Study on the Ionic Transport Properties of 3D Printed Concrete
Abstract
:1. Introduction
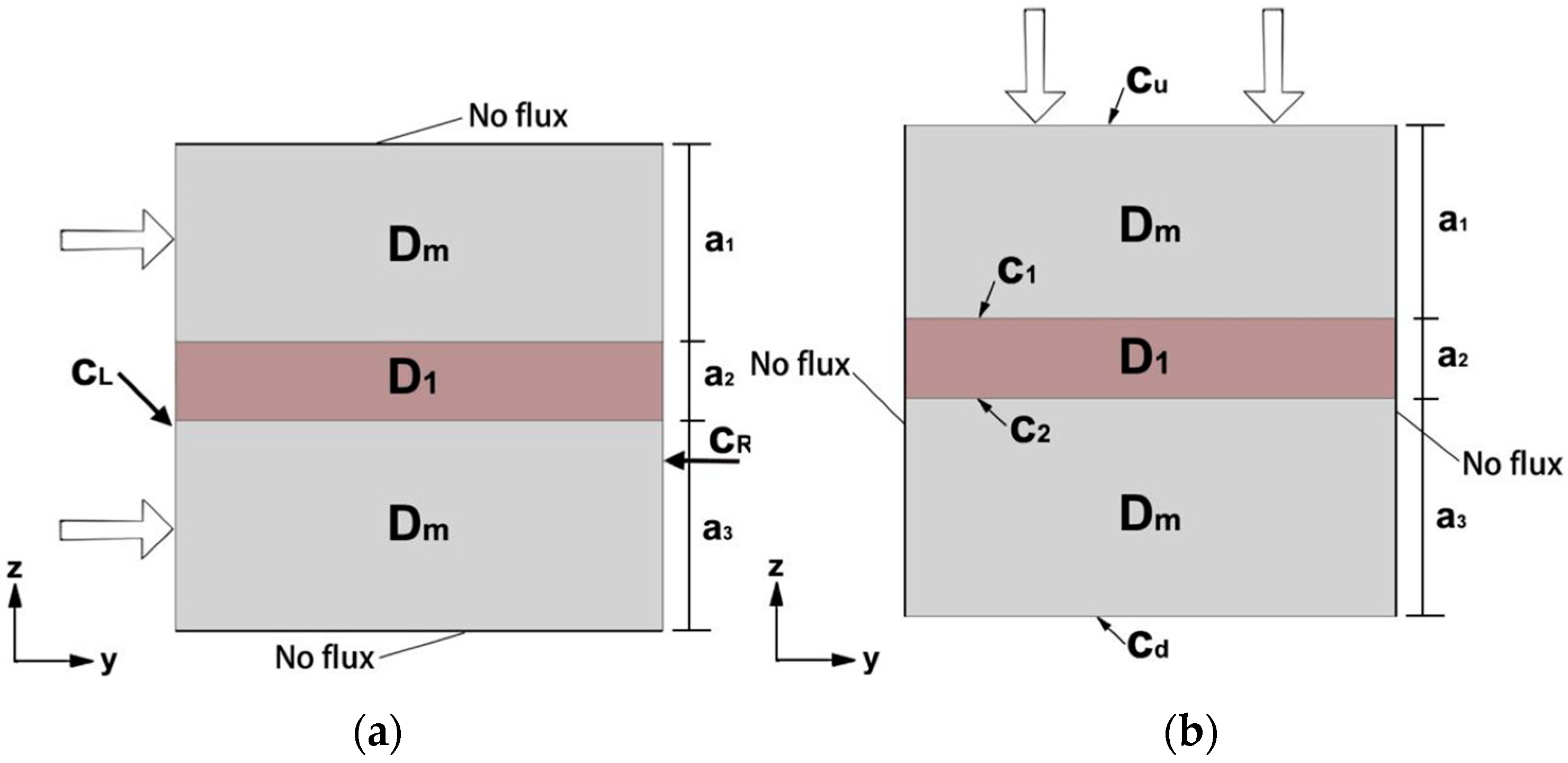
2. Equivalent Diffusion Coefficient Calculation Model
2.1. Parallel Model and Series Model
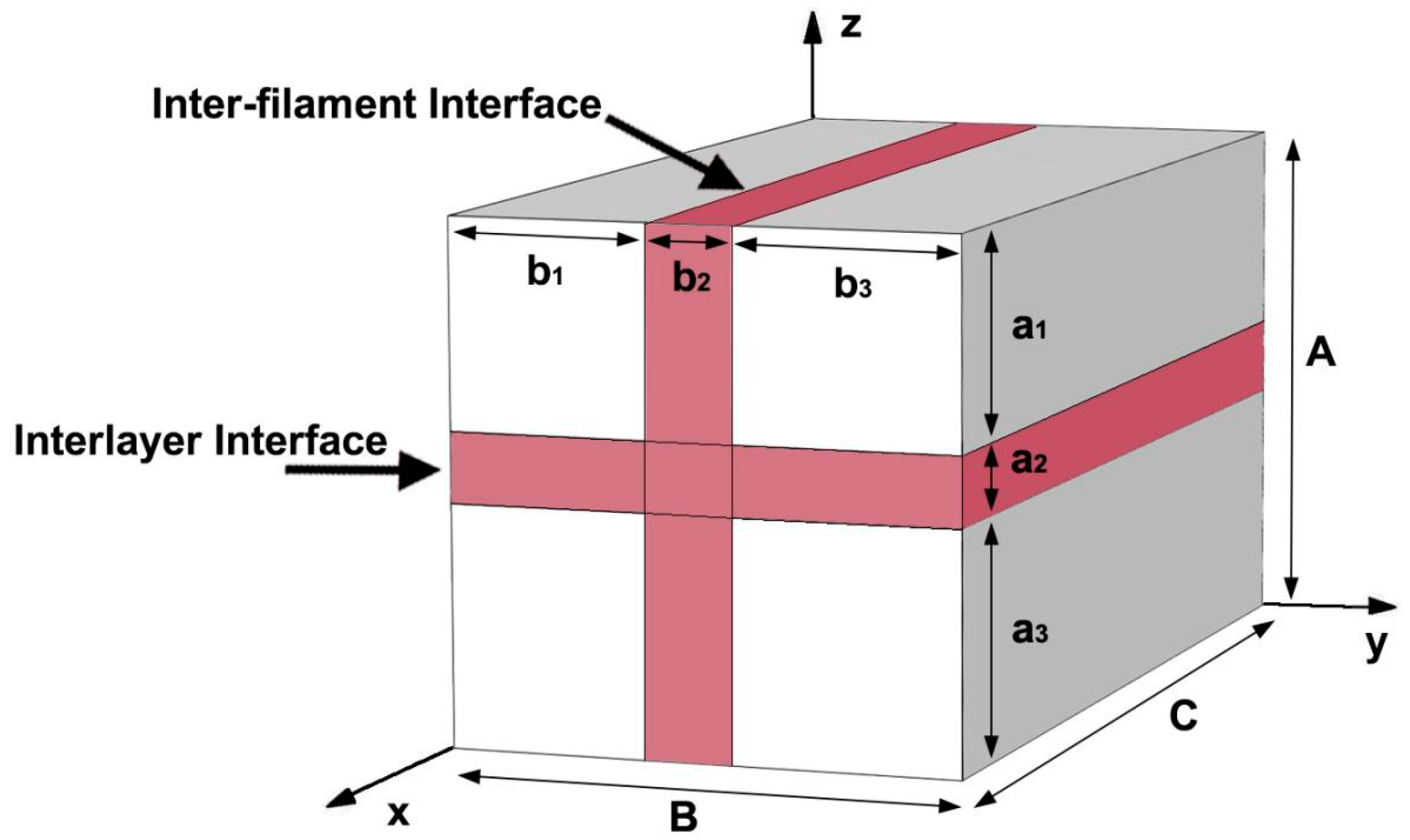
2.2. 3DPC Equivalent Diffusion Coefficient
2.3. Nondimensionalization of Equations
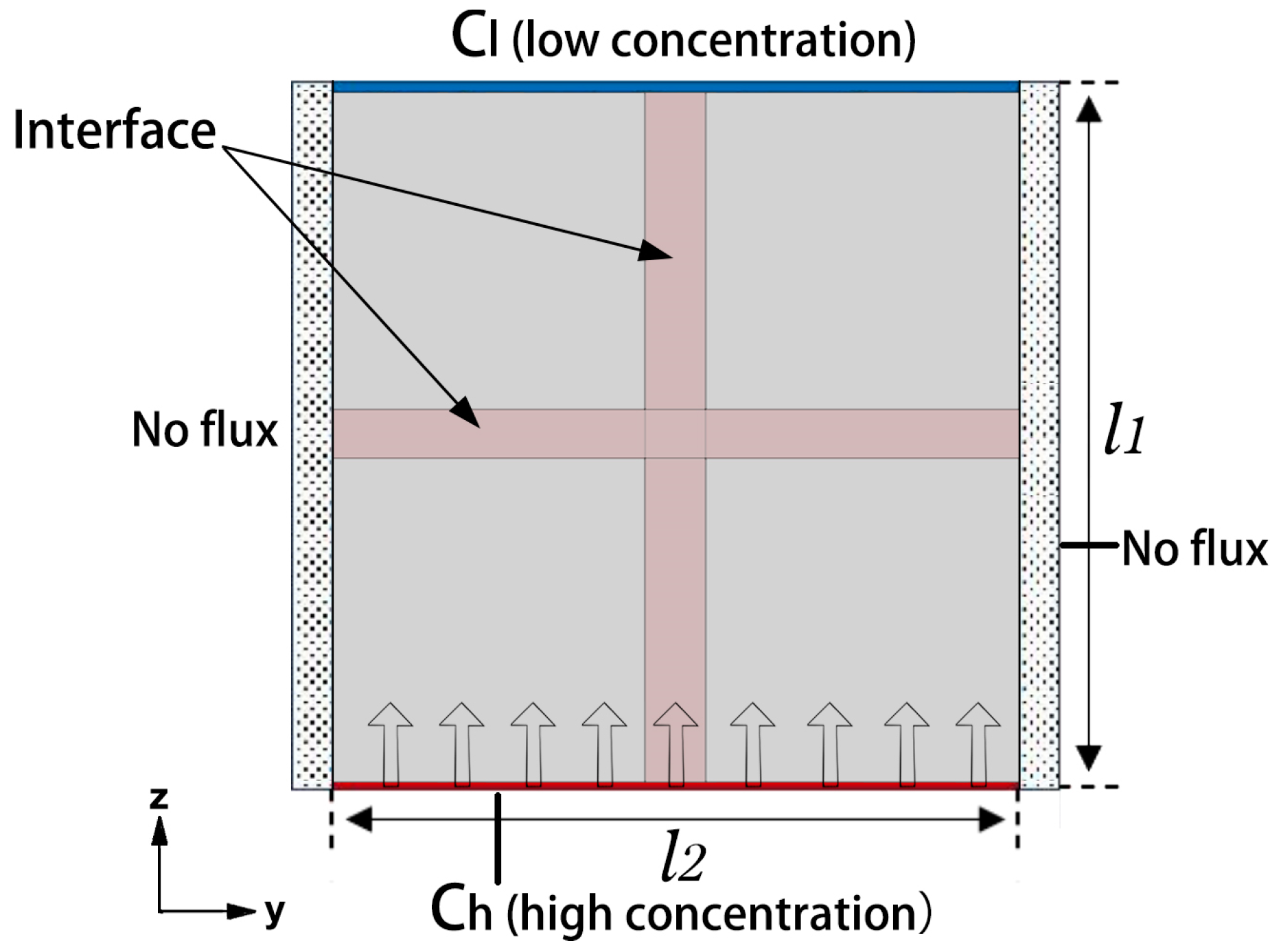
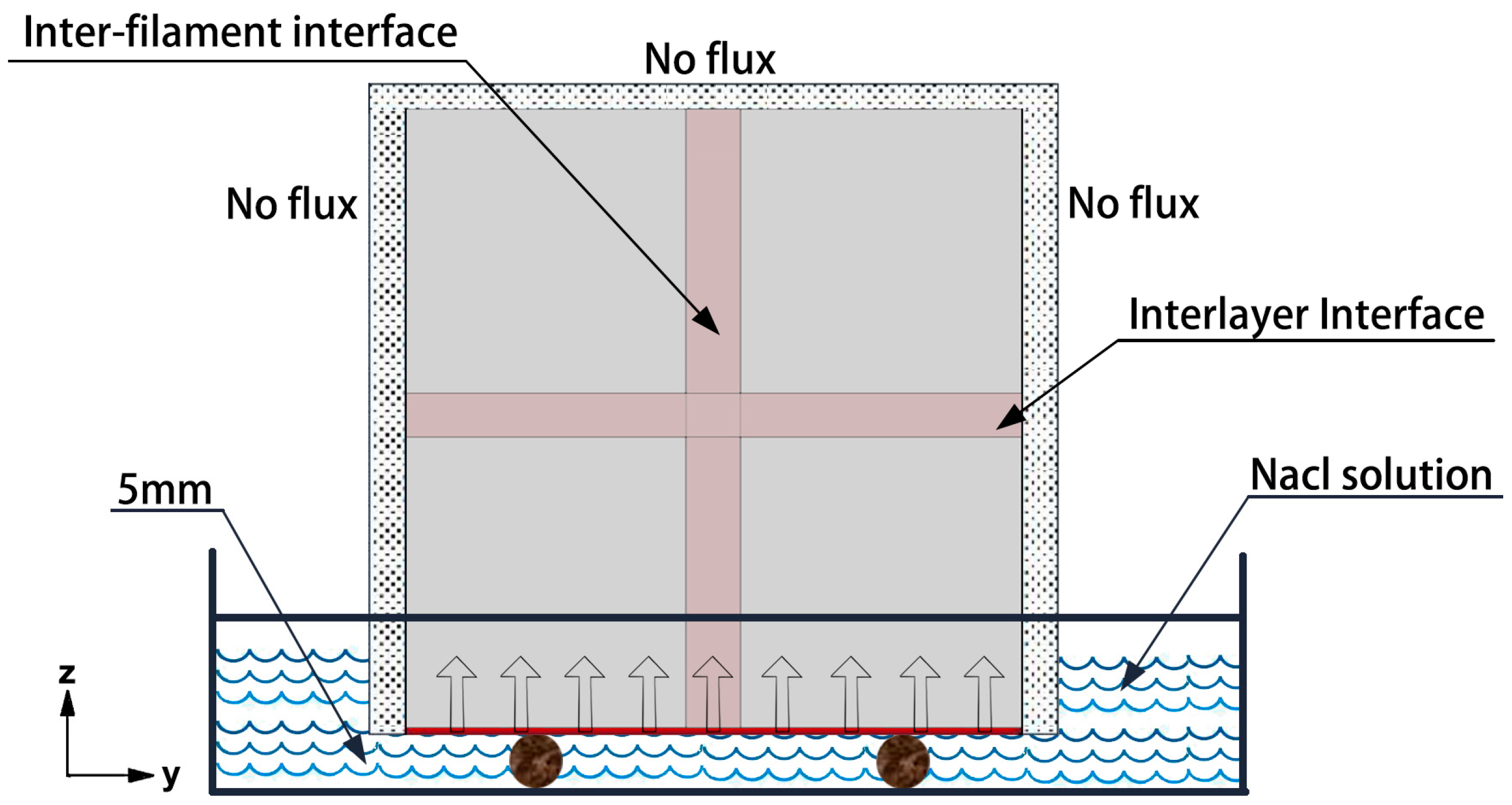
3. Finite Element Model
4. Analysis of Results
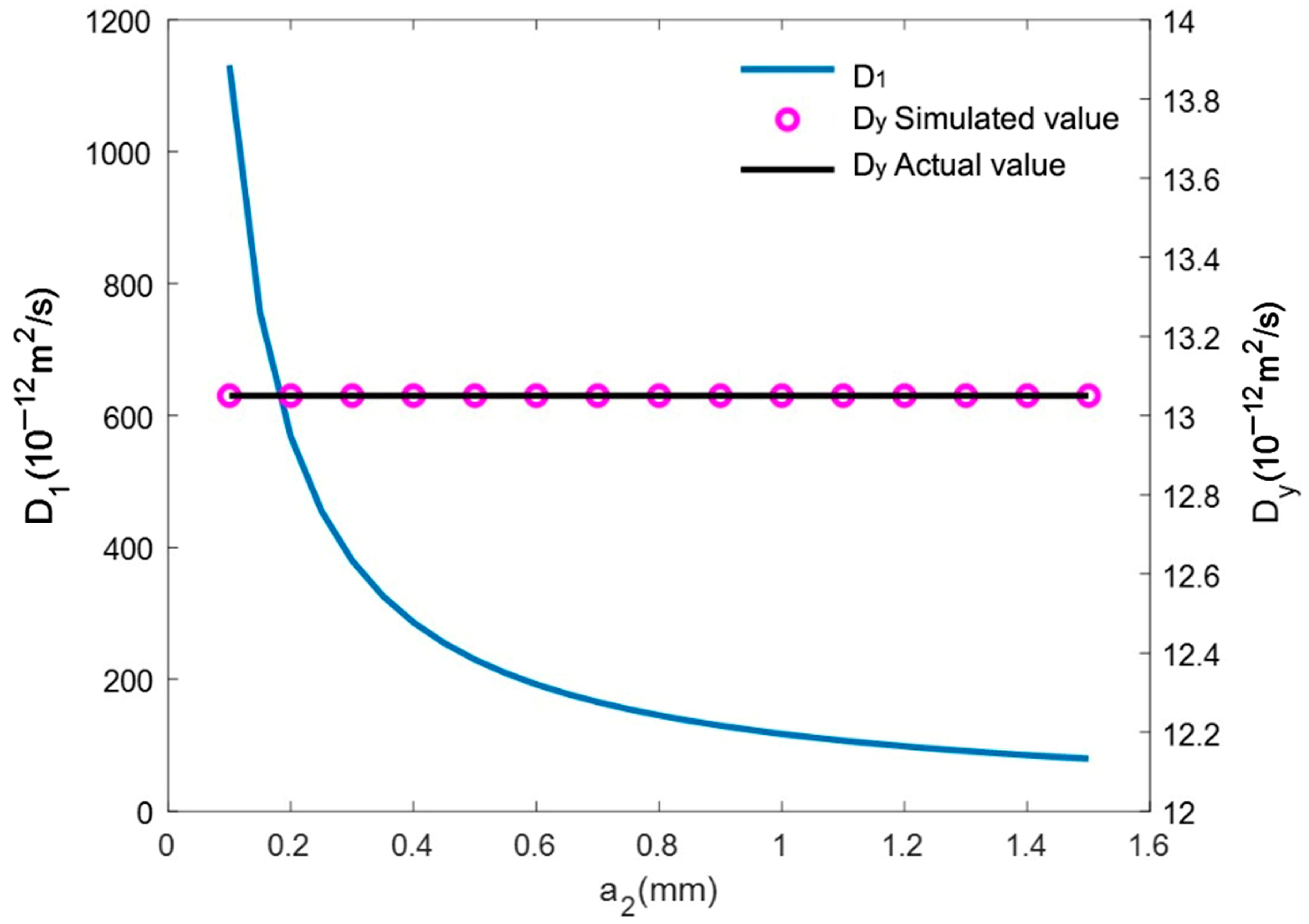
4.1. Theoretical Model Validation
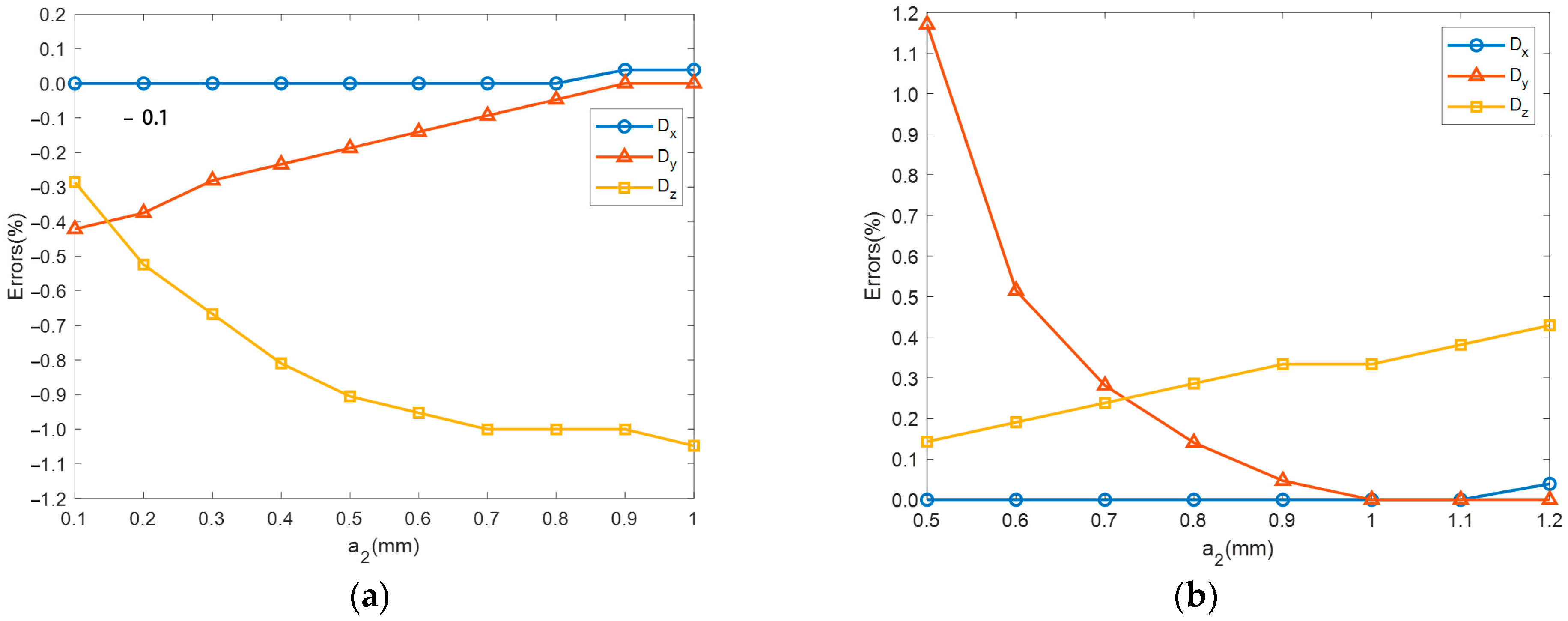
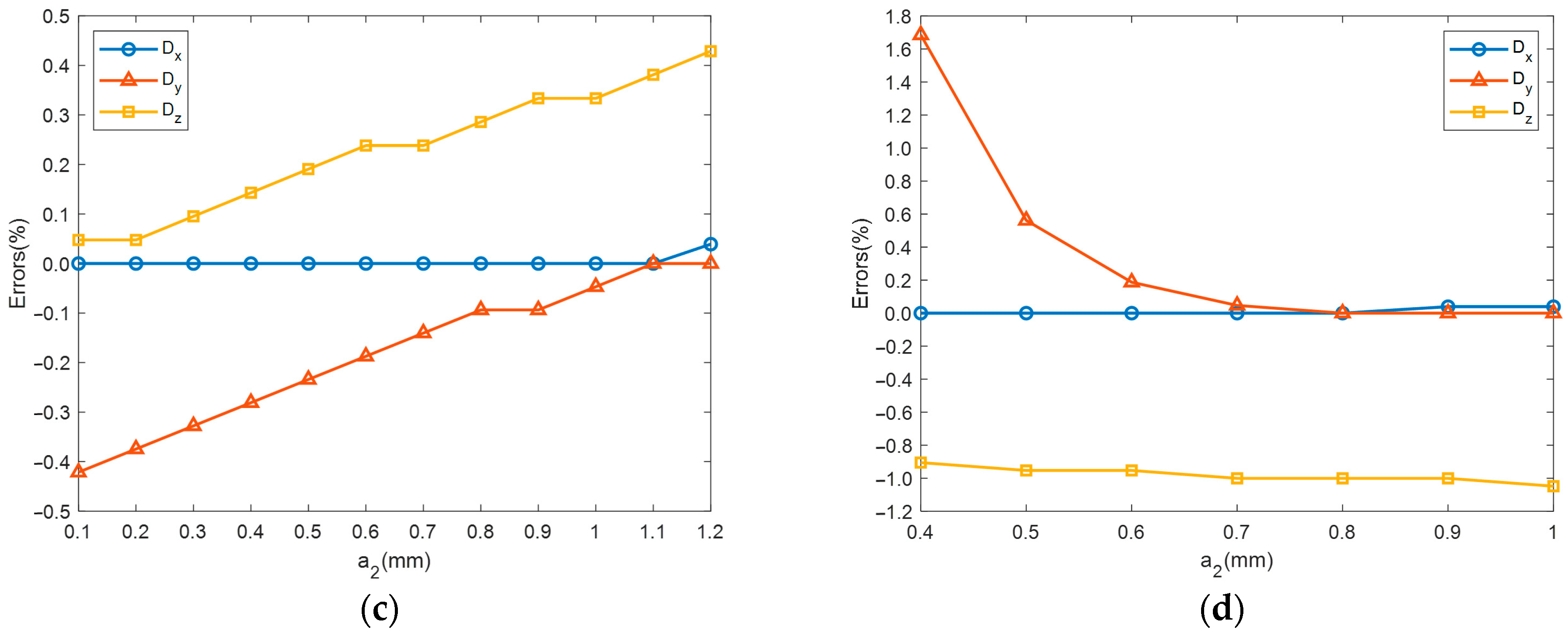
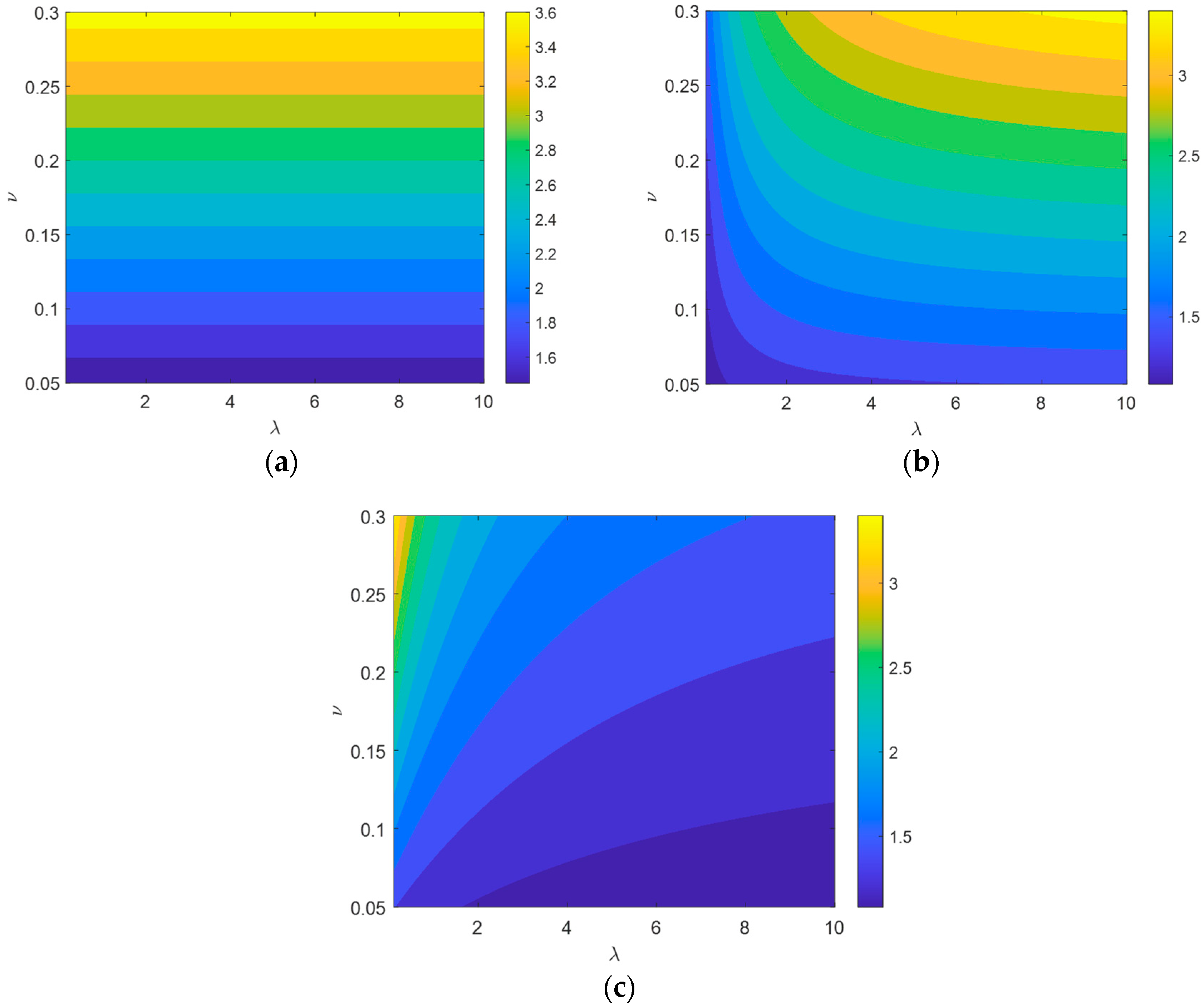
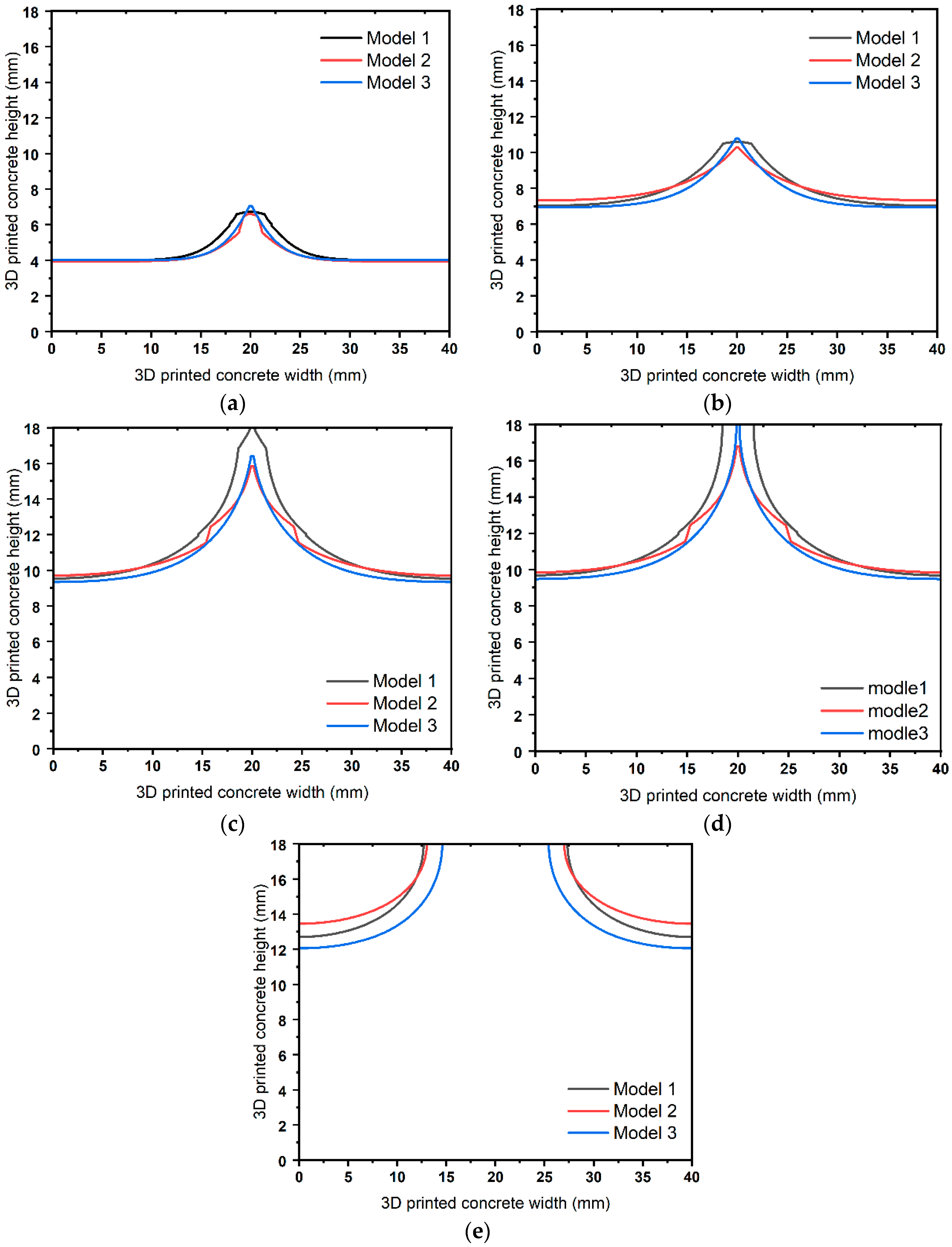
4.2. Static Analyses
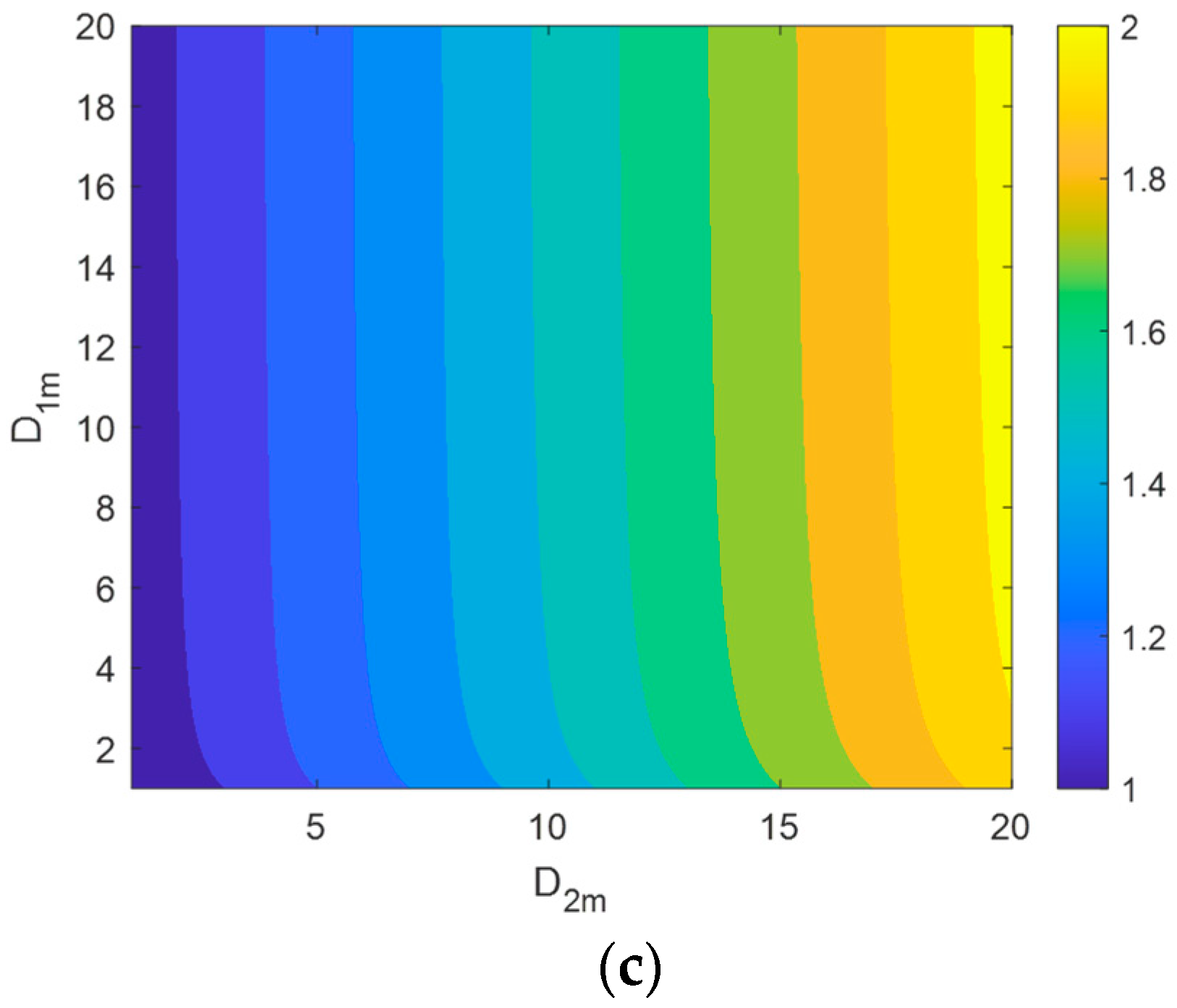
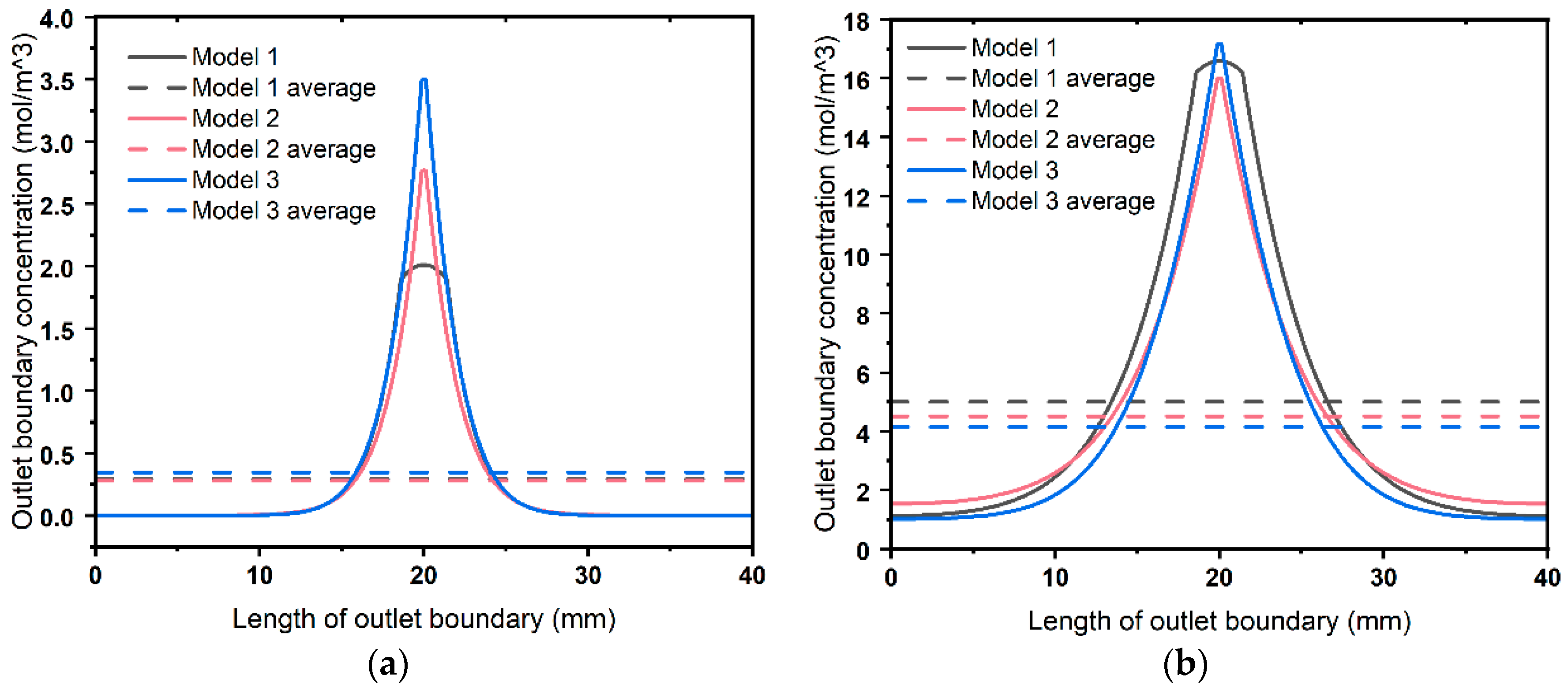
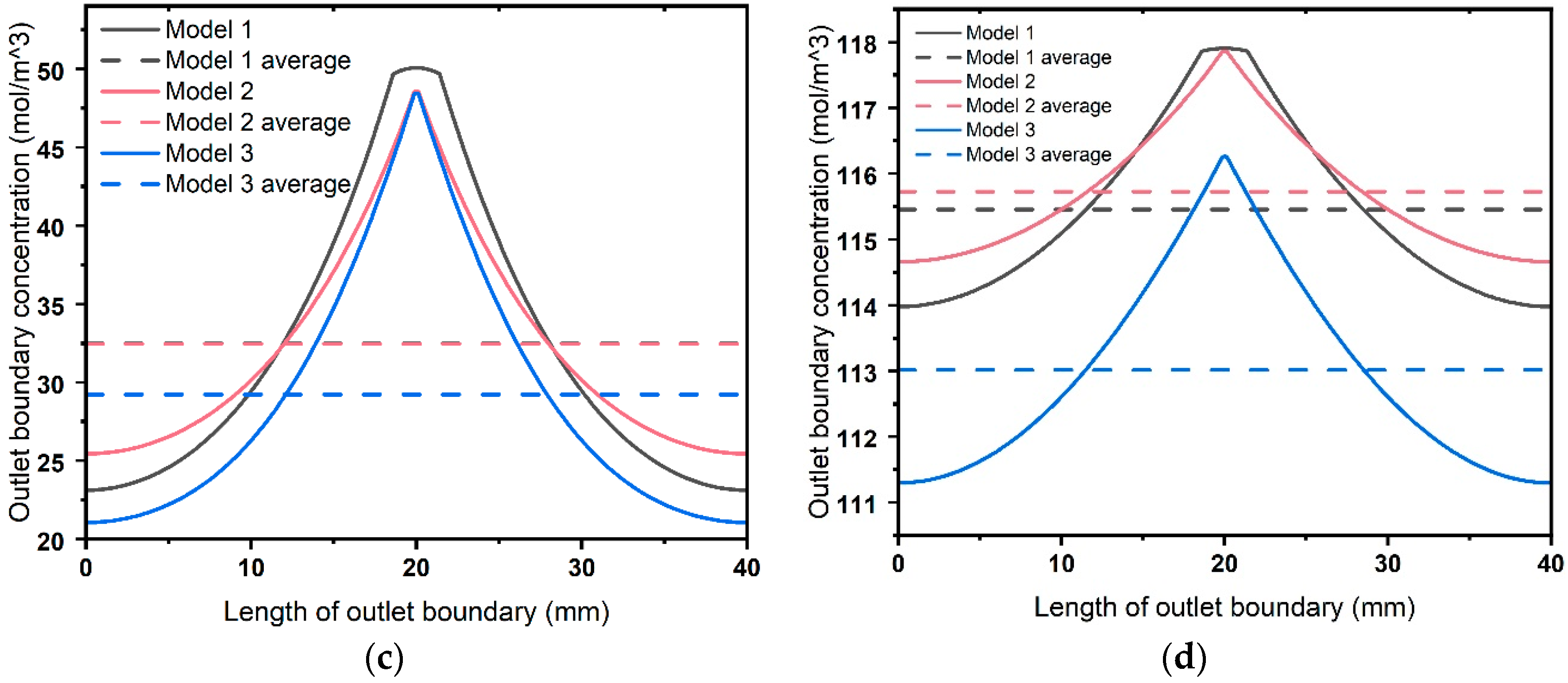
4.3. Dynamic Analysis
5. Conclusions and Perspectives
- (1)
- Numerical calculations have confirmed that the theoretical model provides precise computational results for single-interface 3DPC models, which possess either interlayer interfaces or inter-filament interfaces. For dual-interface 3DPC models, incorporating both interlayer and inter-filament interfaces, the theoretical calculation results for are accurate, while the theoretical results for and show an error of no more than 2%.
- (2)
- The impact of material parameters and on static performance is as follows: and linearly increase with , with minimal impact on , and and linearly increase with , while has little effect on . As for the impact of structural parameters and on static performance, , , and all linearly increase with , with being most affected, followed by and , has no impact on , rapidly increases with before stabilizing, and rapidly decreases with before reaching a steady state.
- (3)
- Interfaces have a significant impact on the dynamic ion transport performance in 3DPC. The capacity of an interface to transport ions is not only related to the interface diffusion coefficient but also to the channel size. The larger the product of these two factors, the stronger the ion transport capacity of the interface, leading to higher ion concentrations over time. Lateral interfaces accelerate ion diffusion from the center toward the sides, making the vertical concentration contour lines flatter. Consequently, for a period, a 3DPC with a lower effective diffusion coefficient may exhibit higher ion concentrations in its central part.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Wang, L.B. Preparation and Properties of 3D Printed Carbon Fiber Reinforced Green Concrete. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Zhao, H. 3D Printing Technology Research Status and Key Technologies. J. Mater. Eng. 2016, 44, 122–128. [Google Scholar]
- Zalp, F.; Yilmaz, H.D. Fresh and Hardened Properties of 3D High-Strength Printing Concrete and Its Recent Applications. Iran. J. Sci. Technol.-Trans. Civ. Eng. 2020, 44, 319–330. [Google Scholar]
- Soar, R.; Andreen, D. The Role of Additive Manufacturing and Physiomimetic Computational Design for Digital Construction. Archit. Des. 2012, 82, 126–135. [Google Scholar] [CrossRef]
- Jianchao, Z.; Zhang, T.; Faried, M.; Wengang, C. 3D Printing Cement Based Ink, and It’s Application within the Construction Industry. In Proceedings of the MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 120, p. 02003. [Google Scholar]
- Surehali, S.; Tripathi, A.; Nimbalkar, A.S.; Neithalath, N. Anisotropic Chloride Transport in 3D Printed Concrete and Its Dependence on Layer Height and Interface Types. Addit. Manuf. 2023, 62, 103405. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Q.; Li, Z.; Shi, C.; Wu, Q.; Huang, Y. Correlation of Interlayer Properties and Rheological Behaviors of 3DPC with Various Printing Time Intervals. Addit. Manuf. 2021, 47, 102327. [Google Scholar] [CrossRef]
- Moelich, G.; Kruger, P.; Combrinck, R. The Effect of Restrained Early Age Shrinkage on the Interlayer Bond and Durability of 3D Printed Concrete. J. Build. Eng. 2021, 43, 102857. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, J.; Wang, Q.; Shi, J.; Wang, H. PVA Fibre Reinforced High-Strength Cementitious Composite for 3D Printing: Mechanical Properties and Durability. Addit. Manuf. 2022, 49, 102500. [Google Scholar] [CrossRef]
- Rodriguez, F.B.; Lopez, C.G.; Wang, Y.; Olek, J.; Zavattieri, P.D.; Youngblood, J.P.; Falzone, G.; Cotrell, J. Evaluation of Durability of 3D-Printed Cementitious Materials for Potential Applications in Structures Exposed to Marine Environments. In Proceedings of the Third RILEM International Conference on Concrete and Digital Fabrication: Digital Concrete 2022; Springer: Berlin/Heidelberg, Germany, 2022; pp. 175–181. [Google Scholar]
- Nodehi, M.; Aguayo, F.; Nodehi, S.E.; Gholampour, A.; Ozbakkaloglu, T.; Gencel, O. Durability Properties of 3D Printed Concrete (3DPC). Autom. Constr. 2022, 142, 104479. [Google Scholar] [CrossRef]
- Bos, F.P.; Ahmed, Z.Y.; Jutinov, E.R.; Salet, T.A. Experimental Exploration of Metal Cable as Reinforcement in 3D Printed Concrete. Materials 2017, 10, 1314. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Grafe, J.; Nerella, V.N.; Spaniol, E.; Hertel, M.; Füssel, U. 3D-Printed Steel Reinforcement for Digital Concrete Construction–Manufacture, Mechanical Properties and Bond Behaviour. Constr. Build. Mater. 2018, 179, 125–137. [Google Scholar] [CrossRef]
- Marchment, T.; Sanjayan, J. Mesh Reinforcing Method for 3D Concrete Printing. Autom. Constr. 2020, 109, 102992. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Yao, L.; Ma, G. Interfacial Bonding Properties of 3D Printed Permanent Formwork with the Post-Casted Concrete. Cem. Concr. Compos. 2022, 128, 104457. [Google Scholar] [CrossRef]
- Zhu, B.; Nematollahi, B.; Pan, J.; Zhang, Y.; Zhou, Z.; Zhang, Y. 3D Concrete Printing of Permanent Formwork for Concrete Column Construction. Cem. Concr. Compos. 2021, 121, 104039. [Google Scholar] [CrossRef]
- Malan, J.D.; van Rooyen, A.S.; van Zijl, G.P. Chloride Induced Corrosion and Carbonation in 3D Printed Concrete. Infrastructures 2021, 7, 1. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sadati, S.; Shekarchi, M. Quantifying Maximum Phenomenon in Chloride Ion Profiles and Its Influence on Service-Life Prediction of Concrete Structures Exposed to Seawater Tidal Zone—A Field Oriented Study. Constr. Build. Mater. 2018, 180, 109–116. [Google Scholar] [CrossRef]
- Malan, J.D. Chloride Induced Corrosion and Concrete Carbonation of 3D Printed Concrete with Reinforced Connections. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2022. [Google Scholar]
- Van Der Putten, J.; De Volder, M.; Van den Heede, P.; Deprez, M.; Cnudde, V.; De Schutter, G.; Van Tittelboom, K. Transport Properties of 3D Printed Cementitious Materials with Prolonged Time Gap between Successive Layers. Cem. Concr. Res. 2022, 155, 106777. [Google Scholar] [CrossRef]
- Ding, T.; Xiao, J.; Mechtcherine, V. Microstructure and Mechanical Properties of Interlayer Regions in Extrusion-Based 3D Printed Concrete: A Critical Review. Cem. Concr. Compos. 2023, 141, 105154. [Google Scholar] [CrossRef]
- Han, N.; Xiao, J.; Zhang, L.; Peng, Y. A Microscale-Based Numerical Model for Investigating Hygro-Thermo-Mechanical Behaviour of 3D Printed Concrete at Elevated Temperatures. Constr. Build. Mater. 2022, 344, 128231. [Google Scholar] [CrossRef]
- Shakor, P.; Gowripalan, N.; Rasouli, H. Experimental and Numerical Analysis of 3D Printed Cement Mortar Specimens Using Inkjet 3DP. Arch. Civ. Mech. Eng. 2021, 21, 58. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, Z.; Duan, Z. Experimental Investigation on Pore Structure of 3d Printed Concrete with Full Recycled Coarse Aggregate. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4329339 (accessed on 24 March 2024).
- Zhang, J.; Zou, D.; Wang, H.; Sun, X. Tensile properties and constitutive model of inter-layer interface of 3D printed concrete. J. Zhejiang Univ. (Eng. Sci.) 2021, 55, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, H.; Qian, R.; Xue, C.; Feng, Q.; Su, L.; Zhang, Y.; Liu, G.; Du, H. Relationship between Water Transport Behaviour and Interlayer Voids of 3D Printed Concrete. Constr. Build. Mater. 2022, 326, 126731. [Google Scholar] [CrossRef]
- Katsuya, K.; Makihiko, I.; Liang, C.; Yan, T.; Li, Z.; Li, Y. Critical concentration and specification of chloride ions for corrosion of reinforcing bars in concrete. Concr. World 2011, 34–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yang, L.; Liu, G.; Chen, Y.; Yu, S.; Du, H. Hardened Properties and Durability of Large-Scale 3D Printed Cement-Based Materials. Mater. Struct. 2021, 54, 45. [Google Scholar] [CrossRef]
- Bran-Anleu, P.; Wangler, T.; Nerella, V.N.; Mechtcherine, V.; Trtik, P.; Flatt, R.J. Using Micro-XRF to Characterize Chloride Ingress through Cold Joints in 3D Printed Concrete. Mater. Struct. 2023, 56, 51. [Google Scholar] [CrossRef] [PubMed]
- Van Der Putten, J.; De Volder, M.; Van den Heede, P.; De Schutter, G.; Van Tittelboom, K. 3D Printing of Concrete: The Influence on Chloride Penetration. In Proceedings of the Second RILEM International Conference on Concrete and Digital Fabrication: Digital Concrete 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 500–507. [Google Scholar]
- Wang, H.; Shao, J.; Zhang, J.; Zou, D.; Sun, X. Bond Shear Performances and Constitutive Model of Interfaces between Vertical and Horizontal Filaments of 3D Printed Concrete. Constr. Build. Mater. 2022, 316, 125819. [Google Scholar] [CrossRef]
- Liu, C.; Liu, G.; Liu, Z.; Yang, L.; Zhang, M.; Zhang, Y. Numerical Simulation of the Effect of Cement Particle Shapes on Capillary Pore Structures in Hardened Cement Pastes. Constr. Build. Mater. 2018, 173, 615–628. [Google Scholar] [CrossRef]
- Zhang, J.; Bian, F.; Zhang, Y.; Fang, Z.; Fu, C.; Guo, J. Effect of Pore Structures on Gas Permeability and Chloride Diffusivity of Concrete. Constr. Build. Mater. 2018, 163, 402–413. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Li, L.; Xie, F. Analysis of chloride ion diffusion in concrete structures under non-penetrating fractures. J. Tongji Univ. (Nat. Sci. Ed.) 2019, 47, 1260–1267. [Google Scholar]
- Nedjar, B. Incremental Viscoelasticity at Finite Strains for the Modelling of 3D Concrete Printing. Comput. Mech. 2022, 69, 233–243. [Google Scholar] [CrossRef]
- Nedjar, B. On a Geometrically Nonlinear Incremental Formulation for the Modelling of 3D Concrete Printing. Mech. Res. Commun. 2021, 116, 103748. [Google Scholar] [CrossRef]



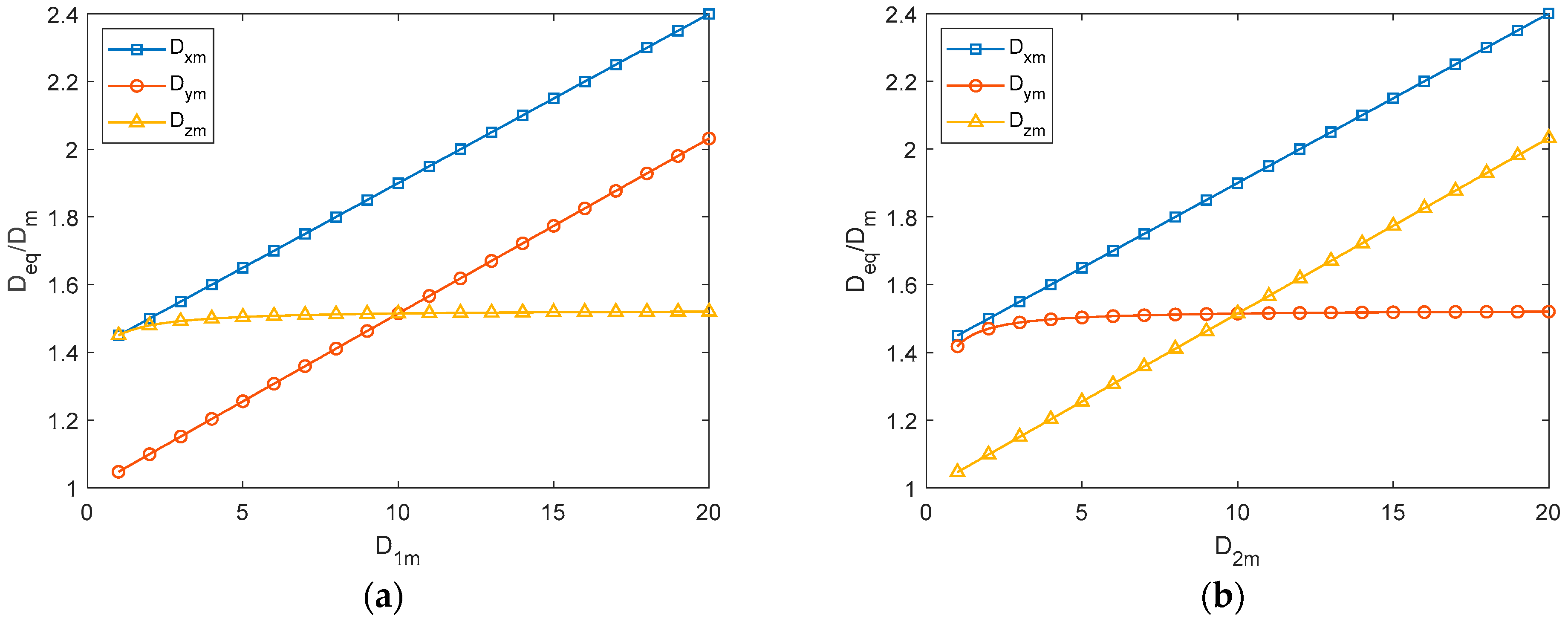


| Layers | Thickness of Single-Layer Matrix | Reference | ||
|---|---|---|---|---|
| 4 | 10 mm | 4.603 × 10−12 m2/s | 13.05 × 10−12 m2/s | J. Van Der Putten et al. [20] |
| Thickness of Single-Layer Matrix | Width of Single-Layer Matrix | Reference | ||||
|---|---|---|---|---|---|---|
| 6 mm | 20 mm | 15.03 × 10−12 m2/s | 25.55 × 10−12 m2/s | 21.36 × 10−12 m2/s | 20.99 × 10−12 m2/s | S. Surehali et al. [6] |
| Scenarios 1 | Scenarios 2 | Scenarios 3 | Scenarios 4 | ||
|---|---|---|---|---|---|
| Theoretical model | parallel connection | parallel connection | parallel connection | parallel connection | |
| Equation | (9) | (9) | (9) | (9) | |
| Theoretical model | parallel-then-series | series-then-parallel | parallel-then-series | series-then-parallel | |
| Equation | (13) | (17) | (13) | (17) | |
| Theoretical model | parallel-then-series | series-then-parallel | series-then-parallel | series-then-parallel | |
| Equation | (18) | (19) | (19) | (18) |
| Scenarios 1 | Scenarios 2 | |||||
| (×10−12 m2/s) | (×10−12 m2/s) | (×10−12 m2/s) | (×10−12 m2/s) | |||
| 0.1 | 2.807 | 477.94 | 96.79 | No solution | ||
| 0.2 | 2.371 | 253.60 | 108.38 | No solution | ||
| 0.3 | 1.981 | 178.48 | 122.88 | No solution | ||
| 0.4 | 1.629 | 140.73 | 141.75 | No solution | ||
| 0.5 | 1.308 | 117.95 | 167.57 | 2.402 | 116.10 | 102.31 |
| 0.6 | 1.014 | 102.68 | 205.35 | 1.816 | 100.23 | 127.34 |
| 0.7 | 0.743 | 91.72 | 266.35 | 1.416 | 89.17 | 155.12 |
| 0.8 | 0.492 | 83.45 | 382.25 | 1.093 | 80.97 | 191.39 |
| 0.9 | 0.259 | 76.99 | 690.27 | 0.815 | 74.62 | 244.96 |
| 1.0 | 0.0418 | 71.80 | 4054.2 | 0.565 | 69.57 | 336.68 |
| 1.1 | No solution | 0.338 | 65.44 | 537.21 | ||
| 1.2 | No solution | 0.128 | 62.01 | 1353.8 | ||
| Scenarios 3 | Scenarios 4 | |||||
| (×10−12 m2/s) | (×10−12 m2/s) | (×10−12 m2/s) | (×10−12 m2/s) | |||
| 0.1 | 2.963 | 472.98 | 93.44 | No solution | ||
| 0.2 | 2.641 | 249.16 | 100.77 | No solution | ||
| 0.3 | 2.335 | 174.48 | 109.52 | No solution | ||
| 0.4 | 2.043 | 137.09 | 120.22 | 2.468 | 143.54 | 98.68 |
| 0.5 | 1.765 | 114.62 | 133.62 | 1.609 | 118.76 | 139.04 |
| 0.6 | 1.499 | 99.62 | 150.95 | 1.147 | 102.98 | 183.34 |
| 0.7 | 1.244 | 88.89 | 174.31 | 0.800 | 91.83 | 248.34 |
| 0.8 | 1.001 | 80.84 | 207.59 | 0.513 | 83.49 | 367.01 |
| 0.9 | 0.768 | 74.56 | 258.99 | 0.264 | 77.00 | 677.08 |
| 1.0 | 0.544 | 69.54 | 349.04 | 0.0419 | 71.80 | 4042.6 |
| 1.1 | 0.331 | 65.43 | 548.23 | No solution | ||
| 1.2 | 0.127 | 62.01 | 1363.8 | No solution | ||
| Number of Layers | Number of Strips | Matrix Thickness (mm) | Matrix Width (mm) | Interlayer Interface Thickness (mm) | Interlayer Interface Diffusion Coefficient (10−12 m2/s) | Interlayer Interface Horizontal Ion Transport Capacity (10−15 m3/s) | |
| Model 1 | 3 | 2 | 6 | 20 | 0.1 | 477.94 | 47.8 |
| Model 2 | 3 | 2 | 6 | 20 | 0.9 | 76.99 | 69.3 |
| Model 3 | 1 | 2 | 18 | 20 | \ | \ | \ |
| Inter-Filament Interface Thickness (mm) | Inter-Filament Interface Diffusion Coefficient (10−12 m2/s) | Inter-Filament Interface Vertical Ion Transport Capacity (10−15 m3/s) | |||||
| Model 1 | 2.807 | 96.79 | 271.7 | ||||
| Model 2 | 0.259 | 690.27 | 178.8 | ||||
| Model 3 | 0.259 | 690.27 | 178.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Peng, Z.; Wang, M.; Feng, S. Study on the Ionic Transport Properties of 3D Printed Concrete. Buildings 2024, 14, 1216. https://doi.org/10.3390/buildings14051216
Huang T, Peng Z, Wang M, Feng S. Study on the Ionic Transport Properties of 3D Printed Concrete. Buildings. 2024; 14(5):1216. https://doi.org/10.3390/buildings14051216
Chicago/Turabian StyleHuang, Tao, Zhongqi Peng, Mengge Wang, and Shuang Feng. 2024. "Study on the Ionic Transport Properties of 3D Printed Concrete" Buildings 14, no. 5: 1216. https://doi.org/10.3390/buildings14051216




