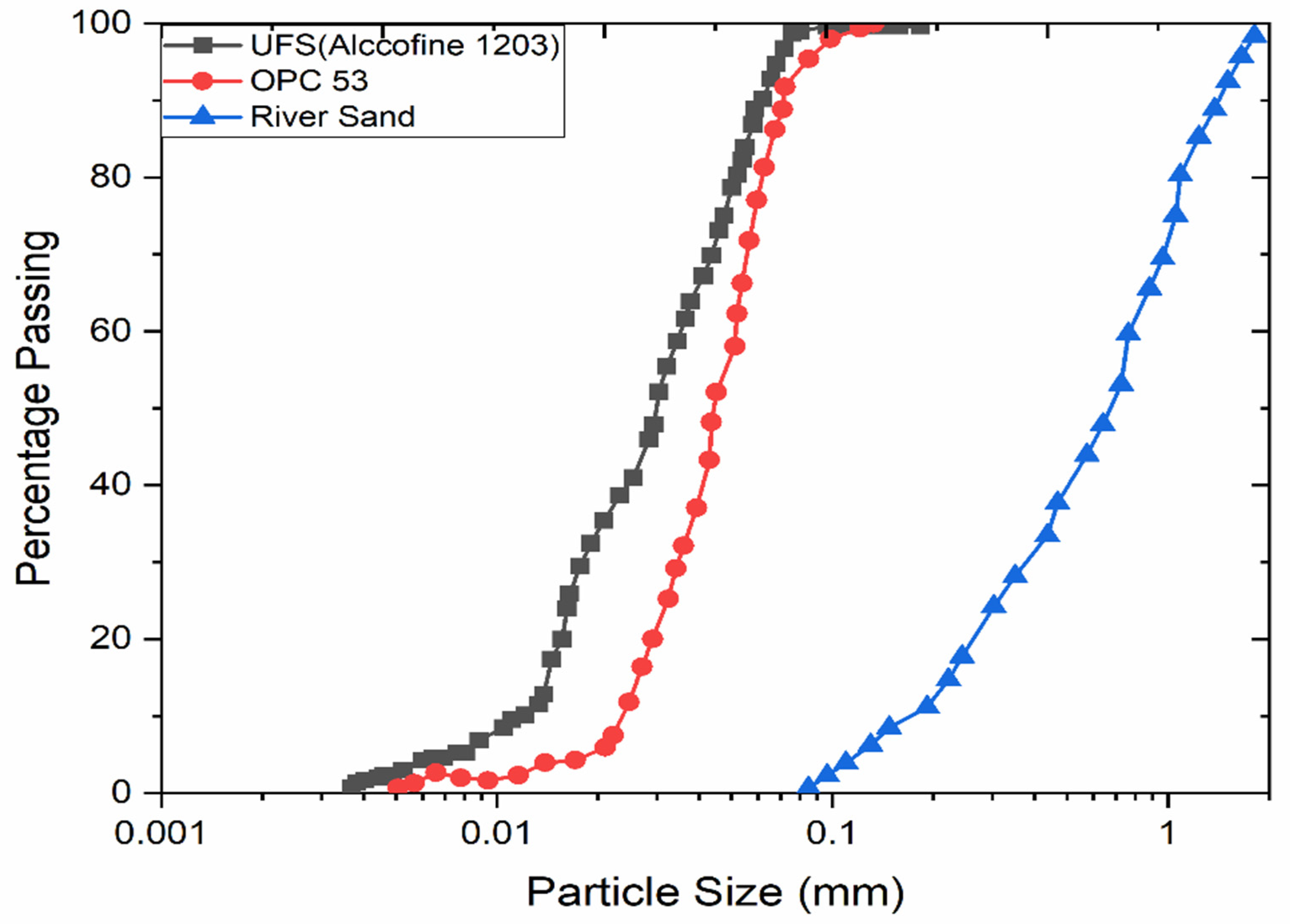
2.1. Materials
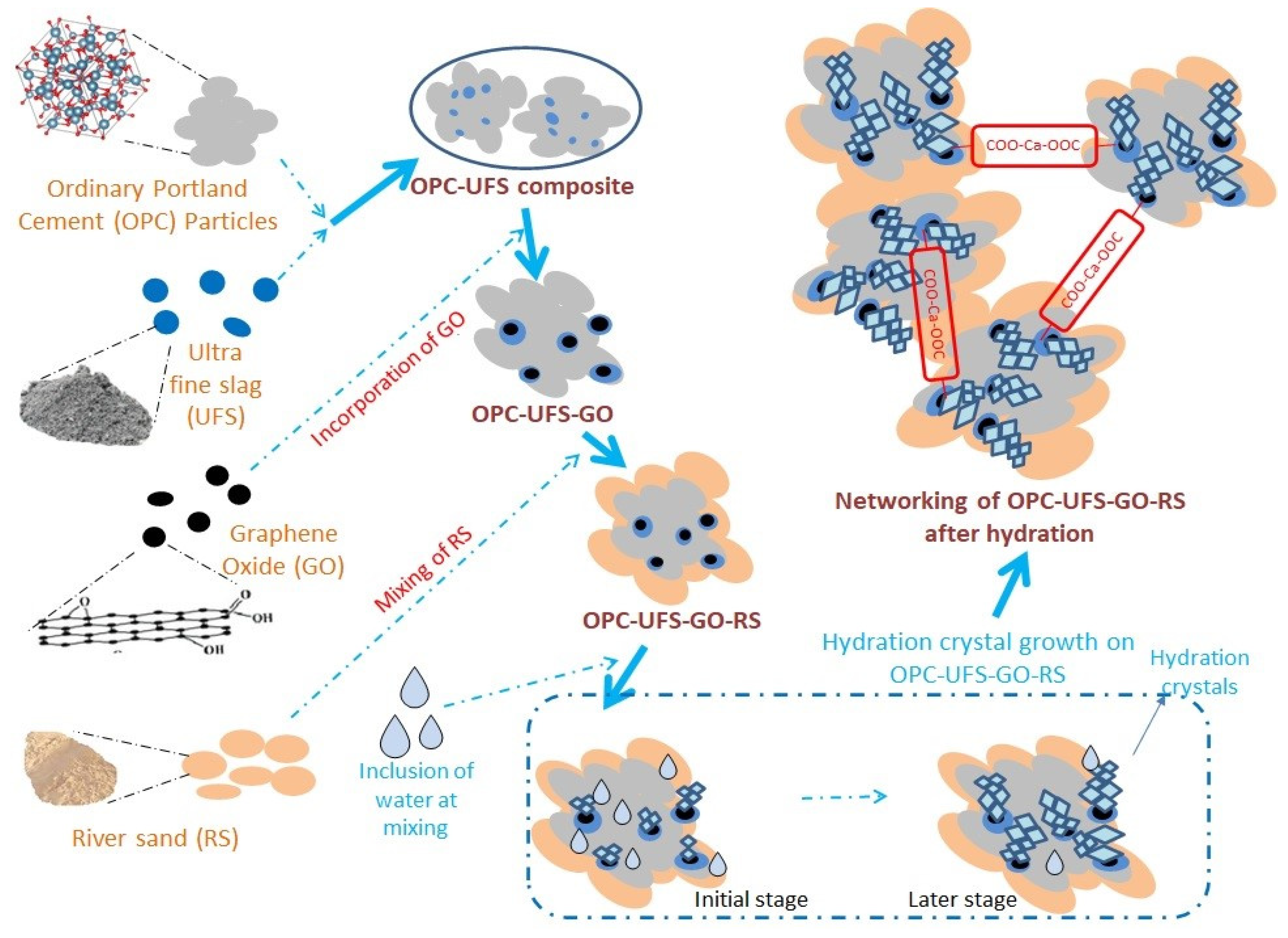
2.2. CM-UFS-GO Mix Composition
2.3. Compression Strength
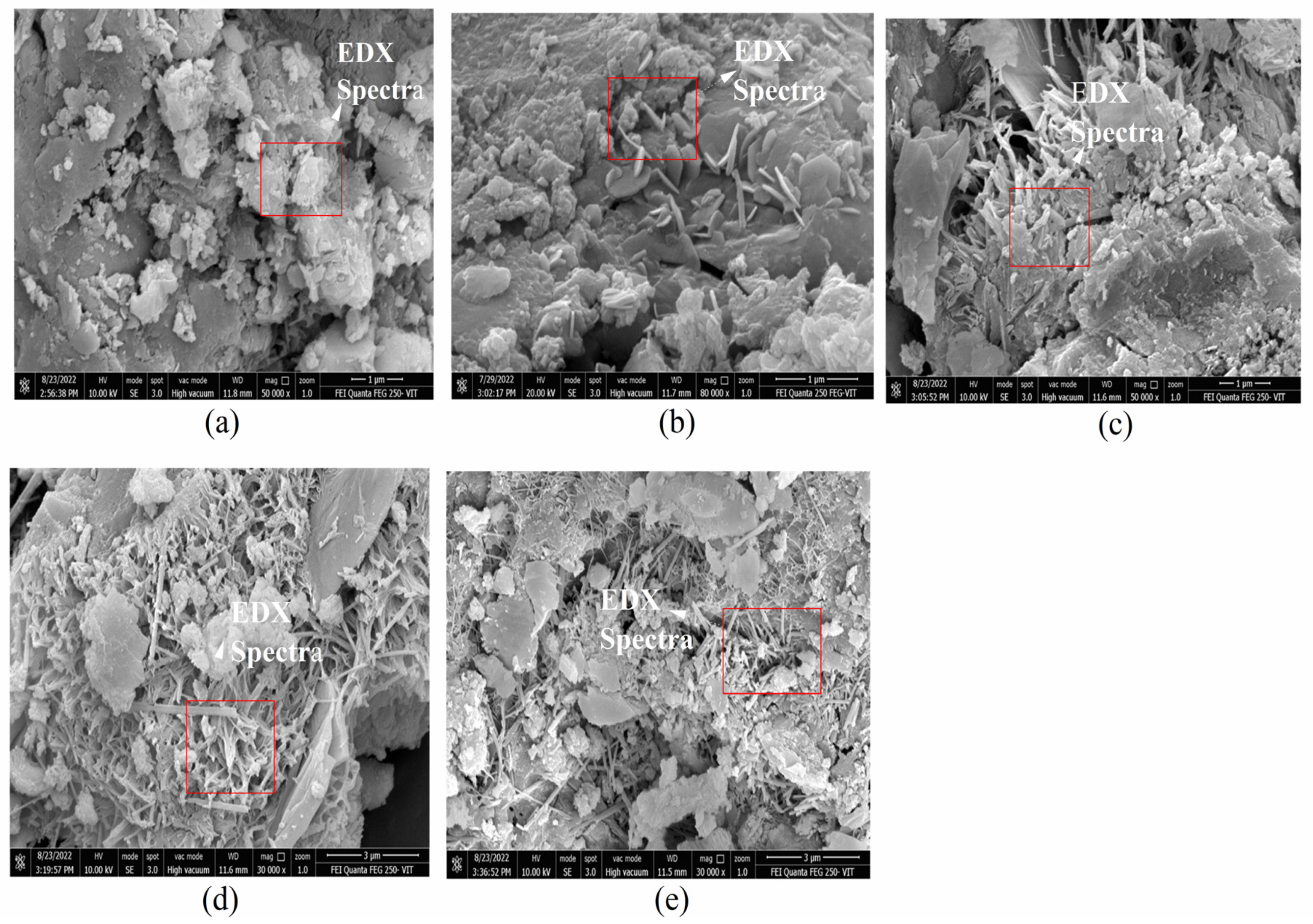
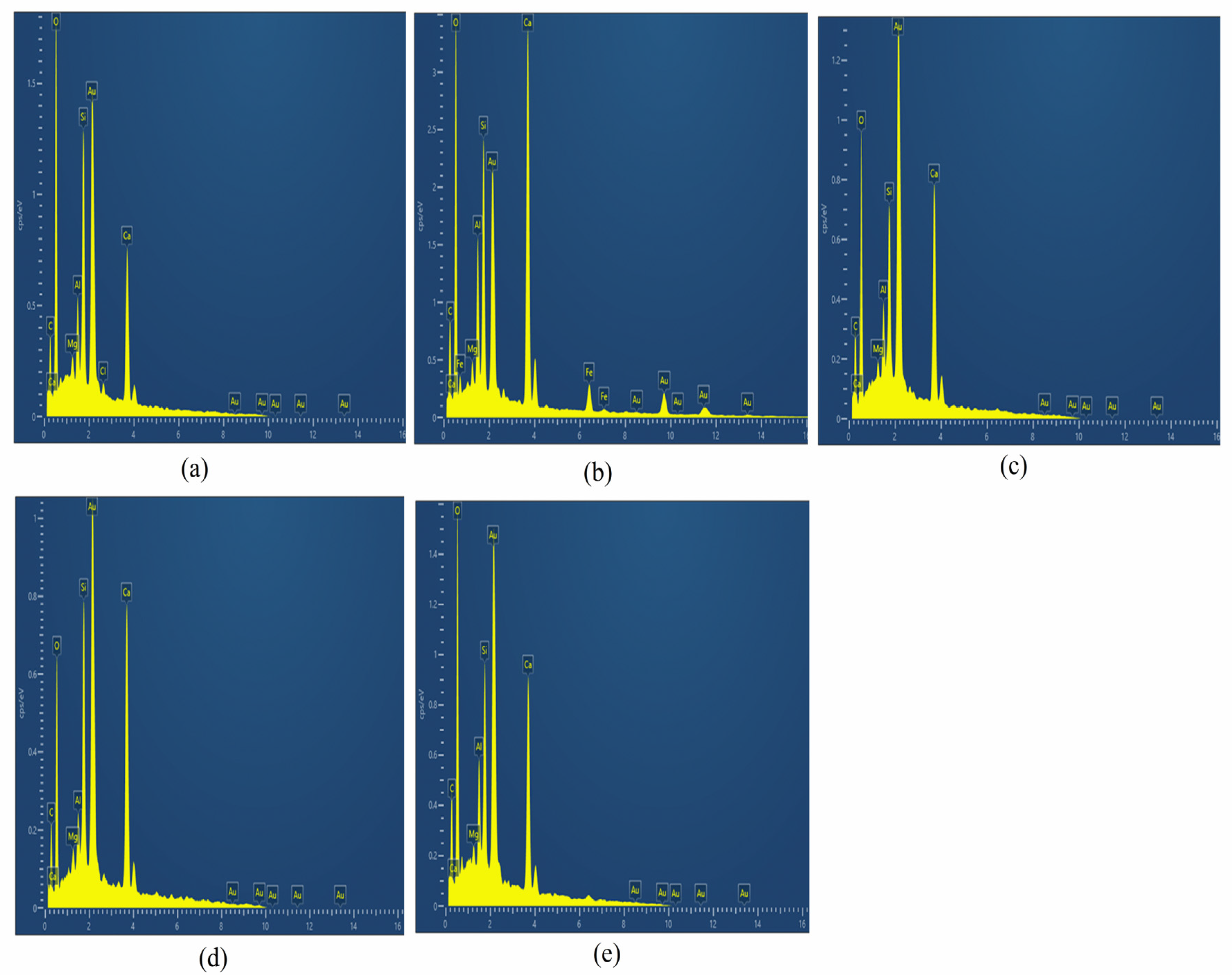
2.4. Field-Emission Scanning Electron Microscope (FE-SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
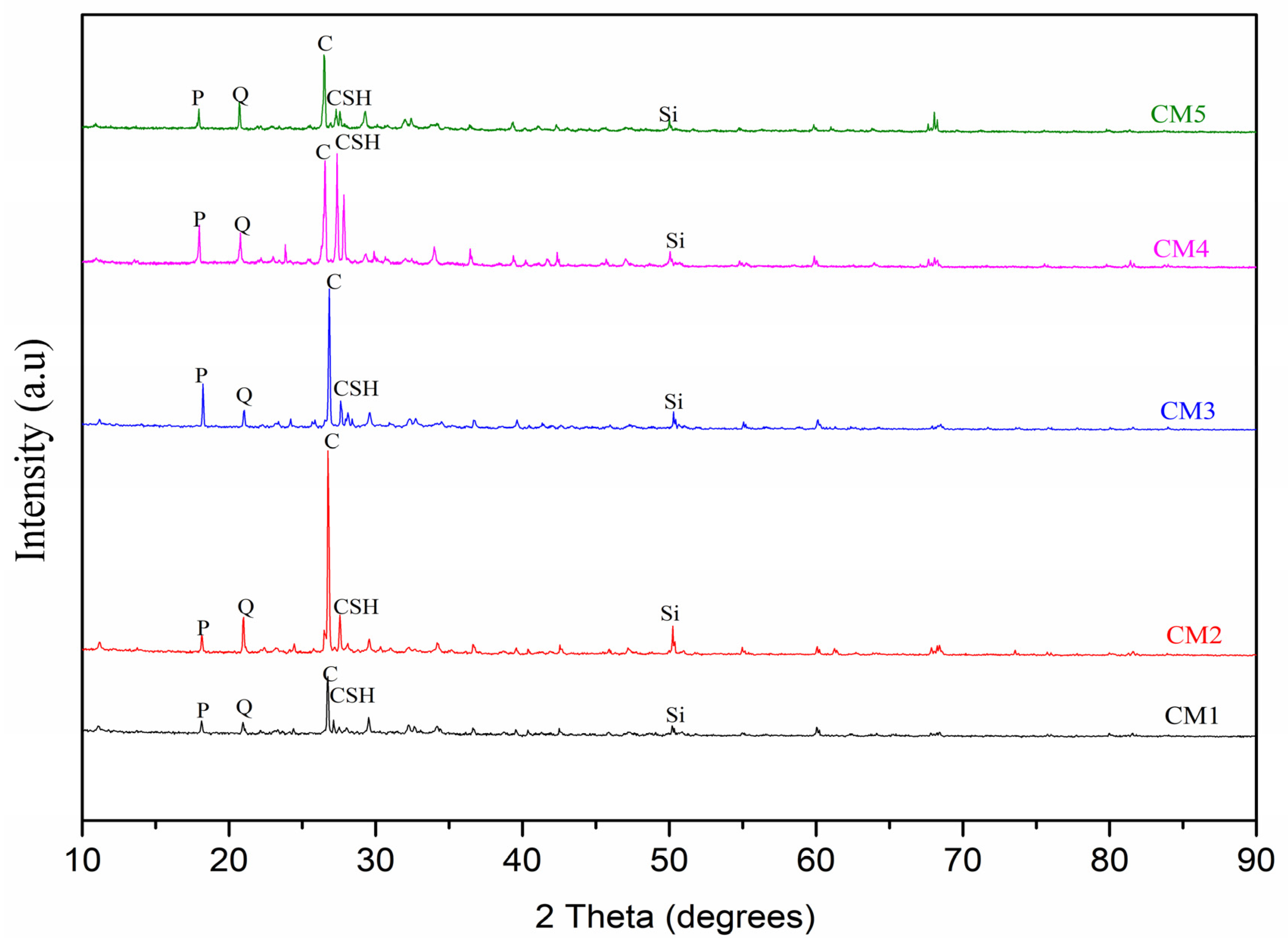
2.5. X-ray Diffraction Analysis (XRD)
2.6. Water Absorption
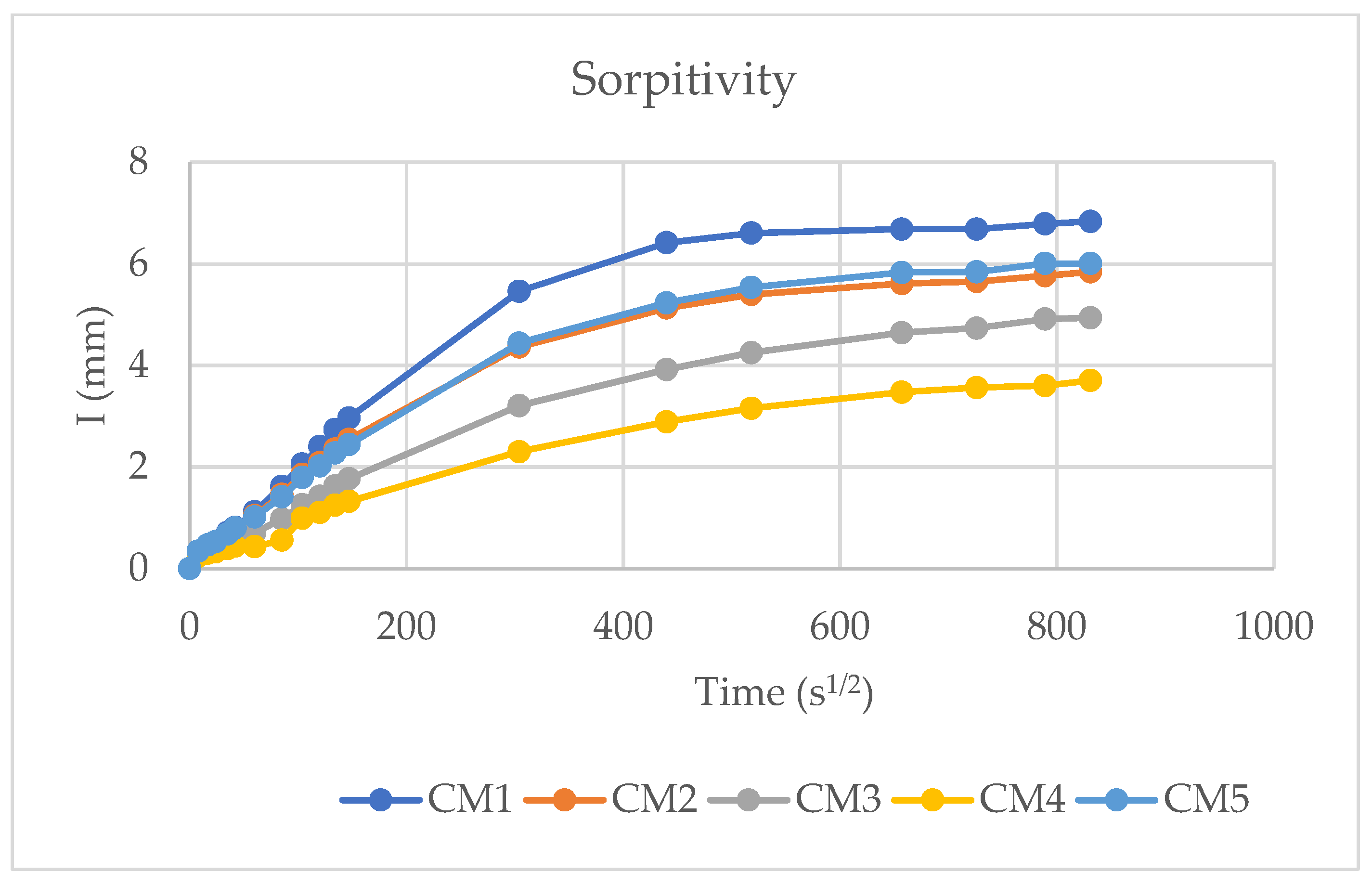
2.7. Sorptivity
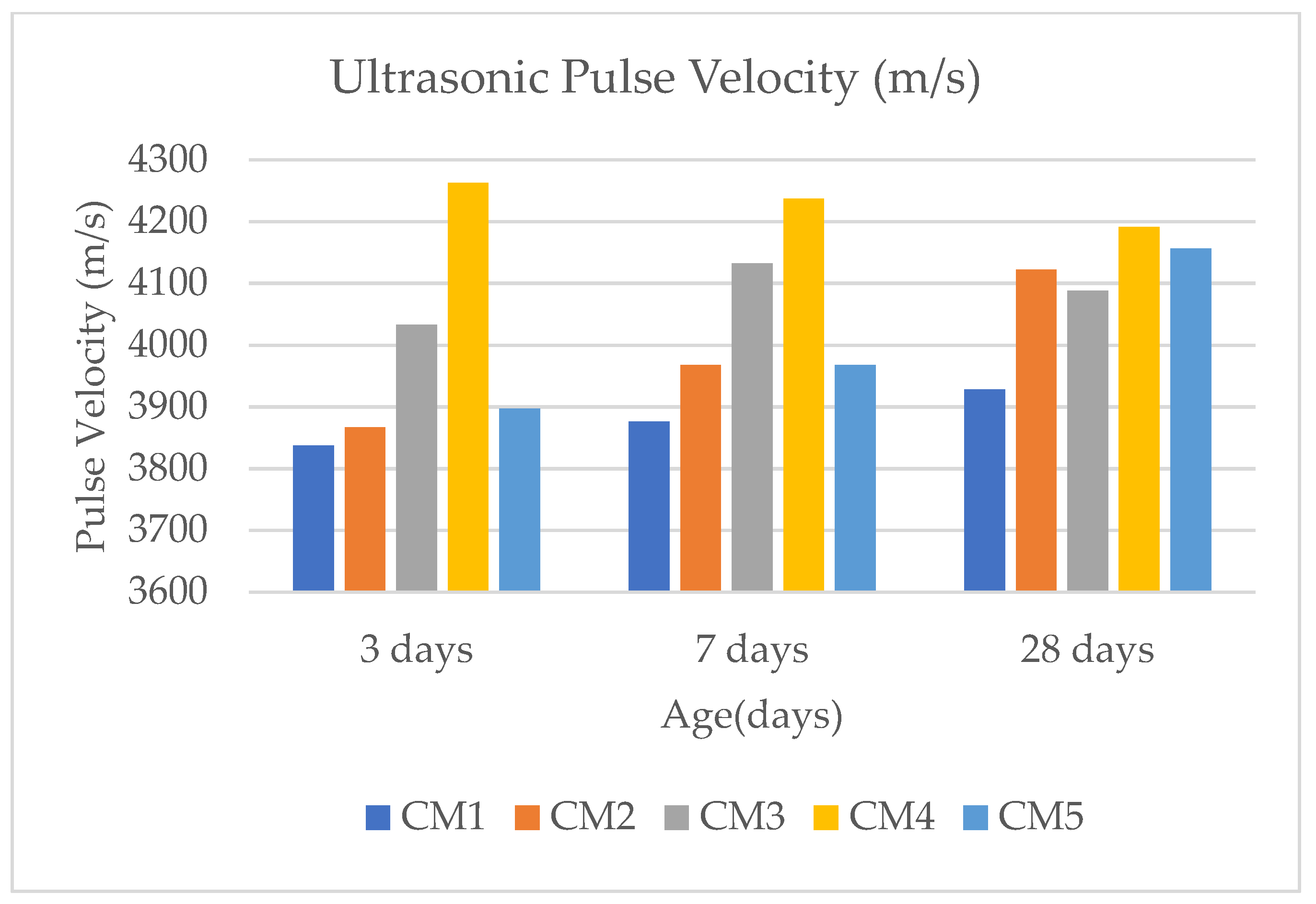
2.8. Ultrasonic Pulse Velocity
2.9. Rapid Chloride Permeability Test
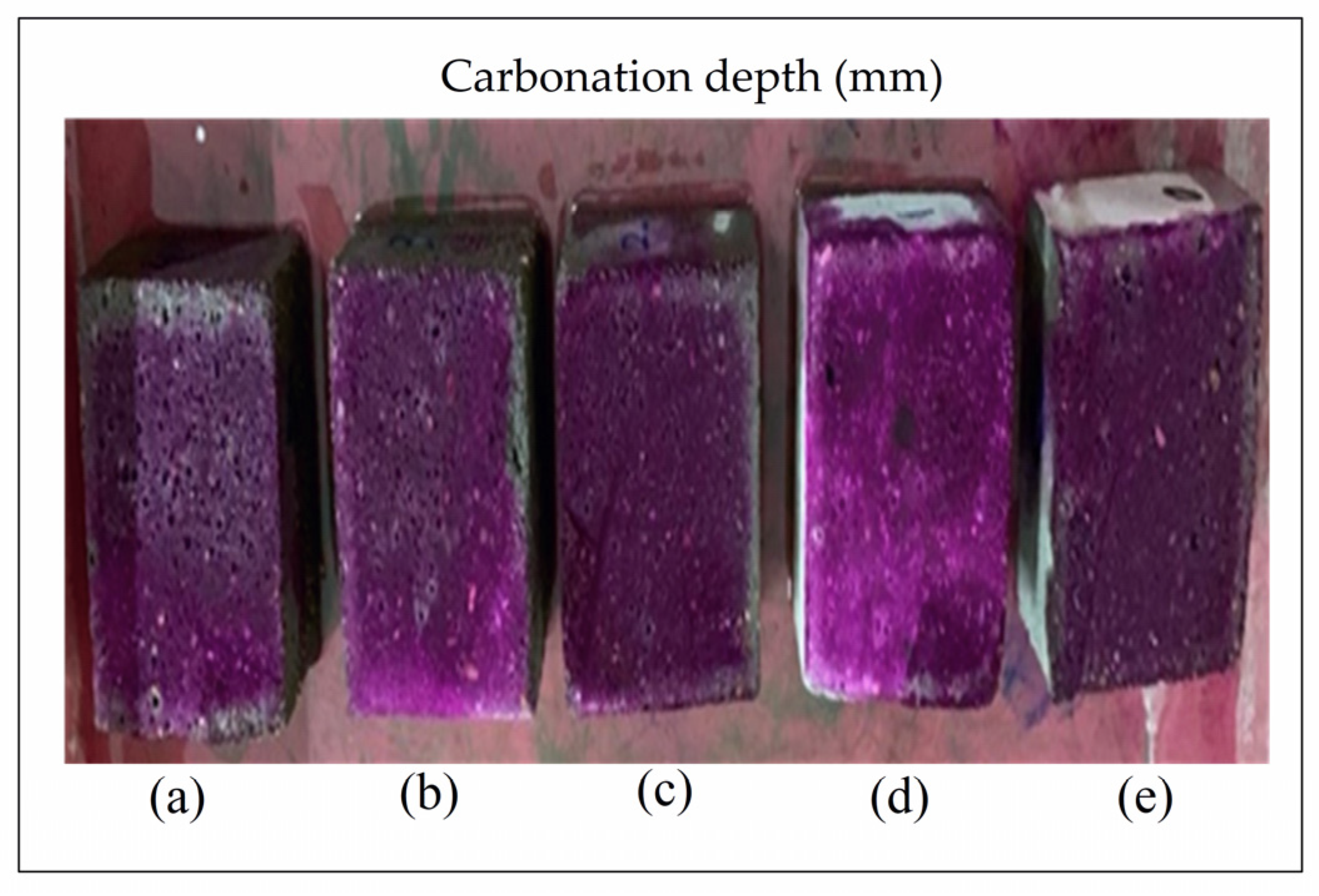
2.10. Carbonation
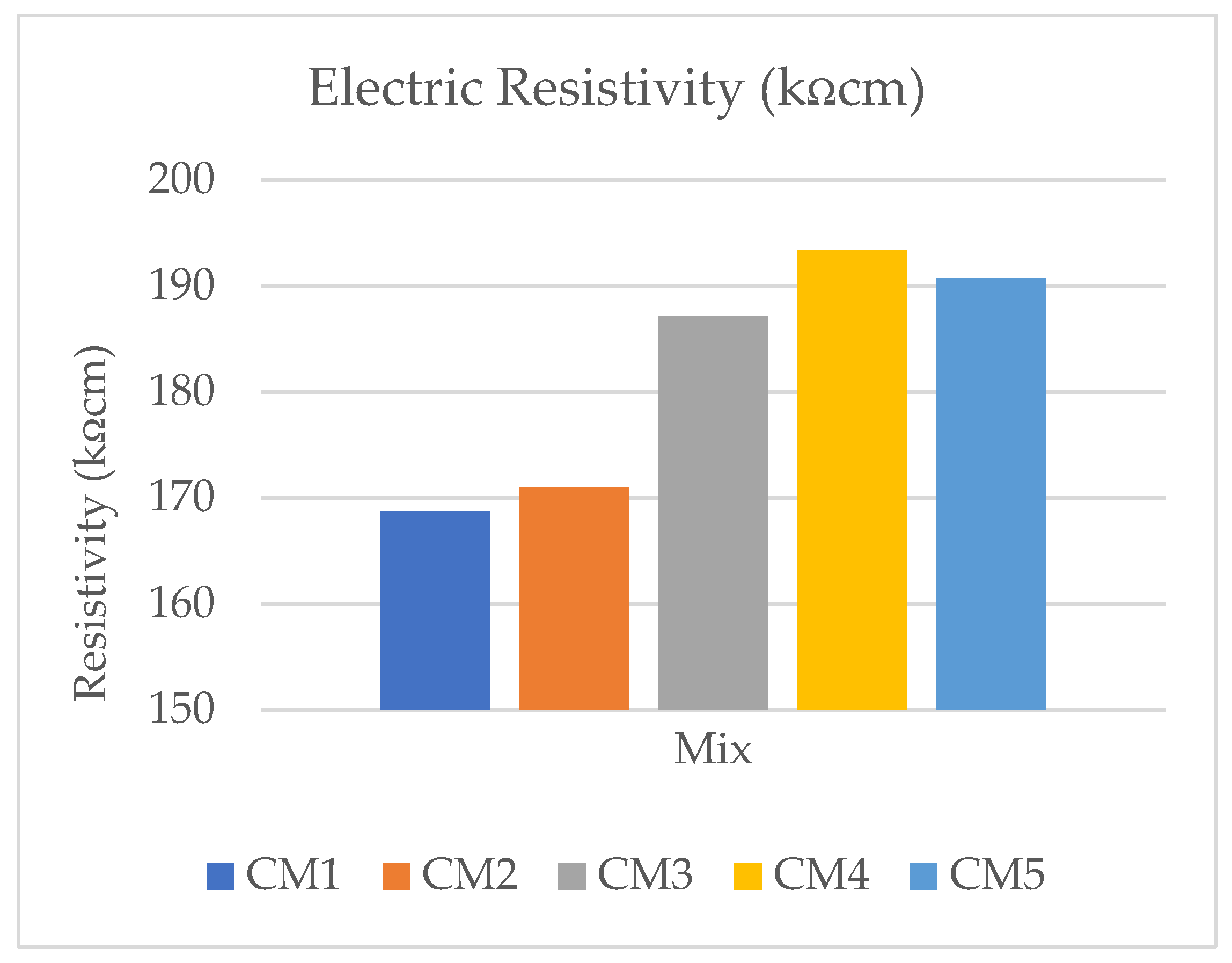
2.11. Electric Resistivity
3. Results and Discussion
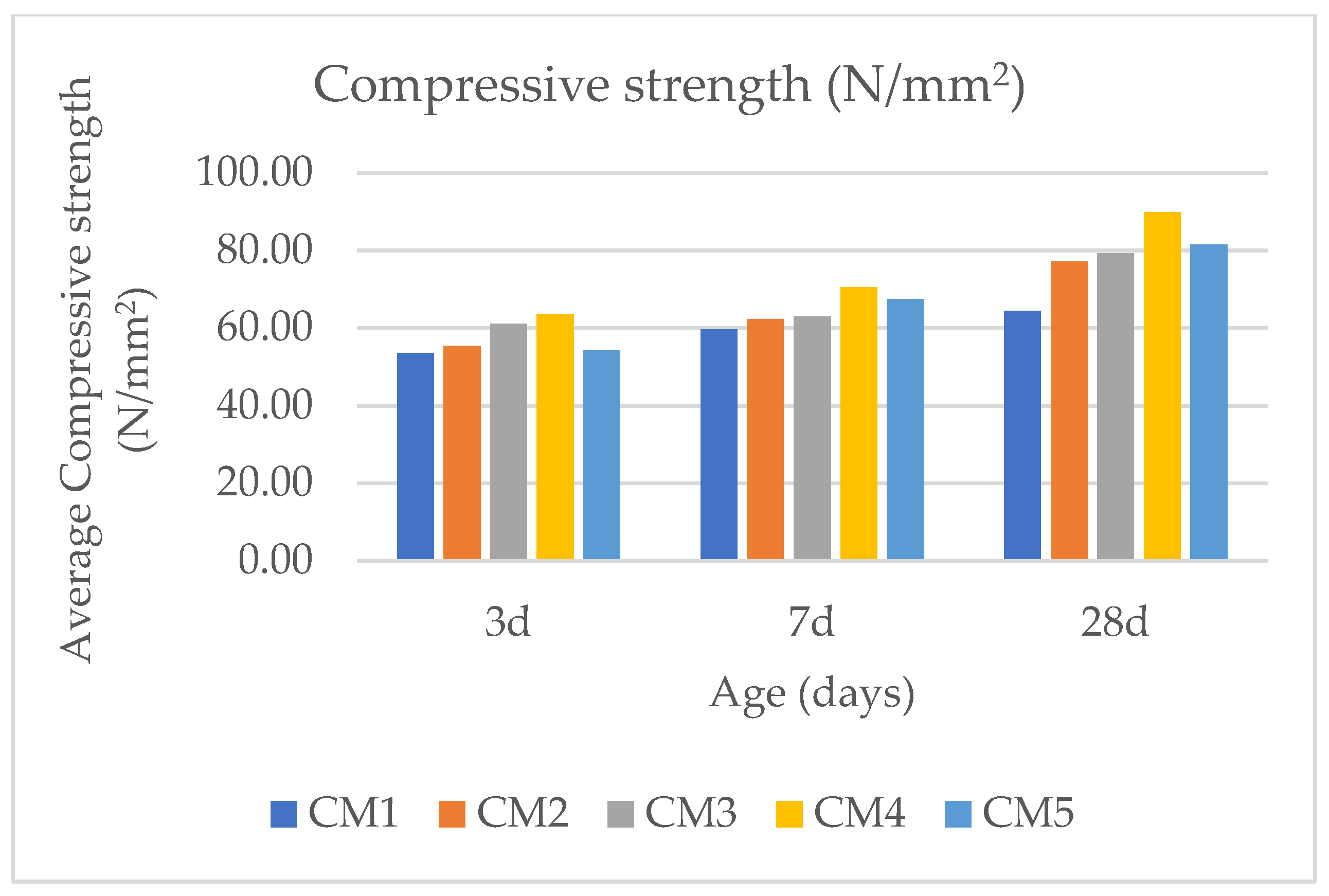
3.1. Compressive Strength of CM-UFS-GO
3.2. FE-SEM and EDX of CM-UFS-GO
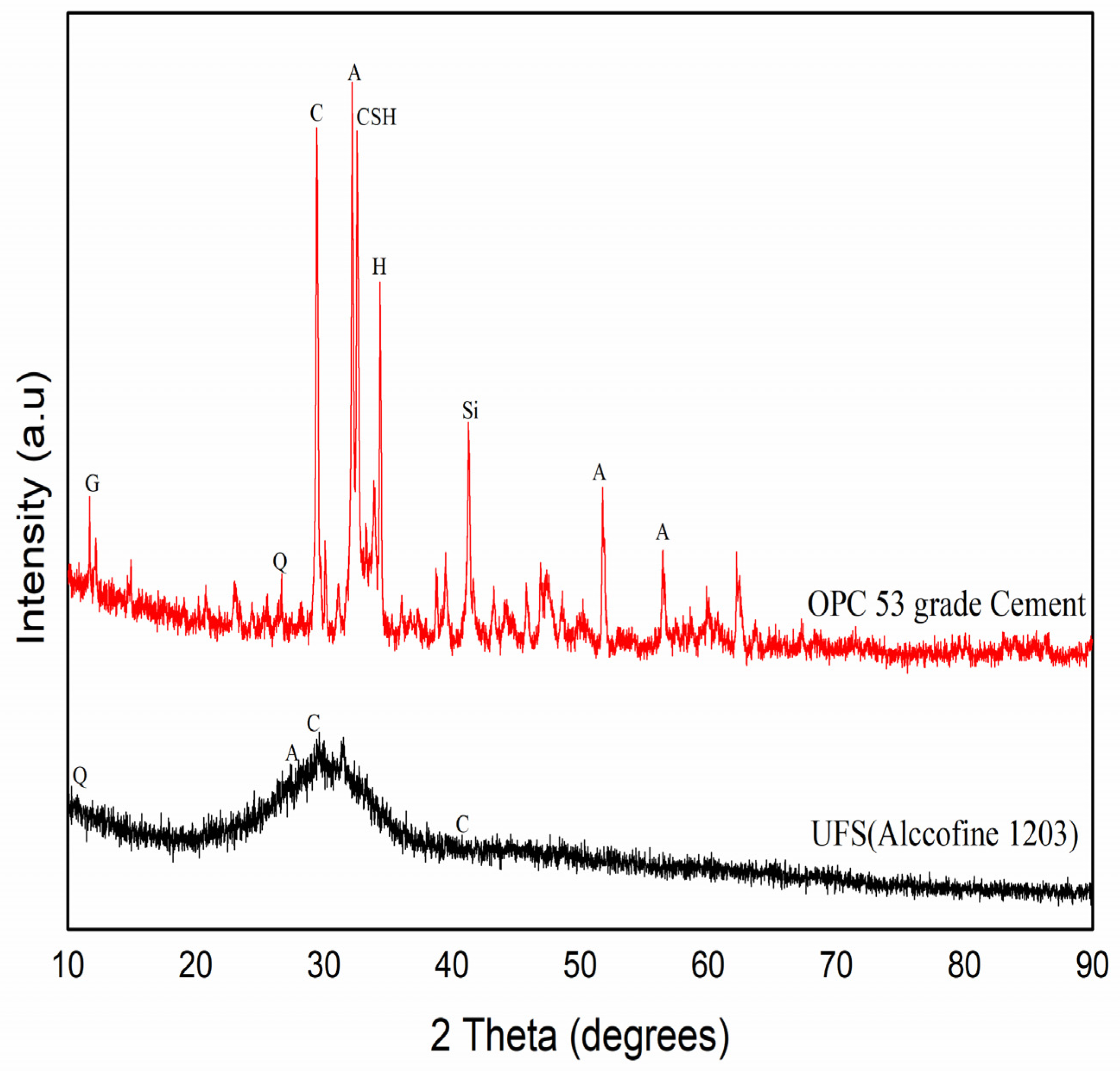
3.3. XRD of CM-UFS-GO
3.4. Water Absorption
3.5. Water Sorptivity
3.6. Ultrasonic Pulse Velocity
3.7. Rapid Chloride Permeability Test
3.8. Carbonation
3.9. Electric Resistivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhushan Jindal, B.; Jangra, P.; Garg, A. Effects of Ultra Fine Slag as Mineral Admixture on the Compressive Strength, Water Absorption and Permeability of Rice Husk Ash Based Geopolymer Concrete. Mater. Today Proc. 2020, 32, 871–877. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical Properties and Microstructure of a Graphene Oxide–Cement Composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Silvestro, L.; Jean Paul Gleize, P. Effect of Carbon Nanotubes on Compressive, Flexural and Tensile Strengths of Portland Cement-Based Materials: A Systematic Literature Review. Constr. Build. Mater. 2020, 264, 120237. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Hanjitsuwan, S.; Hatanaka, S. The Effect of Adding Nano-SiO2 and Nano-Al2O3 on Properties of High Calcium Fly Ash Geopolymer Cured at Ambient Temperature. Mater. Des. 2014, 55, 58–65. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Ariffin, N.F.; Hussin, M.W.; Mohd Sam, A.R.; Bhutta, M.A.R.; Nur, N.H.; Mirza, J. Strength Properties and Molecular Composition of Epoxy-Modified Mortars. Constr. Build. Mater. 2015, 94, 315–322. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Nam, B.H.; Alharbi, Y.; Cho, B.H.; Khawaji, M. Edge-Oxidized Graphene Oxide (EOGO) in Cement Composites: Cement Hydration and Microstructure. Compos. Part B Eng. 2019, 173, 106795. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, F.; Liu, R.; Zhang, R.; Liu, Z.; Liu, H. Effects of Pozzolanic and Non-Pozzolanic Nanomaterials on Cement-Based Materials. Constr. Build. Mater. 2019, 213, 1–9. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Zhang, J.; Geng, Y.; Guo, H. Preparation and Evaluation of Exhaust-Purifying Cement Concrete Employing Titanium Dioxide. Materials 2019, 12, 2182. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; She, X.; Tan, G.; Tanner, J.E. Modification effects of nano-SiO2 on early compressive strength and hydration characteristics of high-volume fly ash concrete. J. Mater. Civ. Eng. 2019, 31, 04019057. [Google Scholar] [CrossRef]
- da Rocha Segundo, I.G.; Dias, E.A.L.; Fernandes, F.D.P.; de Freitas, E.F.; Costa, M.F.; Carneiro, J.O. Photocatalytic Asphalt Pavement: The Physicochemical and Rheological Impact of TiO2 Nano/Microparticles and ZnO Microparticles onto the Bitumen. Road Mater. Pavement Des. 2019, 20, 1452–1467. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, M.; Dehghani, A.; Sherif, M.M. Carbon Nanotube Reinforced Cementitious Composites: A Comprehensive Review. Constr. Build. Mater. 2022, 315, 125100. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of Bacteria on the Compressive Strength, Water Absorption and Rapid Chloride Permeability of Fly Ash Concrete. Constr. Build. Mater. 2012, 28, 351–356. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Li, W.G.; Li, C.Y.; Sanjayan, J.G.; Duan, W.H.; Li, Z. Effects of Graphene Oxide Agglomerates on Workability, Hydration, Microstructure and Compressive Strength of Cement Paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- e Silva, R.A.; de Castro Guetti, P.; da Luz, M.S.; Rouxinol, F.; Gelamo, R.V. Enhanced Properties of Cement Mortars with Multilayer Graphene Nanoparticles. Constr. Build. Mater. 2017, 149, 378–385. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. Dispersion and Stability of Graphene Nanoplatelet in Water and Its Influence on Cement Composites. Constr. Build. Mater. 2018, 167, 403–413. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano Reinforced Cement and Concrete Composites and New Perspective from Graphene Oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Wei, Y.; Miao, Z.; Jia, Z.; Wang, Y.; Zhou, Y.; Zhang, H.; Wei, J. Synergy of Reduced Graphene Oxide and Metal Oxides Improves the Power Factor of Thermoelectric Cement Matrix Composites. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 801–813. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Sun, T.; Ma, Y.; Zhou, Q. Effect of GO Nanosheets on Shapes of Cement Hydration Crystals and Their Formation Process. Constr. Build. Mater. 2014, 64, 231–239. [Google Scholar] [CrossRef]
- Saloni; Parveen; Lim, Y.Y.; Pham, T.M. Effective Utilisation of Ultrafine Slag to Improve Mechanical and Durability Properties of Recycled Aggregates Geopolymer Concrete. Clean. Eng. Technol. 2021, 5, 100330. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Kalenczuk, R.J.; Aleksandrzak, M.; Mijowska, S. Nanocomposite of Cement/Graphene Oxide—Impact on Hydration Kinetics and Young’s Modulus. Constr. Build. Mater. 2015, 78, 234–242. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide Using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- ASTM C109/109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or Cube Specimens). Annual Book of ASTM Standards: West Conshohocken, PA, USA, 2016; Volume 4, pp. 1–10.
- Kavyateja, B.V.; Guru Jawahar, J.; Sashidhar, C. Effectiveness of Alccofine and Fly Ash on Mechanical Properties of Ternary Blended Self Compacting Concrete. Mater. Today Proc. 2020, 33, 73–79. [Google Scholar] [CrossRef]
- Indukuri, C.S.R.; Nerella, R. Enhanced Transport Properties of Graphene Oxide Based Cement Composite Material. J. Build. Eng. 2021, 37, 102174. [Google Scholar] [CrossRef]
- Jumate, E.; Manea, D.L. Application of X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) Methods to the Portland Cement Hydration Processes. J. Appl. Eng. Sci. 2012, 2, 35–42. [Google Scholar]
- Chintalapudi, K.; Mohan, R.; Pannem, R. Enhanced Microstructural Characteristics of Binary and Ternary Blended Cements Reinforced with Graphene Oxide. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 987–1001. [Google Scholar] [CrossRef]
- ASTMC642-21 C 642-21; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International Standard: West Conshohocken, PA, USA, 2021; pp. 11–13. [CrossRef]
- ASTM C1585-13; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes. ASTM International Standard: West Conshohocken, PA, USA, 2013; Volume 41, pp. 1–6. [CrossRef]
- Prabavathy, S.; Jeyasubramanian, K.; Prasanth, S.; Hikku, G.S.; Robert, R.B.J. Enhancement in Behavioral Properties of Cement Mortar Cubes Admixed with Reduced Graphene Oxide. J. Build. Eng. 2020, 28, 101082. [Google Scholar] [CrossRef]
- ASTM C 597-02; Pulse Velocity Through Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016; Volume 4, pp. 3–6. [CrossRef]
- ASTM C1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. The American Society for Testing and Materials: West Conshohocken, PA, USA, 2012; pp. 1–8. [CrossRef]
- Chintalapudi, K.; Pannem, R.M.R. The Effects of Graphene Oxide Addition on Hydration Process, Crystal Shapes, and Microstructural Transformation of Ordinary Portland Cement. J. Build. Eng. 2020, 32, 101551. [Google Scholar] [CrossRef]
- Malikov, E.Y. The Effect of Polyvinyl Alcohol Functionalized Multiwall Carbon Nanotubes on the Improvement of the Compressive Strength of Concrete. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 781–785. [Google Scholar] [CrossRef]
- Yeswanth Sai, T.; Jagadeesh, P. Effect of Graphene Oxide on the Microstructure and Hydration Characteristics of Ultrafine Slag Cement Composites. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 1054–1065. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Tsai, S.-L.; Hung, C.-C.; Jian, T.-Y. Research on Engineering Properties of Cement Mortar Adding Stainless Steel Reduction Slag and Pozzolanic Materials. Case Stud. Constr. Mater. 2022, 16, e01144. [Google Scholar] [CrossRef]
- Ghazanlou, S.I.; Ghazanlou, S.I.; Ashraf, W. Improvement in the Physical and Mechanical Properties of the Cement-Based Composite with the Addition of Nanostructured BN–Fe3O4 Reinforcement. Sci. Rep. 2021, 11, 19358. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.F.; Zhang, L.W.; Liew, K.M. Graphene and Graphene Oxide in Calcium Silicate Hydrates: Chemical Reactions, Mechanical Behavior and Interfacial Sliding. Carbon 2019, 146, 181–193. [Google Scholar] [CrossRef]
- Singh, M.; Siddique, R. Effect of Low-Calcium Coal Bottom Ash as Fine Aggregate on Microstructure and Properties of Concrete. ACI Mater. J. 2015, 112, 693–703. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Motahari Karein, S.M.; Vosoughi, P.; Pilvar, A.; Isapour, S.; Moodi, F. Effects of Calcined Perlite Powder as a SCM on the Strength and Permeability of Concrete. Constr. Build. Mater. 2014, 66, 222–228. [Google Scholar] [CrossRef]
- Basquiroto de Souza, F.; Yao, X.; Lin, J.; Naseem, Z.; Tang, Z.Q.; Hu, Y.; Gao, W.; Sagoe-Crentsil, K.; Duan, W. Effective Strategies to Realize High-Performance Graphene-Reinforced Cement Composites. Constr. Build. Mater. 2022, 324, 126636. [Google Scholar] [CrossRef]
- Zhai, S.; Pang, B.; Liu, G.; Zhang, Y.; Xu, K.; She, W.; Zhang, Y. Investigation on Preparation and Multifunctionality of Reduced Graphene Oxide Cement Mortar. Constr. Build. Mater. 2021, 275, 122119. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Zhang, Q.; Sagoe-Crentsil, K.; Duan, W. Graphene Oxide-Coated Sand for Improving Performance of Cement Composites. Cem. Concr. Compos. 2021, 124, 104279. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Pan, S.; Cui, X.; Corr, D.J.; Shah, S.P. Effect of Graphene Oxide on the Hydration and Microstructure of Fly Ash-Cement System. Constr. Build. Mater. 2019, 198, 106–119. [Google Scholar] [CrossRef]
- He, W.; Liao, G. Effects of Nano-C-S-H Seed Crystal on Early-Age Hydration Process of Portland Cement. Fuller. Nanotub. Carbon Nanostruct. 2021, 30, 365–372. [Google Scholar] [CrossRef]
- Jing, G.; Wu, J.; Lei, T.; Wang, S.; Strokova, V.; Nelyubova, V.; Wang, M.; Ye, Z. From Graphene Oxide to Reduced Graphene Oxide: Enhanced Hydration and Compressive Strength of Cement Composites. Constr. Build. Mater. 2020, 248, 118699. [Google Scholar] [CrossRef]
- Pei, C.; Ueda, T.; Zhu, J. Investigation of the Effectiveness of Graphene/Polyvinyl Alcohol on the Mechanical and Electrical Properties of Cement Composites. Mater. Struct. 2020, 53, 66. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Qi, X.-Q.; Guo, S.-Y.; Ren, J.; Chen, J.-Z.; Chi, B.; Wang, X.-C. Effect of a Novel Hybrid TiO2-Graphene Composite on Enhancing Mechanical and Durability Characteristics of Alkali-Activated Slag Mortar. Constr. Build. Mater. 2021, 275, 122154. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Hanif, A.; Hao, W.; Li, Z.; Sun, G. Comparative Evaluation on the Dispersion and Stability of Graphene Oxide in Water and Cement Pore Solution by Incorporating Silica Fume. Cem. Concr. Compos. 2018, 94, 33–42. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental Impacts and Decarbonization Strategies in the Cement and Concrete Industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Mowlaei, R.; Lin, J.; Basquiroto de Souza, F.; Fouladi, A.; Habibnejad Korayem, A.; Shamsaei, E.; Duan, W. The Effects of Graphene Oxide-Silica Nanohybrids on the Workability, Hydration, and Mechanical Properties of Portland Cement Paste. Constr. Build. Mater. 2021, 266, 121016. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Fan, J.; Li, P.; Chen, D. Dispersion of Triethanolamine-Functionalized Graphene Oxide (TEA-GO) in Pore Solution and Its Influence on Hydration, Mechanical Behavior of Cement Composite. Constr. Build. Mater. 2019, 216, 128–136. [Google Scholar] [CrossRef]
- Wang, B.; Pang, B. Mechanical Property and Toughening Mechanism of Water Reducing Agents Modified Graphene Nanoplatelets Reinforced Cement Composites. Constr. Build. Mater. 2019, 226, 699–711. [Google Scholar] [CrossRef]
- Soldado, E.; Antunes, A.; Costa, H.; do Carmo, R.; Júlio, E. Durability of Mortar Matrices of Low-Cement Concrete with Specific Additions. Constr. Build. Mater. 2021, 309, 125060. [Google Scholar] [CrossRef]




| S No | Test Details | Total Number of Specimens Casted | Age (Days) | Size of Specimen |
|---|---|---|---|---|
| 1 | Compression strength (CS) | 45 | 3, 7, 28 days | Cubes of 50 mm × 50 mm × 50 mm |
| 2 | Water absorption | 15 | 3, 7, 28 days | Circular discs of diameter 100 mm and height 50 mm |
| 3 | Sorptivity | 15 | 28 days | Circular discs of diameter 100 mm and height 50 mm |
| 4 | Ultrasonic pulse velocity (UPV) | 15 | 3, 7, 28 days | Cubes of 50 mm × 50 mm × 50 mm |
| 5 | Rapid chloride permeability test (RCPT) | 15 | 28 days | Circular discs of diameter 100 mm and height 50 mm |
| 6 | Carbonation | 5 | 28 days | Cubes of 50 mm × 50 mm × 50 mm |
| 7 | Electric resistivity | 5 | 28 days | Cylinder of diameter 100 mm and height 200 mm |
| MIX | OPC (kg·m−3) | River Sand (kg·m−3) | UFS (kg·m−3) | GO (kg·m−3) | Water (kg·m−3) | Superplasticizer Dosage (%) |
|---|---|---|---|---|---|---|
| CM1 | 599.76 | 1833.28 | 66.64 | - | 322.4 | 1 |
| CM2 | 0.066 | |||||
| CM3 | 0.132 | |||||
| CM4 | 0.199 | |||||
| CM5 | 0.266 |
| MIX | 3 Days | 7 Days | 28 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Avg CS (N/mm2) | SD | CoV (%) | Avg CS (N/mm2) | SD | CoV (%) | Avg CS (N/mm2) | SD | CoV (%) | |
| CM1 | 53.47 | 2.84 | 5.31 | 59.60 | 2.29 | 3.84 | 64.40 | 2.23 | 3.46 |
| CM2 | 55.33 | 1.94 | 3.51 | 62.27 | 2.00 | 3.22 | 77.07 | 1.92 | 2.49 |
| CM3 | 61.07 | 1.9 | 3.11 | 62.93 | 1.79 | 2.85 | 79.20 | 1.56 | 1.97 |
| CM4 | 63.60 | 1.26 | 1.98 | 70.53 | 1.22 | 1.73 | 89.87 | 0.42 | 0.46 |
| CM5 | 54.27 | 1.30 | 2.40 | 67.47 | 1.81 | 2.69 | 81.47 | 1.21 | 1.48 |
| Mix | CM1 | CM2 | CM3 | CM4 | CM5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % |
| C | 9.27 | 15.94 | 16.62 | 24.91 | 8.21 | 15.1 | 8.29 | 16.04 | 8.89 | 15.57 |
| O | 45.63 | 55.66 | 52.88 | 59.49 | 37.39 | 51.63 | 30.88 | 44.84 | 41.91 | 55.14 |
| Mg | 0.89 | 0.75 | 0.85 | 0.63 | 0.77 | 0.7 | 0.97 | 0.93 | 0.77 | 0.67 |
| Al | 4.01 | 3.46 | 4.06 | 2.71 | 3.63 | 2.98 | 2.08 | 1.79 | 4.4 | 3.43 |
| Si | 11.49 | 9.45 | 9.26 | 3.95 | 8.67 | 6.82 | 11.68 | 9.66 | 9.23 | 6.92 |
| Ca | 28.71 | 14.74 | 16.33 | 8.31 | 41.33 | 22.78 | 46.1 | 26.73 | 34.8 | 18.27 |
| Total (%) | 100 | 100 | 100 | 100 | 100 | |||||
| Mix | Avg Charge Passed (Coulombs) | % Variation | SD | CoV (%) |
|---|---|---|---|---|
| CM1 | 2527.7 | - | 4.47 | 0.18 |
| CM2 | 2253.1 | 10.86 | 4.22 | 0.19 |
| CM3 | 2238.2 | 11.45 | 2.65 | 0.12 |
| CM4 | 1480.7 | 41.42 | 2.06 | 0.15 |
| CM5 | 2123.4 | 15.99 | 2.71 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatineni, Y.S.; Putta, J. Enhanced Strength, Durability, and Microstructural Attributes of Graphene Oxide-Modified Ultrafine Slag Cement Mortar. Buildings 2022, 12, 2199. https://doi.org/10.3390/buildings12122199
Tatineni YS, Putta J. Enhanced Strength, Durability, and Microstructural Attributes of Graphene Oxide-Modified Ultrafine Slag Cement Mortar. Buildings. 2022; 12(12):2199. https://doi.org/10.3390/buildings12122199
Chicago/Turabian StyleTatineni, Yeswanth Sai, and Jagadeesh Putta. 2022. "Enhanced Strength, Durability, and Microstructural Attributes of Graphene Oxide-Modified Ultrafine Slag Cement Mortar" Buildings 12, no. 12: 2199. https://doi.org/10.3390/buildings12122199





