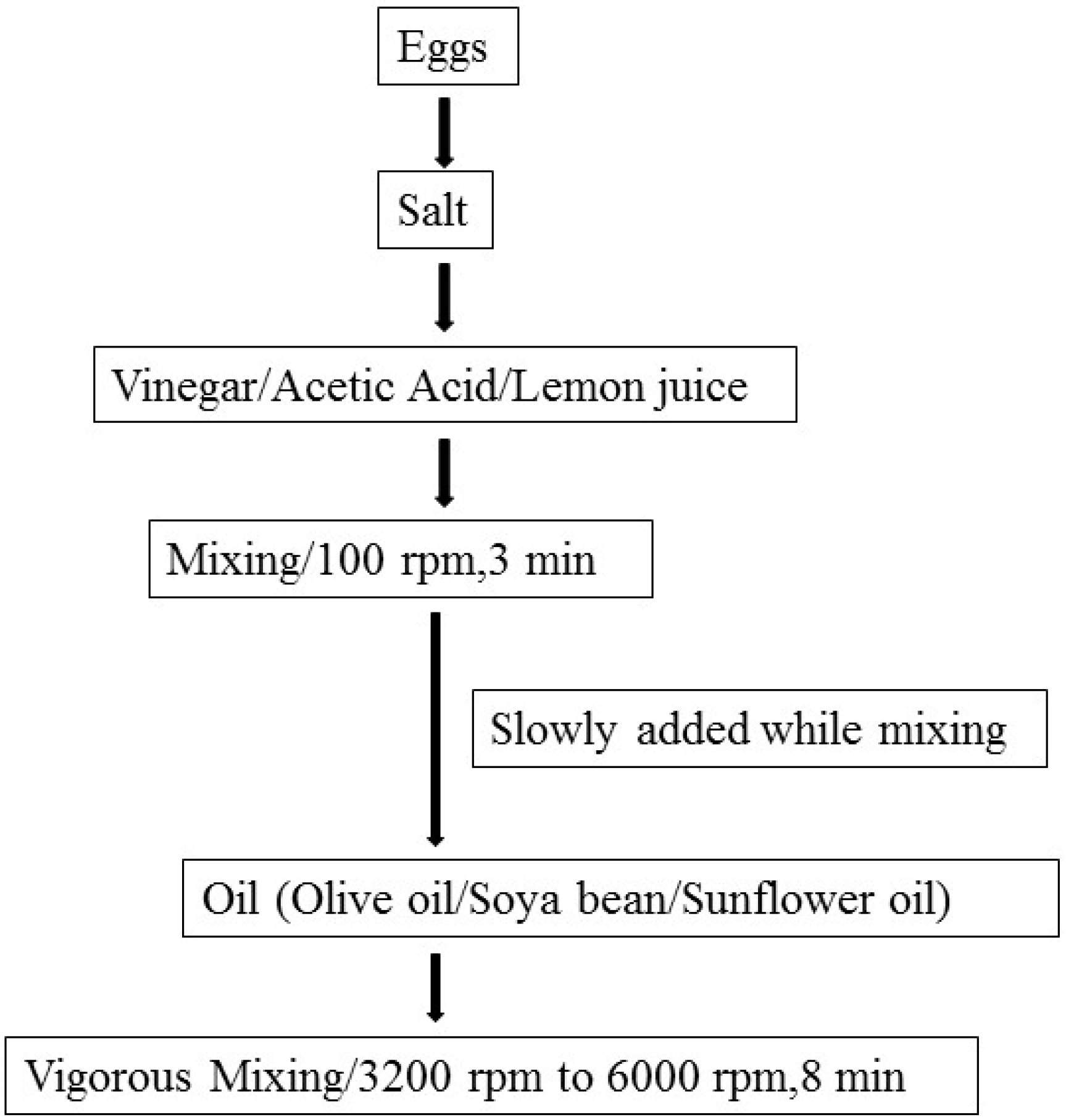
A Review of Temperature, pH, and Other Factors that Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products
Abstract
:1. Introduction
2. pH/Acid Tolerance and Temperature
3. Vinegar vs. Lemon Juice
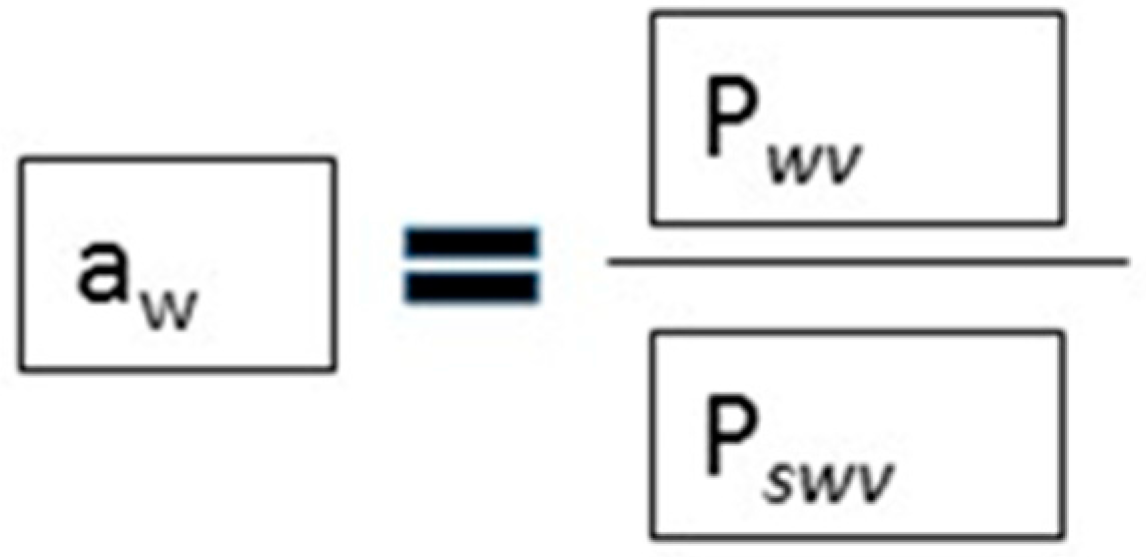
4. Addition of NaCl and Reduction of Water Activity
5. Garlic (Allium sativum)
6. Oils
7. Fat Content
8. Conclusions
Author Contributions
Conflicts of Interest
Appendix A
| Country | Salmonella spp. | Food | Control Mechanisms | Comments | Reference |
|---|---|---|---|---|---|
| U.K. | S. Enteritidis | Mayonnaise | pH | 20 mL vinegar (6% w/v acetic acid) per fresh egg yolk, 40 mL per fresh egg white or 60 mL per fresh whole egg was used. Should be held at 20 °C or above for at least 48 h before refrigeration or consumption. | [77] |
| U.K. | S. Enteritidis | Mayonnaise-based shrimp salad | pH/preservatives chitosan and acetic acid | Chitosan could be useful as a preservative combined with acetic acid. | [54] |
| China/U.S. | S. typhimurium S. heidelberg S. Enteritidis | Home-Style Mayonnaise | pH commercial wine vinegar, lemon juice, and acetic or citric acid | Salmonella counts in acid solutions at 4 °C were reported as significantly higher than those in samples at 25 °C. Viability of Salmonella decreased as the amounts of vinegar and lemon juice in mayonnaise increased. | [35] |
| France | S. typhimurium | Reduced-calorie mayonnaise | pH/Temperature | Higher temperature with a low pH, greater the inactivation of the organism. | [78] |
| U.S. | Salmonella | Mayonnaise-based potato salad, macaroni salad, and coleslaw | pH, NaCl, and temperature | Decreased pH and increased the bactericidal activity irrespective of sodium concentration or storage temperature Sodium concentrations had little or no effect on the behavior of Salmonella when stored at 4 or 10 °C for up to 27 days. | [50] |
| U.K. | S. Enteritidis | Mayonnaise | pH acetic acid (vinegar) | Mayonnaise made with vinegar to a pH of 4.1 or less controlled S. Enteritidis. Storage of mayonnaise at refrigeration temperatures protected Salmonella spp. from acidulants and therefore a holding time of 24 h at 18–22 °C was advised before refrigeration. | [79] |
| Spain | S. Enteritidis | Home-made Mayonnaise | pH/temperature | Vinegar was used as an acidulant to achieve a pH of 3.6–4 and storage in a warm place is recommended. | [52] |
| U.S. | S. senftenberg | Egg salads | pH/temperature | Significant decrease in Salmonella numbers, particularly during storage at room temperature (22 °C) at the acidity ranging from pH 4.25–4.30 was recorded. | [80] |
| Brazil | S. Enteritidis | Mayonnaise | oil oregano essential oil | Natural antimicrobial to reduce the S. Enteritidis growth | [81] |
| Spain | S. Enteritidis | Egg mayonnaise | oil (virgin olive oil) | Egg mayonnaise made with virgin olive oil required more than 48 h to reduce the number of microorganisms to an undetectable level. | [54] |
| U.K. | S. Enteritidis | Homemade Mayonnaise | Temperature, pH, oils (olive oil with garlic, basil, soya, grapeseed, rapeseed, groundnut, sunflower, hazelnut) | The death rate of the S. Enteritidis differed with the various oils. | [82] |
| Brazil | S. Enteritidis | Mayonnaise | oil oregano essential oils (OEO1/OEO2) Nisin EDTA | Antimicrobial activity of OEO against S. Enteritidis was improved when combined with nisin or EDTA. | [74] |
| U.K, | S. Enteritidis | Mayonnaise | garlic | Garlic (1%) reduced the viable cells of S. Enteritidis in mayonnaise by a factor of 10. | [67] |
| Greece | S. Enteritidis | Mayonnais- based Aubergine salad | sorbic/benzoic acids | Addition of preservatives decreased the pathogen survival. | [83] |
| U.K. | S. Enteritidis | Mayonnaise | pH | Within the pH 4.2–4.5 S. Enteritidis remained stable for 4 weeks at 4 °C. | [43] |
References
- Newman, K.L.; Leon, J.S.; Rebolledo, P.A.; Scallan, E. The impact of socioeconomic status on foodborne illness in high-income countries: A systematic review. Epidemiol. Infect. 2015, 143, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the united states. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Murray, R.; Flockhart, L.; Pintar, K.; Pollari, F.; Fazil, A.; Nesbitt, A.; Marshall, B. Estimates of the burden of foodborne illness in canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog. Dis. 2013, 10, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.; Kirk, M.D.; Becker, N.; Gregory, J.E.; Unicomb, L.; Millard, G.; Stafford, R.; Lalor, K.; Group, O.W. Estimating foodborne gastroenteritis, australia. Emerg. Infect. Dis. 2005, 11, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease Burden of Illness Studies. The global burden of nontyphoidal salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The family enterobacteriaceae. In Practical Handbook of Microbiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 307. [Google Scholar]
- Batista, D.F.A.; Freitas Neto, O.C.; Barrow, P.A.; de Oliveira, M.T.; Almeida, A.M.; Ferraudo, A.S.; Berchieri, A., Jr. Identification and characterization of regions of difference between the salmonella gallinarum biovar gallinarum and the salmonella gallinarum biovar pullorum genomes. Infect. Genet. Evol. 2015, 30, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Rabsch, W.; Andrews, H.L.; Kingsley, R.A.; Prager, R.; Tschäpe, H.; Adams, L.G.; Bäumler, A.J. Salmonella enterica serotype typhimurium and its host-adapted variants. Infect. Immun. 2002, 70, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention. Multistate outbreak of salmonella serotype tennessee infections associated with peanut butter—United states, 2006–2007. Morb. Mortal. Wkly. Rep. 2007, 56, 521–524. [Google Scholar]
- Wilson, M.R.; Brown, E.; Keys, C.; Strain, E.; Luo, Y.; Muruvanda, T.; Grim, C.; Beaubrun, J.J.-G.; Jarvis, K.; Ewing, L. Whole genome DNA sequence analysis of salmonella subspecies enterica serotype tennessee obtained from related peanut butter foodborne outbreaks. PLoS ONE 2016, 11, e0146929. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Ross, K. Salmonella and eggs: From production to plate. Int. J. Environ. Res. Public Health 2015, 12, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Mishu, B.; Koehler, J.; Lee, L.A.; Rodrigue, D.; Brenner, F.H.; Blake, P.; Tauxe, R.V. Outbreaks of salmonella enteritidis infections in the united states, 1985–1991. J. Infect. Dis. 1994, 169, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Delarocque-Astagneau, E.; Desenclos, J.-C.; Bouvet, P.; Grimont, P. Risk factors for the occurrence of sporadic salmonella enterica serotype enteritidis infections in children in france: A national case-control study. Epidemiol. Infect. 1998, 121, 561–567. [Google Scholar] [CrossRef] [PubMed]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.; Petersen, C. The effect of egg shell quality on penetration by various salmonellae. Poult. Sci. 1974, 53, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Berrang, M.; Frank, J.; Buhr, R.; Bailey, J.; Cox, N.; Mauldin, J. Eggshell characteristics and penetration by salmonella through the productive life of a broiler breeder flock. Poult. Sci. 1998, 77, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.; Cranstoun, S.; Solomon, S. Relationship between shell structure and movement of salmonella enteritidis across the eggshell wall. Br. Poult. Sci. 1992, 33, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Johnson, A. The bacterial flora of unhatched eggs. Br. Poult. Sci. 1978, 19, 681–689. [Google Scholar] [CrossRef]
- De Oliveira Elias, S.; Tomasco, P.V.; Alvarenga, V.O.; de Souza Sant’Ana, A.; Tondo, E.C. Contributor factors for the occurrence of salmonellosis during preparation, storage and consumption of homemade mayonnaise salad. Food Res. Int. 2015, 78, 266–273. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention. Outbreaks of salmonella serotype enteritidis infection associated with consumption of raw shell eggs—United states, 1994–1995. Morb. Mortal. Wkly. Rep. 1996, 45, 737–742. [Google Scholar]
- Sinclair, M.; Hellard, M.; Wolfe, R.; Fairley, C. Pathogens Causing Community Gastroenteritis in Australia [Poster Abstract]. In Proceedings of the Australian Society for Infectious Disease Conference, Barossa Valley, South Australia, 13–17 April 2002.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Depree, J.; Savage, G. Physical and flavour stability of mayonnaise. Trends Food Sci. Technol. 2001, 12, 157–163. [Google Scholar] [CrossRef]
- Di Mattia, C.; Balestra, F.; Sacchetti, G.; Neri, L.; Mastrocola, D.; Pittia, P. Physical and structural properties of extra-virgin olive oil based mayonnaise. LWT-Food Scie. Technol. 2015, 62, 764–770. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the united states. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated salmonella enterica serotypes and food commodities, united states, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Ivarsson, S.; Lofdahl, M.; Nauta, M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the european union, 2009. Epidemiol. Infect. 2013, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- OzFoodNet Working Group. Monitoring the incidence and causes of diseases potentially transmitted by food in australia: Annual report of the ozfoodnet network, 2011. Commun. Dis. Intell. Q. Rep. 2015, 39, E236–E264. [Google Scholar]
- Mitchell, E.; O’Mahony, M.; Lynch, D.; Ward, L.; Rowe, B.; Uttley, A.; Rogers, T.; Cunningham, D.; Watson, R. Large outbreak of food poisoning caused by salmonella typhimurium definitive type 49 in mayonnaise. Br. Med. J. 1989, 298, 99–101. [Google Scholar] [CrossRef]
- Mason, B.; Williams, N.; Salmon, R.; Lewis, A.; Price, J.; Johnston, K.; Trott, R. Outbreak of salmonella indiana associated with egg mayonnaise sandwiches at an acute nhs hospital. Commun. Dis. Public Health/PHLS 2001, 4, 300–304. [Google Scholar]
- Carneiro, M.R.P.; Cabello, P.H.; Albuquerque-Junior, R.L.C.; Jain, S.; Candido, A.L. Characterization of a foodborne outbreak caused by salmonella enteritidis in aracaju, state of sergipe, brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Von Wissmann, B.; Klinc, C.; Schulze, R.; Wolf, A.; Schreiner, H.; Rabsch, W.; Prager, R.; Hautmann, W. Outbreak of salmonellosis after a wedding party, bavaria, germany, summer 2010: The importance of implementing food safety concepts. Eurosurveillance 2012, 17, 20076. [Google Scholar] [PubMed]
- Jung, Y.; Beuchat, L. Sensitivity of multidrug-resistant salmonella typhimurium dt104 to organic acids and thermal inactivation in liquid egg products. Food Microbiol. 2000, 17, 63–71. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Chen, J. Survival of salmonella in home-style mayonnaise and acid solutions as affected by acidulant type and preservatives. J. Food Prot. 2012, 75, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Khoo, W.J.; Zheng, Q.; Chung, H.-J.; Yuk, H.-G. Growth temperature alters salmonella enteritidis heat/acid resistance, membrane lipid composition and stress/virulence related gene expression. Int. J. Food Microbiol. 2014, 172, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mattick, K.; Jørgensen, F.; Legan, J.; Cole, M.; Porter, J.; Lappin-Scott, H.; Humphrey, T. Survival and filamentation of salmonella entericaserovar enteritidis pt4 and salmonella enterica serovar typhimurium dt104 at low water activity. Appl. Environ. Microbiol. 2000, 66, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Indu, M.; Hatha, A.; Abirosh, C.; Harsha, U.; Vivekanandan, G. Antimicrobial activity of some of the south-indian spices against serotypes of escherichia coli, salmonella, listeria monocytogenes and aeromonas hydrophila. Braz. J. Microbiol. 2006, 37, 153–158. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial food-borne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, R.; Matsumoto, M.; Sakae, K.; Miyazaki, Y. Ability of shiga toxin-producing escherichia coli and salmonella spp. To survive in a desiccation model system and in dry foods. Appl. Environ. Microbiol. 2005, 71, 6657–6663. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of desiccation on tolerance of salmonella enterica to multiple stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Leyer, G.; Johnson, E. Acid adaptation induces cross-protection against environmental stresses in salmonella typhimurium. Appl. Environ. Microbiol. 1993, 59, 1842–1847. [Google Scholar] [PubMed]
- Leuschner, R.G.; Boughtflower, M.P. Standardized laboratory-scale preparation of mayonnaise containing low levels of salmonella enterica serovar enteritidis. J. Food Prot. 2001, 64, 623–629. [Google Scholar] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial ph sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Booth, I.R. Regulation of cytoplasmic ph in bacteria. Microbiol. Rev. 1985, 49, 359–378. [Google Scholar] [PubMed]
- Foster, J.W.; Hall, H.K. Inducible ph homeostasis and the acid tolerance response of salmonella typhimurium. J. Bacteriol. 1991, 173, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Hall, H.K. Adaptive acidification tolerance response of salmonella typhimurium. J. Bacteriol. 1990, 172, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Fernández, A.; Bernardo, A.; López, M. Acid tolerance in salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 2010, 27, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Ikeda, J.; Sofos, J. Evaluation of the ph-dependent, stationary-phase acid tolerance in listeria monocytogenes and salmonella typhimurium dt104 induced by culturing in media with 1% glucose: A comparative study with Escherichia coli o157: H7. J. Appl. Microbiol. 2003, 95, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Alali, W.Q.; Mann, D.A.; Beuchat, L.R. Viability of salmonella and listeria monocytogenes in delicatessen salads and hummus as affected by sodium content and storage temperature. J. Food Prot. 2012, 75, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Modeling the boundaries of growth of salmonella typhimurium in broth as a function of temperature, water activity, and ph. J. Food Prot. 2004, 67, 53–59. [Google Scholar] [PubMed]
- Perales, I.; Garcia, M. The influence of ph and temperature on the behaviour of salmonella enteritidis phage type 4 in home-made mayonnaise. Lett. Appl. Microbiol. 1990, 10, 19–22. [Google Scholar] [CrossRef]
- Lock, J.; Board, R. The fate of salmonella enteritidis pt4 in home-made mayonnaise prepared from artificially inoculated eggs. Food Microbiol. 1995, 12, 181–186. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antimicrobial properties of chitosan in mayonnaise and mayonnaise-based shrimp salads. J. Food Prot. 2000, 63, 202–209. [Google Scholar] [PubMed]
- Csonka, L.N.; Hanson, A.D. Prokaryotic osmoregulation: Genetics and physiology. Annu. Rev. Microbiol. 1991, 45, 569–606. [Google Scholar] [CrossRef] [PubMed]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [PubMed]
- Finn, S.; Händler, K.; Condell, O.; Colgan, A.; Cooney, S.; McClure, P.; Amézquita, A.; Hinton, J.C.; Fanning, S. Prop is required for the survival of desiccated salmonella enterica serovar typhimurium cells on a stainless steel surface. Appl. Environ. Microbiol. 2013, 79, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Hurdle Technology in Food Preservation. In Minimally Processed Foods; Springer: Cham, Switzerland, 2015; pp. 17–33. [Google Scholar]
- Henry, J.E.; Taylor, C.L. Strategies to Reduce Sodium Intake in the United States; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology; American Society of Microbiology: Washington, DC, USA, 2013; pp. 765–801. [Google Scholar]
- Manas, P.; Pagan, R.; Leguérinel, I.; Condon, S.; Mafart, P.; Sala, F. Effect of sodium chloride concentration on the heat resistance and recovery of salmonella typhimurium. Int. J. Food Microbiol. 2001, 63, 209–216. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Berwal, J. Sensitivity of food pathogens to garlic (allium sativum). J. Appl. Microbiol. 1998, 84, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Vaughn, R.H. Death of salmonella typhimurium and escherichia coli in the presence of freshly reconstituted dehydrated garlic and onion. Appl. Microbiol. 1969, 17, 903–905. [Google Scholar] [PubMed]
- Bernbom, N.; Ng, Y.Y.; Paludan-Müller, C.; Gram, L. Survival and growth of salmonella and vibrio in som-fak, a thai low-salt garlic containing fermented fish product. Int. J. Food Microbiol. 2009, 134, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ekwenye, U.; Elegalam, N. Antibacterial activity of ginger (zingiber officinale roscoe and garlic (allium sativum l.) extracts on escherichia coli and salmonella typhi. Int. J. Mol. Adv. Sci. 2005, 1, 411–416. [Google Scholar]
- Leuschner, R.G.; Zamparini, J. Effects of spices on growth and survival of escherichia coli 0157 and salmonella enterica serovar enteritidis in broth model systems and mayonnaise. Food Control 2002, 13, 399–404. [Google Scholar] [CrossRef]
- Bajpai, V.; Rahman, A.; Dung, N.; Huh, M.; Kang, S. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of magnolia liliflora desr. J. Food Sci. 2008, 73, M314–M320. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wendakoon, C.N.; Sakaguchi, M. Inhibition of amino acid decarboxylase activity of enterobacter aerogenes by active components in spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar]
- Tassou, C.; Drosinos, E.; Nychas, G. Effects of essential oil from mint (mentha piperita) on salmonella enteritidis and listeria monocytogenes in model food systems at 4 and 10 c. J. Appl. Bacteriol. 1995, 78, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Dabbah, R.; Edwards, V.; Moats, W. Antimicrobial action of some citrus fruit oils on selected food-borne bacteria. Appl. Microbiol. 1970, 19, 27–31. [Google Scholar] [PubMed]
- Valverde, M.; Iniesta, F.; Garrido, L.; Rodríguez, A.; García-García, I.; Macanas, H.; Roda, R. Inactivation of Salmonella spp. in Refrigerated Liquid Egg Products Using Essential Oils and Their Active Compounds. In Proceedings of the International Conference on Food Innovation “Food Innova”, Universitydad Politecnica De Valencia, Valencia, Spain, 25–26 October 2010.
- Da Silva, J.P.L.; de Souza, E.F.; Della Modesta, R.C.; Gomes, I.A.; Freitas-Silva, O.; de Melo Franco, B.D.G. Antibacterial activity of nisin, oregano essential oil, edta, and their combination against salmonella enteritidis for application in mayonnaise. Vigil. Sanit. Debate Soc. Ciênc. Tecnol. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Stevens, K.; Klapes, N.; Sheldon, B.; Klaenhammer, T. Antimicrobial action of nisin against salmonella typhimurium lipopolysaccharide mutants. Appl. Environ. Microbiol. 1992, 58, 1786–1788. [Google Scholar] [PubMed]
- Lock, J.; Board, R. The fate of salmonella enteritidis pt4 in deliberately infected commercial mayonnaise. Food Microbiol. 1994, 11, 499–504. [Google Scholar] [CrossRef]
- Xiong, R.; Xie, G.; Edmondson, A. Modelling the ph of mayonnaise by the ratio of egg to vinegar. Food Control 2000, 11, 49–56. [Google Scholar] [CrossRef]
- Membre, J.-M.; Majchrzak, V.; Jolly, I. Effects of temperature, ph, glucose, and citric acid on the inactivation of salmonella typhimurium in reduced calorie mayonnaise. J. Food Prot. 1997, 60, 1497–1501. [Google Scholar]
- Radford, S.; Board, R. Review: Fate of pathogens in home-made mayonnaise and related products. Food Microbiol. 1993, 10, 269–278. [Google Scholar] [CrossRef]
- Simmons, S.E.; Bartolucci, D.P.; Stadelman, W. Formulation and evaluation of a low ph egg salad. J. Food Sci. 1979, 44, 1501–1504. [Google Scholar] [CrossRef]
- Da Silva, J.P.L.; de Melo, B.D.G. Application of oregano essential oil against salmonella enteritidis in mayonnaise salad. Int. J. Food Sci. Nutr. Eng. 2012, 2, 70–75. [Google Scholar] [CrossRef]
- Lock, J.; Board, R. The influence of acidulants and oils on autosterilization of home-made mayonnaise. Food Res. Int. 1995, 28, 569–572. [Google Scholar] [CrossRef]
- Tassou, C.C.; Samaras, F.J.; Arkoudelos, J.S.; Mallidis, C.G. Survival of acid-adapted or non-adapted salmonella enteritidis, listeria monocytogenes and escherichia coli o157: H7, in traditional greek salads. Int. J. Food Sci. Technol. 2009, 44, 279–287. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.; Sacchetti, G.; Neri, L.; Pittia, P. Role of olive oil phenolics in physical properties and stability of mayonnaise-like emulsions. Food Chem. 2016, 213, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; Brenes, M.; de Castro, A. Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. A Review of Temperature, pH, and Other Factors that Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products. Pathogens 2016, 5, 63. https://doi.org/10.3390/pathogens5040063
Keerthirathne TP, Ross K, Fallowfield H, Whiley H. A Review of Temperature, pH, and Other Factors that Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products. Pathogens. 2016; 5(4):63. https://doi.org/10.3390/pathogens5040063
Chicago/Turabian StyleKeerthirathne, Thilini Piushani, Kirstin Ross, Howard Fallowfield, and Harriet Whiley. 2016. "A Review of Temperature, pH, and Other Factors that Influence the Survival of Salmonella in Mayonnaise and Other Raw Egg Products" Pathogens 5, no. 4: 63. https://doi.org/10.3390/pathogens5040063