Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Methods
3. Optimization of Mechanical Properties by Response Surface Methodology
3.1. Single Factor Test
3.2. Optimization of Mechanical Properties by Response Surface Methodology
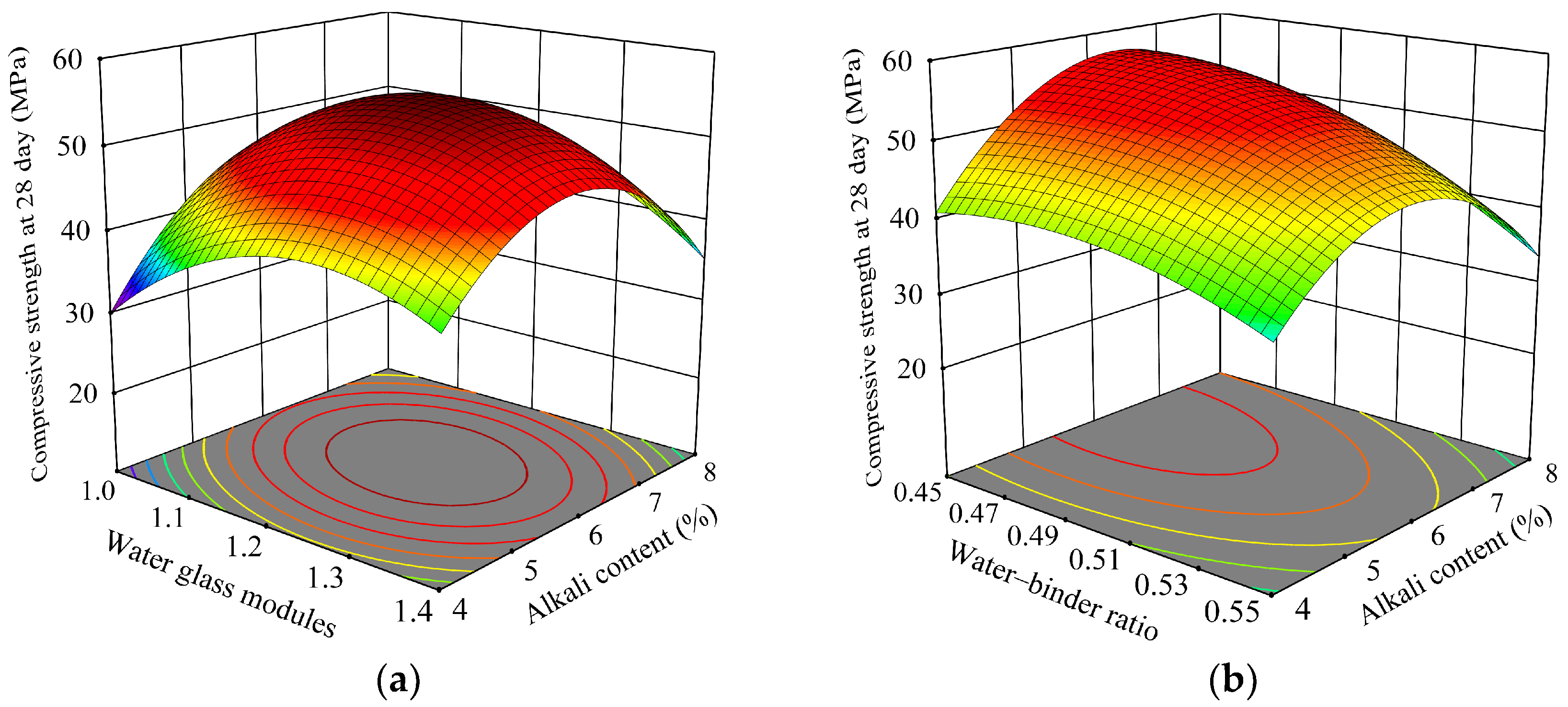
3.2.1. Response Surface Equation Establishment and Variance Analysis
3.2.2. Analysis of Variance for Influencing Factors
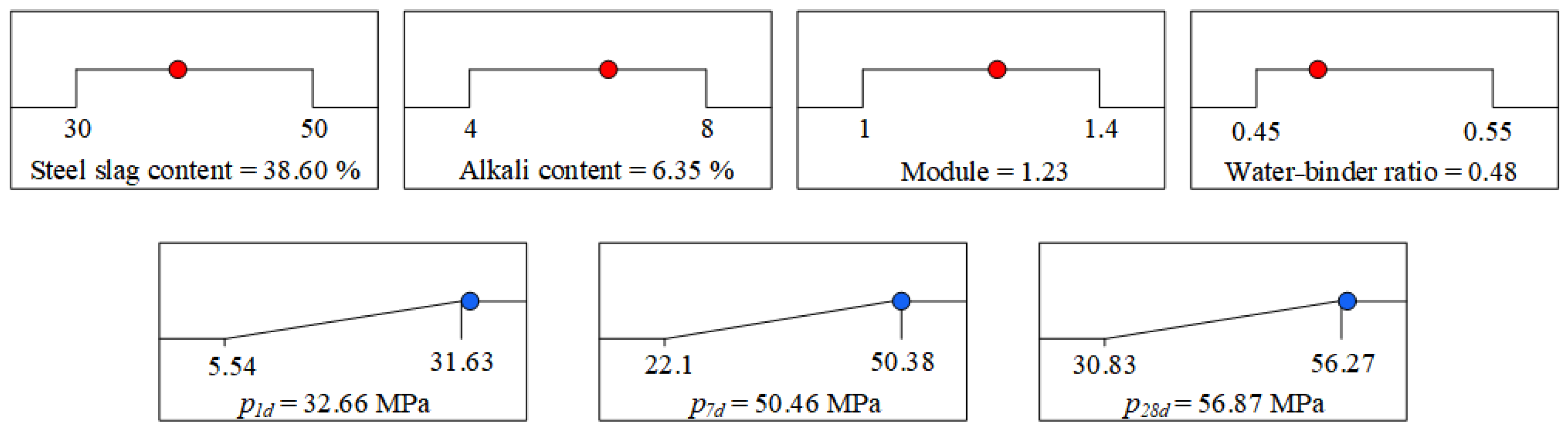
3.2.3. Response Surface Optimization Results and Validation
4. Microscopic Mechanism
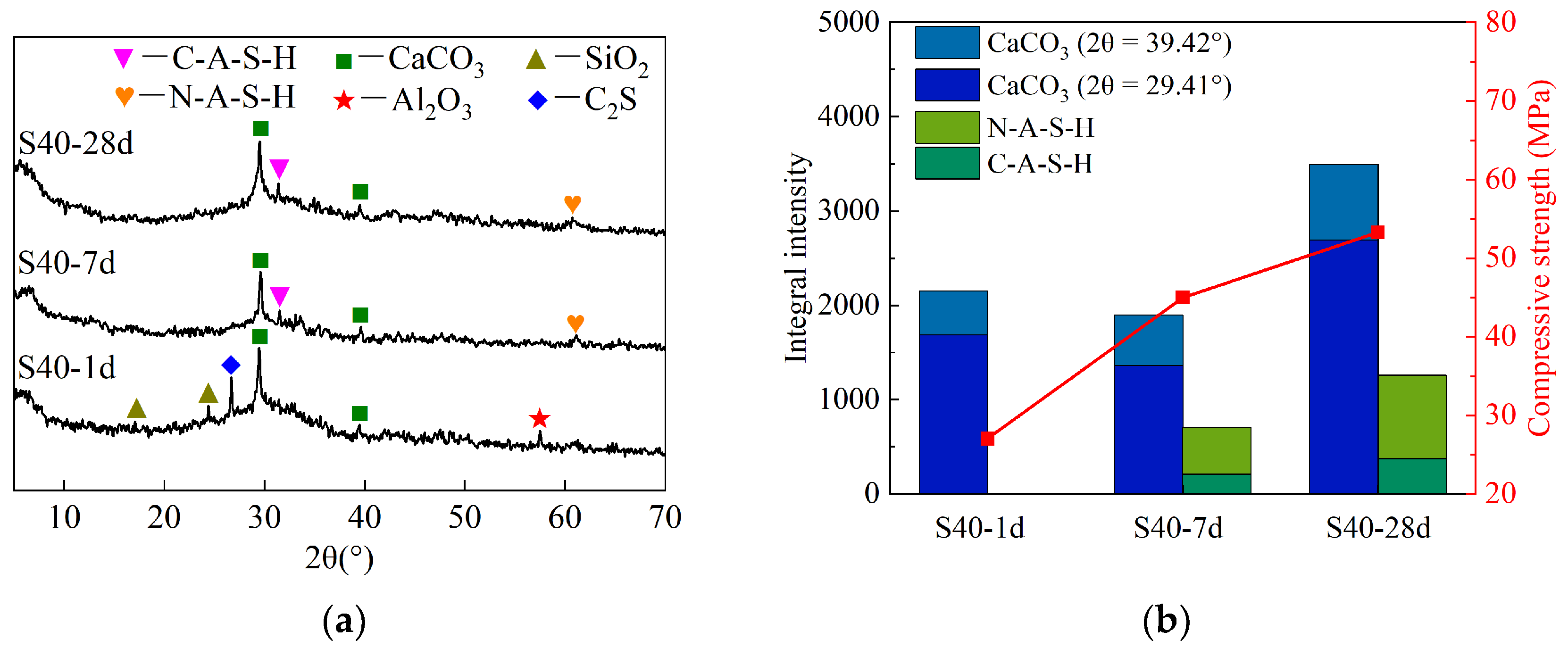
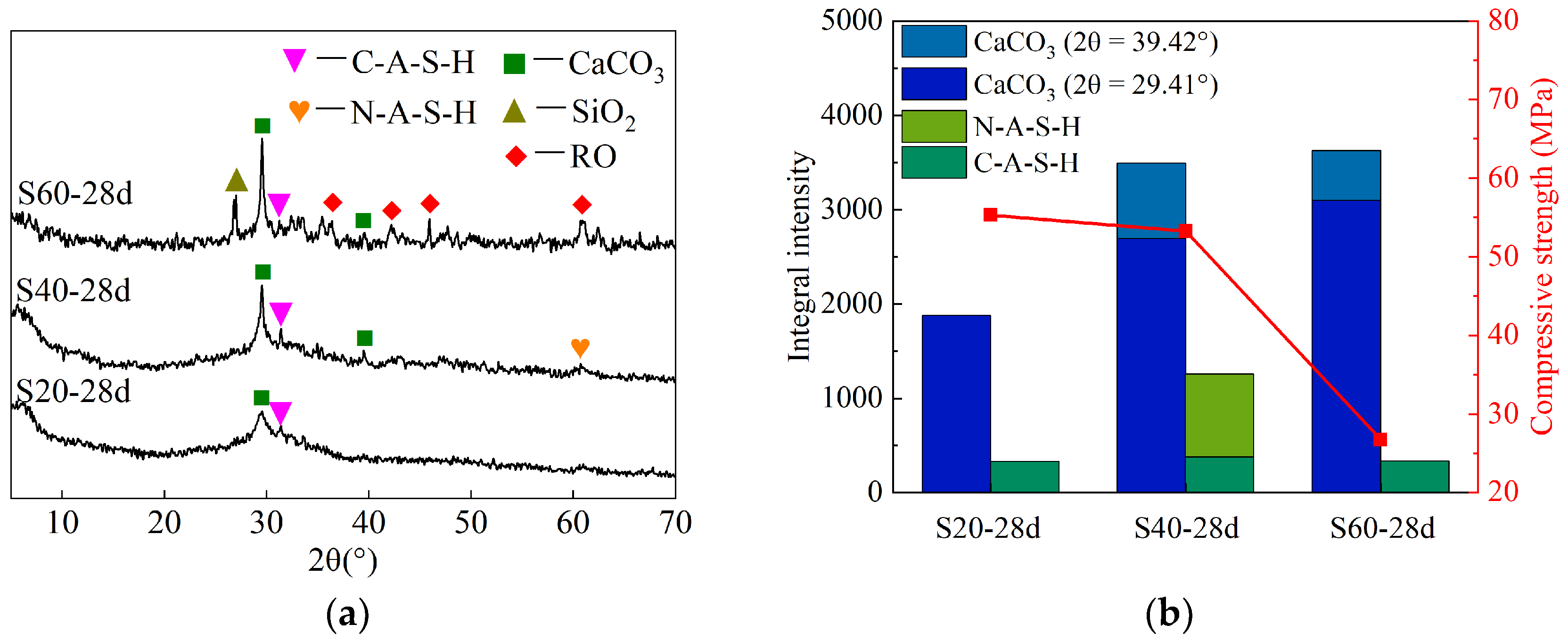
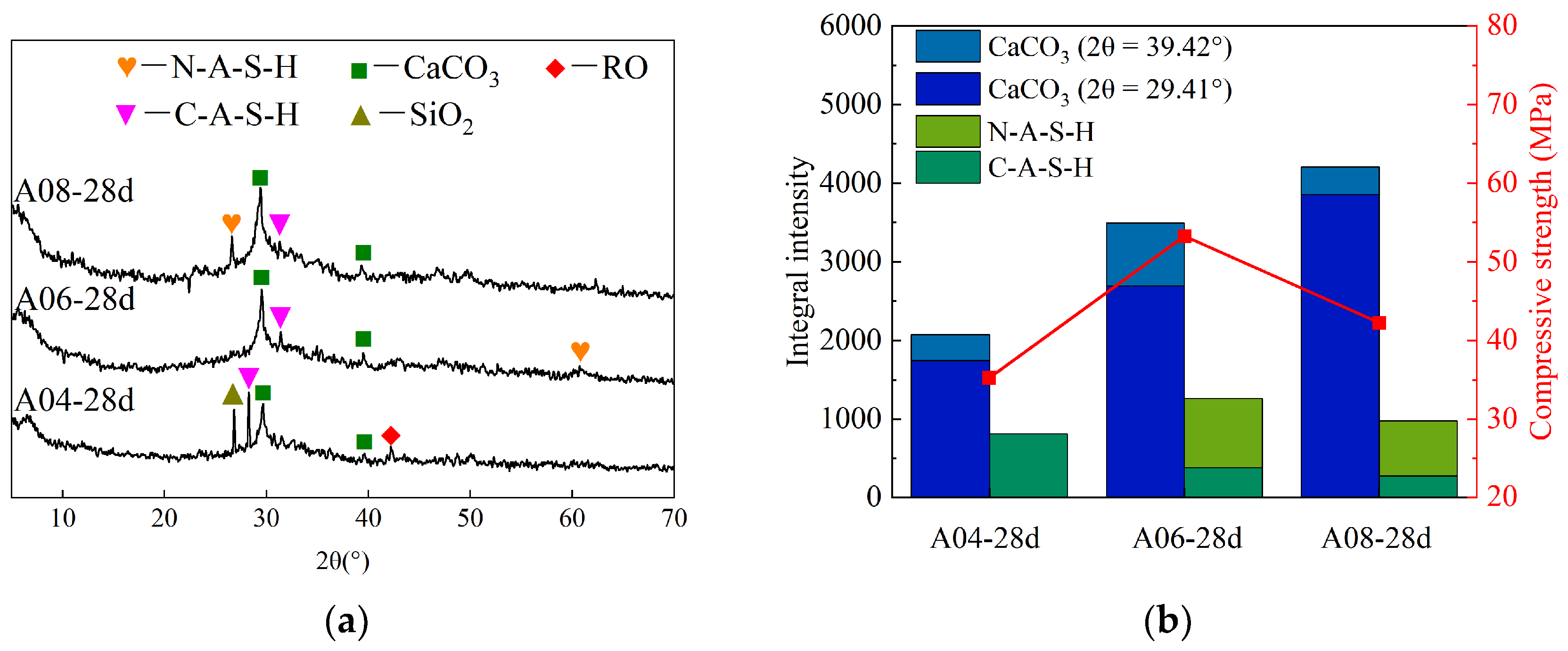
4.1. XRD
4.2. SEM
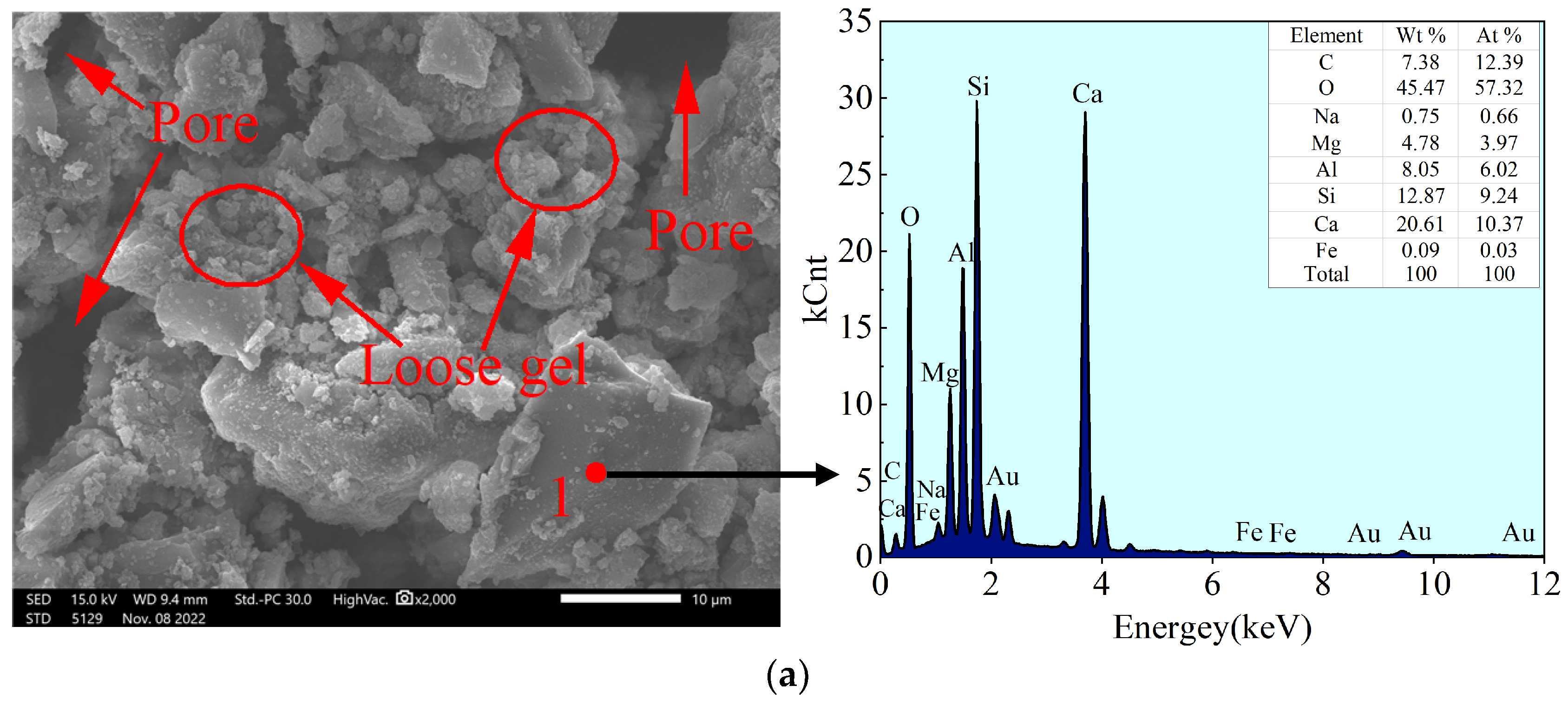
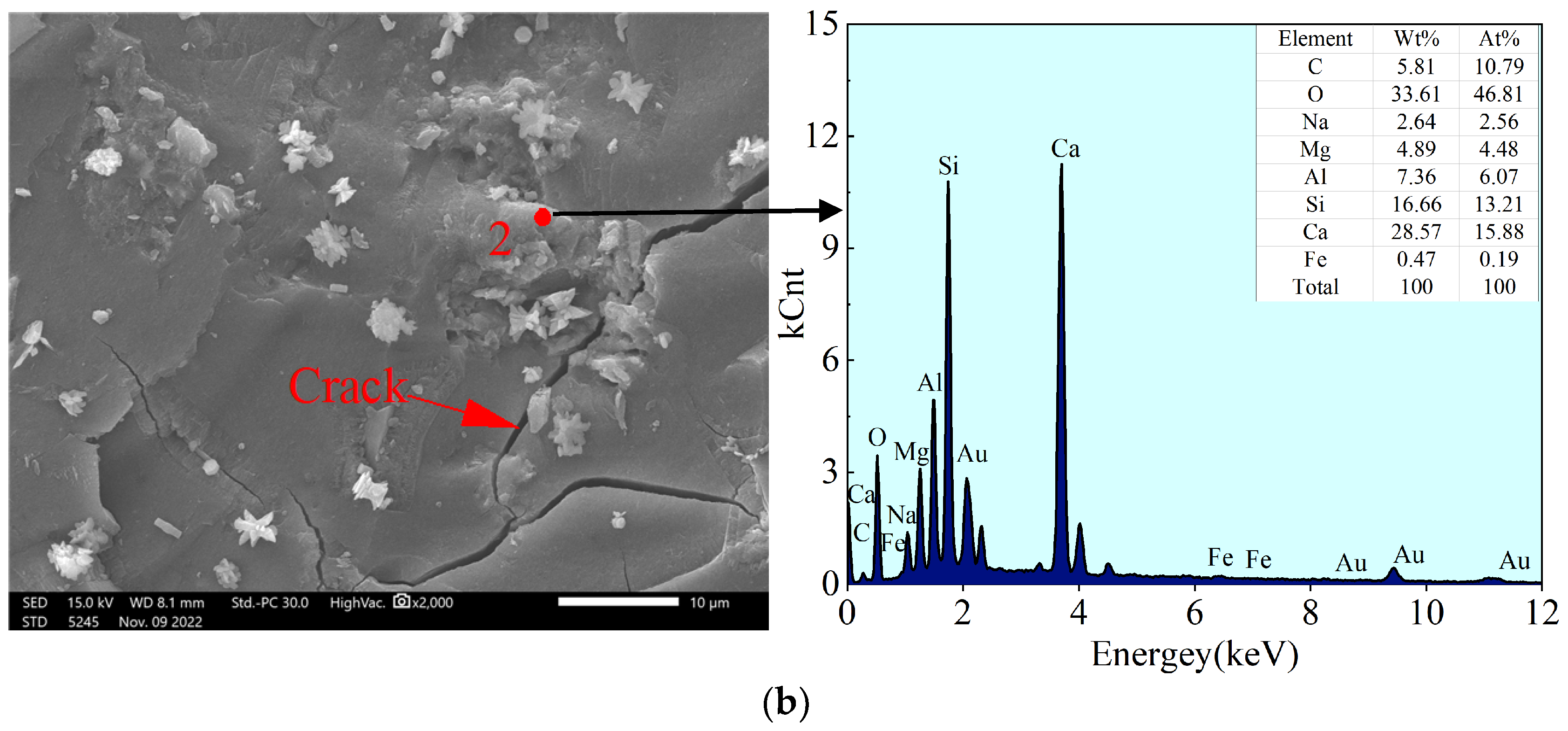
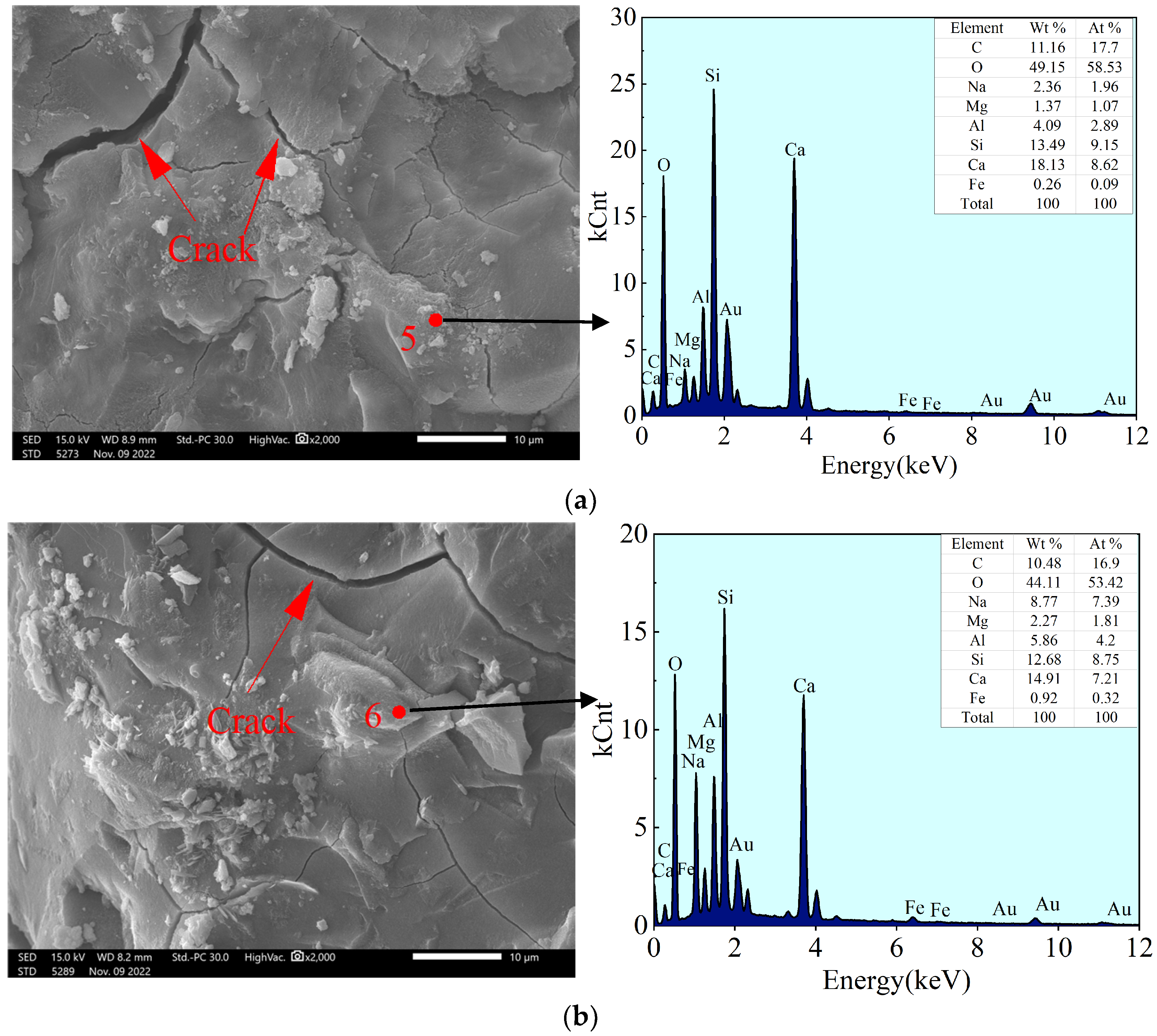
4.2.1. Morphology and Energy Spectrum Analysis
4.2.2. Pore (Crack) Gap Structure
5. Discussion
6. Conclusions
- (1)
- Individual variations in the four factors manifested diverse patterns of influence on the compressive strength of the specimen. The variation range of compressive strength for samples with steel slag content of approximately 40%, alkali content of approximately 6%, water glass modulus near 1.2, and water–binder ratio near 0.5 was significant, making these values suitable as the central-level value for response surface methodology. Based on the principle of maximizing steel slag content and three response values, the optimal mix ratio was determined as follows: a steel slag content of 38.596%, an alkali content of 6.348%, water glass modulus of 1.227, and water–binder ratio of 0.476. With these values, the response surface model predicted that the compressive strength at 1 day, 7 day, and 28 day would be 32.657 MPa, 50.461 MPa, and 56.867 MPa, respectively.
- (2)
- The XRD analysis revealed that the fluctuations in compressive strength among stone bodies at different curing ages primarily stem from the geopolymerization reaction process. The 28-day strength of specimens with varying steel slag contents was influenced by the hydration gel, geopolymer gel, and CaCO3 content, whereas those with varying alkali contents were influenced by the former two types of gels.
- (3)
- The SEM-EDS analysis indicated that the gel formed in this system mainly consisted of C-S-H gel generated by the reaction of volcanic ash and (N, C)-A-S-H gel generated by geopolymerization. The analysis of pores (cracks) in the binary form indicated that the change in compressive strength was mainly influenced by porosity, followed by the pore fractal dimension.
- (4)
- A microreaction model for alkali-activated steel slag–slag cementitious materials was proposed. This suggested that the process of alkaline activation of steel slag and blast furnace slag is essentially a combination of volcanic ash and geopolymerization reactions of active substances in the raw materials under alkaline conditions, resulting in compact adhesion with insoluble particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.L.; Bao, Y.P.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Chen, G.X.; Wang, S.Y. Research on macro-microscopic mechanical evolution mechanism of cement-stabilized steel slag. J. Build. Eng. 2023, 75, 107047. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Dasu, J.; Murthy, Y.R.; Kapure, G.; Pal, A.R.; Filippov, L.O. Utilisation perspective on water quenched and air-cooled blast furnace slags. J. Clean. Prod. 2020, 262, 121354. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, H.; Cui, L.; Yang, S.; Zhang, C.; Liu, W.; Zheng, D. Mutual Activation Mechanism of Cement–GGBS–Steel Slag Ternary System Excited by Sodium Sulfate. Buildings 2024, 14, 631. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B. Repair of ordinary Portland cement concrete using alkali activated slag/fly ash: Freeze-thaw resistance and pore size evolution of adhesive interface. Constr. Build. Mater. 2021, 300, 124334. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.X. Current situation and development trends of comprehensive utilization of steel slag. Multipurp. Util. Miner. Resour. 2019, 2, 21–25. [Google Scholar]
- Huang, Y.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar]
- Sun, J.W.; Zhang, Z.Q.; Zhuang, S.Y.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Liu, Q.S.; Ma, S.H.; Huang, X.X.; Gan, M.; Ji, Z.Y.; Chen, X.; Fan, X. Steel slag for carbon fixation and synthesis of alkali-activated material. Constr. Build. Mater. 2022, 351, 128959. [Google Scholar] [CrossRef]
- Peng, X.Q.; Liu, C.; Li, S.; Jiang, Y.; Zeng, L. Research on the setting and hardening performance of alkali-activated steel slag-slag based cementitious materials. J. Hunan Univ. (Nat. Sci.) 2015, 6, 47–52. [Google Scholar]
- Zhou, Y.Q.; Sun, J.W.; Liao, Y.W. Influence of ground granulated blast furnace slag on the early hydration and microstructure of alkali-activated converter steel slag binder. J. Therm. Anal. Calorim. 2022, 147, 243–252. [Google Scholar] [CrossRef]
- Zhang, T.S.; Liu, F.T.; Li, Y.K.; Zhou, Z.H.; Chen, X. Influence of activators on the properties of steel slag cementitious materials. J. Build. Mater. 2008, 11, 469–474. [Google Scholar]
- Xu, Z.K.; Yue, J.C.; Pang, G.H.; Li, R.X.; Zhang, P.; Xu, S.T. Influence of the activator concentration and solid/liquid ratio on the strength and shrinkage characteristics of alkali-activated slag geopolymer pastes. Adv. Civ. Eng. 2021, 2021, 6631316. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.J.; Zhang, H.B.; Wang, X.F.; Huo, J.Y.; Zhang, T.H. Optimization design and characterization of slag cementitious composites containing carbide slag and desulfurized gypsum based on response surface methodology. J. Build. Eng. 2023, 77, 107441. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Su, Q.; Li, M.Z.; Wang, Q.; Zhou, J.H. Effect of water glass modulus on mechanical properties of geopolymer recycled aggregate concrete. J. Build. Mater. 2023, 26, 61–70. [Google Scholar]
- JGJ/T 70-2009; Standard for Test Method of Performance on Building Mortar. Standards Press of China: Beijing, China, 2009.
- Ye, G.C.; Ma, L.Q.; Li, L.; Liu, J.L.; Yuan, S.J.; Huang, G. Application of Box–Behnken design and response surface methodology for modeling and optimization of batch flotation of coal. Int. J. Coal Prep. Util. 2020, 40, 131–145. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Z. Response surface method and its application in pharmaceutical field. J. Jilin Inst. Chem. Technol. 2012, 29, 20–26. [Google Scholar]
- Bentz, D.P.; Ardani, A.; Barrett, T.; Jones, S.Z.; Lootens, D.; Peltz, M.A.; Sato, T.; Stutzman, P.E.; Tanesi, J.; Weiss, W.J. Multiscale investigation of the performance of limestone in concrete. Constr. Build. Mater. 2015, 75, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, C.X.; Ma, Z.Y.; Li, F. Study on preparation of supplementary cementitious material using microbial CO2 fixation of steel slag powder. Constr. Build. Mater. 2022, 326, 126864. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers-Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. A review on alkali-activated slag concrete. Ain Shams Eng. J. 2021, 12, 1475–1499. [Google Scholar] [CrossRef]
- Hou, Y.F.; Wang, D.M.; Li, Q.; Liu, H.B. Effect of water glass performance on fly ash-based geopolymers. J. Chin. Ceram. Soc. 2008, 36, 61–64. [Google Scholar]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Liu, J.X.; Yang, Y.M.; Wang, S.Q.; Liu, P.; Zhang, M.L.; Hai, R. Effect of chemical activator on mechanical property of red mud geopolymer mortar. Inorg. Chem. Ind. 2020, 52, 72–75. [Google Scholar]
- Wang, Q.; Bian, H.; Li, M.; Dai, M.; Chen, Y.; Jiang, H.; Zhang, Q.; Dong, F.; Huang, J.; Ding, Z. Effects of a water-glass module on compressive strength, size effect and stress–strain behavior of geopolymer recycled aggregate concrete. Crystals 2022, 12, 218. [Google Scholar] [CrossRef]
- Zhu, C.X.; Lu, C. The progress to recognize the hardening mechanism of waterglass. Inorg. Chem. Ind. 2001, 33, 22–25. [Google Scholar]
- Tong, G.Q.; Zhang, W.Y.; Gao, Y.T.; Tang, X.Y. Mechanical properties and micromechanism of alkali-activated fly ash geopolymer. Mater. Rep. 2022, 36, 129–134. [Google Scholar]
- Du, T.L.; Liu, Y.; Yu, Y.Y.; Yan, J.X.; Ji, Y.S. Influence of sodium silicate on fly ash slag geopolymer strength and stimulating mechanism. J. Highw. Transp. Res. Dev. 2021, 38, 41–49. [Google Scholar]
- Zhou, P.; Xie, S.L.; Li, Q. Effect of water-binder ratio on properties and pore structure of concrete. Bull. Chin. Ceram. Soc. 2018, 37, 974–978. [Google Scholar]
- Zhang, L.L.; Xu, J.; Wu, Z.P.; Zhao, Y.J.; Wang, T.H. Cylindrical diffusion model and parameter optimization for NaOH solution reinforcement of collapsible loess. Comput. Geotech. 2023, 158, 105376. [Google Scholar] [CrossRef]
- Yu, Q.Z.; Song, L.K.; Chi, Y.L.; Fan, S.H.; Li, G.H. X-ray diffraction quantitative analysis with polynomial-fit method for polycrystalline materials. Spectrosc. Spectr. Anal. 2004, 24, 248–250. [Google Scholar]
- Davidovits, J. Geopolymer chemistry and sustainable development, in: Geopolymer green chemistry and sustainable development solutions. In Proceedings of the Geopolymer 2005 Conference, Austin, TX, USA, 24–26 January 2005. [Google Scholar]
- Hajimohammadi, A.; Ngo, T.; Kashani, A. Glass waste versus sand as aggregates: The characteristics of the evolving geopolymer binders. J. Clean. Prod. 2018, 193, 593–603. [Google Scholar] [CrossRef]
- Deventer, J.S.J.V.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 13, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Shi, H.S.; Xia, M. Effect of different calcium resouces on reaction mechanism of geopolymer. Chin. J. Mater. Res. 2016, 30, 348–354. [Google Scholar]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.W.; Li, L.; Wang, J.X.; Shao, N.N.; Wang, D.M. Microstructure and phase evolution of alkali-activated steel slag during early age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Wang, Q.; Su, H.L.; Li, C.M.; Lyu, X.J. Recycling of steel slag as an alkali activator for blast furnace slag: Geopolymer preparation and its application in composite cement. Clean Technol. Environ. Policy. 2023, 25, 1617–1629. [Google Scholar] [CrossRef]
- Liu, C.; Shi, B.; Zhou, J.; Tang, C. Quantification and characterization of microporosity by image processing, geometric measurement and statistical methods: Application on SEM images of clay materials. Appl. Clay Sci. 2011, 54, 97–106. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Shi, K.Y.; Deng, H.Y.; Hu, J.X.; Zhou, J.Q.; Cai, X.H.; Liu, Z.W. Effects of steel slag powder content and curing condition on the performance of alkali-activated materials based UHPC matrix. Materials 2023, 16, 3875. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.H.; Liu, S.Y.; Sun, J.J. Research on micro-mechanism of carbonated reactive MgO-stabilized silt. Chin. Civ. Eng. J. 2017, 50, 105–113. [Google Scholar]
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes Part II. Stabilisation of chromium and lead. Cem. Concr. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]









| Category | CaO | Fe2O3 | SiO2 | Al2O3 | MgO | SO3 | MnO | P2O5 | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Steel slag | 28.26 | 18.60 | 18.31 | 6.37 | 5.49 | - | 2.84 | 1.28 | - | 1.05 |
| Slag | 35.06 | - | 25.04 | 12.91 | 6.80 | 2.19 | 0.26 | - | 0.49 | 1.26 |
| Category | SiO2 | Na2O | H2O | Module | Baume Degree |
|---|---|---|---|---|---|
| Water glass | 27.27% | 8.32% | 58.2% | 3.2 | 39 |
| Change Factor | Experimental Ratio | ||||
|---|---|---|---|---|---|
| Slag Content | Steel Slag Content | Alkali Content | Modulus | Water–Binder Ratio | |
| Steel slag content | 80% | 20% | 6% | 1.2 | 0.5 |
| 70% | 30% | 6% | 1.2 | 0.5 | |
| 60% | 40% | 6% | 1.2 | 0.5 | |
| 50% | 50% | 6% | 1.2 | 0.5 | |
| 40% | 60% | 6% | 1.2 | 0.5 | |
| Alkali content | 60% | 40% | 4% | 1.2 | 0.5 |
| 60% | 40% | 6% | 1.2 | 0.5 | |
| 60% | 40% | 8% | 1.2 | 0.5 | |
| 60% | 40% | 10% | 1.2 | 0.5 | |
| 60% | 40% | 12% | 1.2 | 0.5 | |
| Water glass modulus | 60% | 40% | 6% | 0.8 | 0.5 |
| 60% | 40% | 6% | 1 | 0.5 | |
| 60% | 40% | 6% | 1.2 | 0.5 | |
| 60% | 40% | 6% | 1.4 | 0.5 | |
| 60% | 40% | 6% | 1.6 | 0.5 | |
| Water–binder ratio | 60% | 40% | 6% | 1.2 | 0.4 |
| 60% | 40% | 6% | 1.2 | 0.45 | |
| 60% | 40% | 6% | 1.2 | 0.5 | |
| 60% | 40% | 6% | 1.2 | 0.55 | |
| 60% | 40% | 6% | 1.2 | 0.6 | |
| Factor | Parameter | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Steel slag content (%) | X1 | 30 | 40 | 50 |
| Alkali content (%) | X2 | 4 | 6 | 8 |
| Water glass modulus | X3 | 1.0 | 1.2 | 1.4 |
| Water–binder ratio | X4 | 0.45 | 0.50 | 0.55 |
| Number | Steel Slag Content (%) | Alkali Content (%) | Modulus | Water–Binder Ratio | Compressive Strength (MPa) | ||
|---|---|---|---|---|---|---|---|
| p1d | p7d | p28d | |||||
| 1 | 40 | 6 | 1.2 | 0.5 | 27.04 | 47.01 | 56.27 |
| 2 | 40 | 6 | 1.2 | 0.5 | 31.63 | 47.38 | 52.68 |
| 3 | 40 | 6 | 1.2 | 0.5 | 29.94 | 50.38 | 55.31 |
| 4 | 40 | 6 | 1 | 0.45 | 21.12 | 34.44 | 50.06 |
| 5 | 40 | 4 | 1.2 | 0.55 | 7.52 | 22.90 | 34.09 |
| 6 | 40 | 6 | 1.4 | 0.45 | 28.79 | 40.01 | 47.27 |
| 7 | 30 | 8 | 1.2 | 0.5 | 30.02 | 43.21 | 44.25 |
| 8 | 50 | 6 | 1.2 | 0.45 | 24.56 | 32.82 | 46.79 |
| 9 | 40 | 8 | 1.2 | 0.45 | 31.08 | 43.10 | 50.19 |
| 10 | 40 | 6 | 1 | 0.55 | 18.30 | 32.38 | 36.44 |
| 11 | 40 | 4 | 1 | 0.5 | 5.54 | 22.10 | 32.23 |
| 12 | 50 | 6 | 1.2 | 0.55 | 15.40 | 30.59 | 38.03 |
| 13 | 40 | 4 | 1.2 | 0.45 | 9.15 | 29.52 | 42.44 |
| 14 | 30 | 6 | 1.2 | 0.45 | 31.26 | 50.26 | 52.32 |
| 15 | 30 | 6 | 1.2 | 0.55 | 23.58 | 37.09 | 43.19 |
| 16 | 50 | 6 | 1 | 0.5 | 12.25 | 27.10 | 33.69 |
| 17 | 50 | 6 | 1.4 | 0.5 | 22.71 | 36.60 | 40.15 |
| 18 | 40 | 4 | 1.4 | 0.5 | 11.61 | 26.06 | 38.79 |
| 19 | 50 | 8 | 1.2 | 0.5 | 20.28 | 27.10 | 35.63 |
| 20 | 40 | 8 | 1 | 0.5 | 20.29 | 31.13 | 40.06 |
| 21 | 30 | 4 | 1.2 | 0.5 | 13.50 | 30.88 | 34.65 |
| 22 | 30 | 6 | 1 | 0.5 | 21.10 | 38.97 | 42.95 |
| 23 | 40 | 8 | 1.2 | 0.55 | 22.44 | 29.22 | 30.92 |
| 24 | 50 | 4 | 1.2 | 0.5 | 6.34 | 23.88 | 30.83 |
| 25 | 30 | 6 | 1.4 | 0.5 | 30.06 | 43.46 | 44.25 |
| 26 | 40 | 8 | 1.4 | 0.5 | 27.69 | 32.34 | 33.69 |
| 27 | 40 | 6 | 1.4 | 0.55 | 22.08 | 31.21 | 40.73 |
| Model | Equation | R2 | F | p |
|---|---|---|---|---|
| 1 | Y1 = −653.29208 + 2.53633X1 + 39.98542X2 + 320.8375X3 + 1278.21667X4 − 0.03225X1X2 + 0.1875X1X3 − 0.74X1X4 + 0.83125X2X3 − 17.525X2X4 − 97.25X3X4 − 0.032471X12 − 2.23677X22 − 110.92708X32 − 1087.83333X42 | 0.98 | 41.6 | <0.0001 |
| 2 | Y2 = −997.20208 + 0.300917X1 + 54.19875X2 + 549.45417X3 + 2261.53333X4 − 0.113875X1X2 + 0.62625X1X3 + 5.47X1X4 − 1.71875X2X3 − 18.15X2X4 − 168.5X3X4 − 0.045654X12 − 3.0326X22 − 195.88542X32 − 2247.166667X42 | 0.97 | 33.3 | <0.0001 |
| 3 | Y3 = −729.72625 + 4.53908X1 + 61.32333X2 + 387.5X3 + 1222.38333X4 − 0.06X1X2 + 0.645X1X3 + 0.185X1X4 − 8.08125X2X3 − 27.3X2X4 + 177X3X4 − 0.066871X12 − 2.88927X22 − 187.23958X32 − 1387.83333X42 | 0.97 | 24.1 | <0.0001 |
| Source | p1d | p7d | p28d | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| X1 | 64.52 | <0.0001 | 95.23 | <0.0001 | 25.83 | 0.0003 |
| X2 | 269.93 | <0.0001 | 56.70 | <0.0001 | 9.14 | 0.0106 |
| X3 | 55.10 | <0.0001 | 12.22 | 0.0044 | 1.73 | 0.2127 |
| X4 | 37.62 | <0.0001 | 48.12 | <0.0001 | 83.64 | <0.0001 |
| X1X2 | 0.5597 | 0.4688 | 5.48 | 0.0373 | 1.34 | 0.2695 |
| X1X3 | 0.1892 | 0.6713 | 1.66 | 0.2223 | 1.55 | 0.2370 |
| X1X4 | 0.1842 | 0.6754 | 7.90 | 0.0157 | 0.0080 | 0.9304 |
| X2X3 | 0.1487 | 0.7065 | 0.4993 | 0.4933 | 9.73 | 0.0087 |
| X2X4 | 4.13 | 0.0648 | 3.48 | 0.0867 | 6.94 | 0.0218 |
| X3X4 | 1.27 | 0.2814 | 3.00 | 0.1089 | 2.92 | 0.1134 |
| X12 | 18.91 | 0.0009 | 29.36 | 0.0002 | 55.51 | <0.0001 |
| X22 | 143.58 | <0.0001 | 207.25 | <0.0001 | 165.80 | <0.0001 |
| X32 | 35.31 | <0.0001 | 86.47 | <0.0001 | 69.63 | <0.0001 |
| X42 | 13.27 | 0.0034 | 44.45 | <0.0001 | 14.94 | 0.0022 |
| Lack of fit | 0.4621 | 0.8344 | 1.13 | 0.5572 | 1.29 | 0.5129 |
| Number | Steel Slag (%) | Alkali (%) | Water Glass Modulus | Water–Binder Ratio |
|---|---|---|---|---|
| S20 | 20 | 6 | 1.2 | 0.5 |
| S40 (A06) | 40 | 6 | 1.2 | 0.5 |
| S60 | 60 | 6 | 1.2 | 0.5 |
| A04 | 40 | 4 | 1.2 | 0.5 |
| A08 | 40 | 8 | 1.2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Xu, J.; Zhang, X.; Tan, L.; Mei, Y. Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials. Buildings 2024, 14, 1204. https://doi.org/10.3390/buildings14051204
Wang M, Xu J, Zhang X, Tan L, Mei Y. Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials. Buildings. 2024; 14(5):1204. https://doi.org/10.3390/buildings14051204
Chicago/Turabian StyleWang, Mengqi, Jian Xu, Xuejing Zhang, Longzhen Tan, and Yuan Mei. 2024. "Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials" Buildings 14, no. 5: 1204. https://doi.org/10.3390/buildings14051204







