Implications of Neuroinvasive Bacterial Peptides on Rodents Behaviour and Neurotransmission
Abstract
:1. Introduction
2. Results
2.1. Identification of Etiological Agents from CSF
2.2. Physiological Changes
2.3. Home Cage and Open Field Test
2.4. Bioactive Peptides Released by Neuropathogenic Bacteria
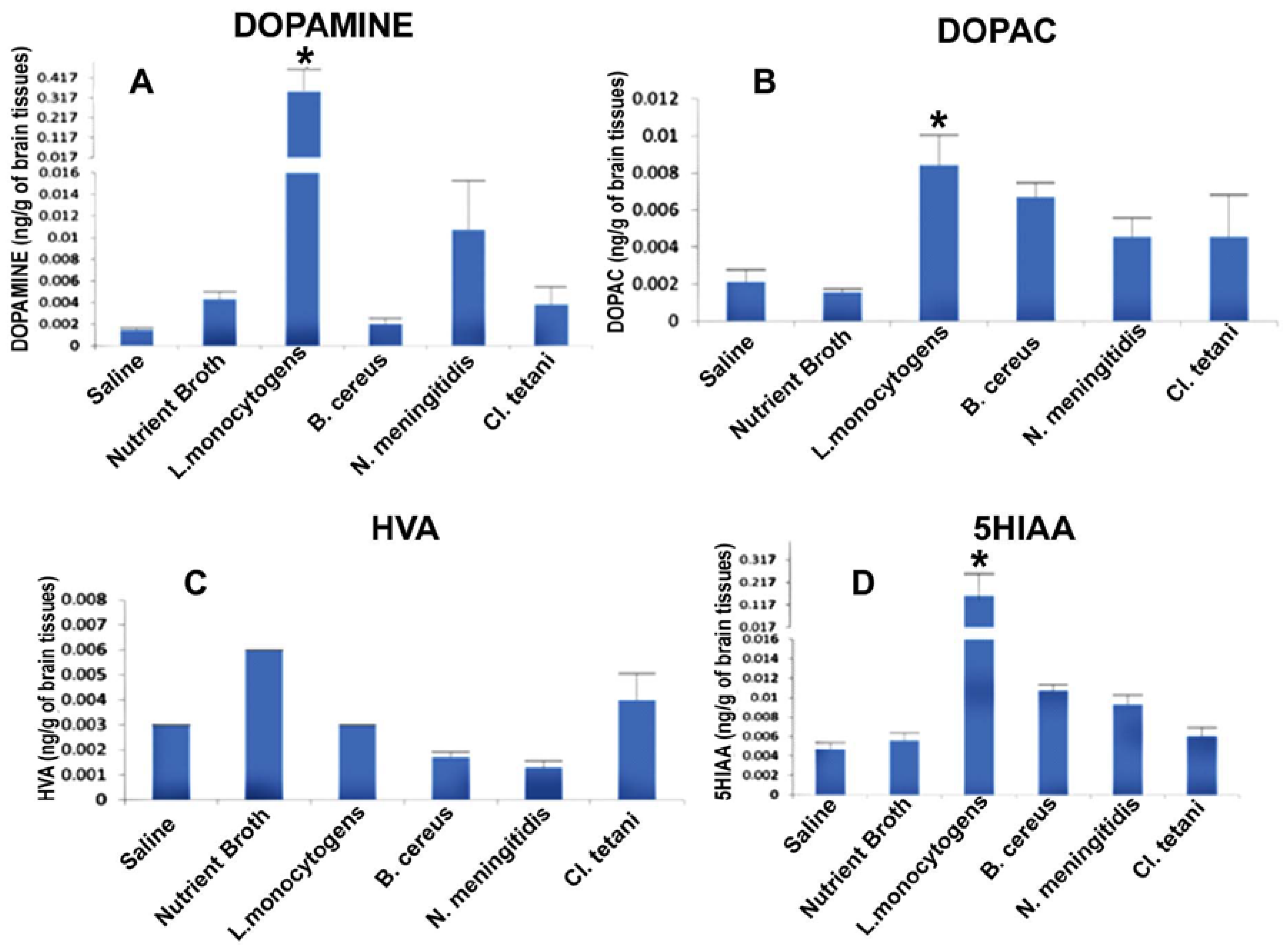
2.5. Quantification of Neurotransmitters in Response to Crude SCFBs
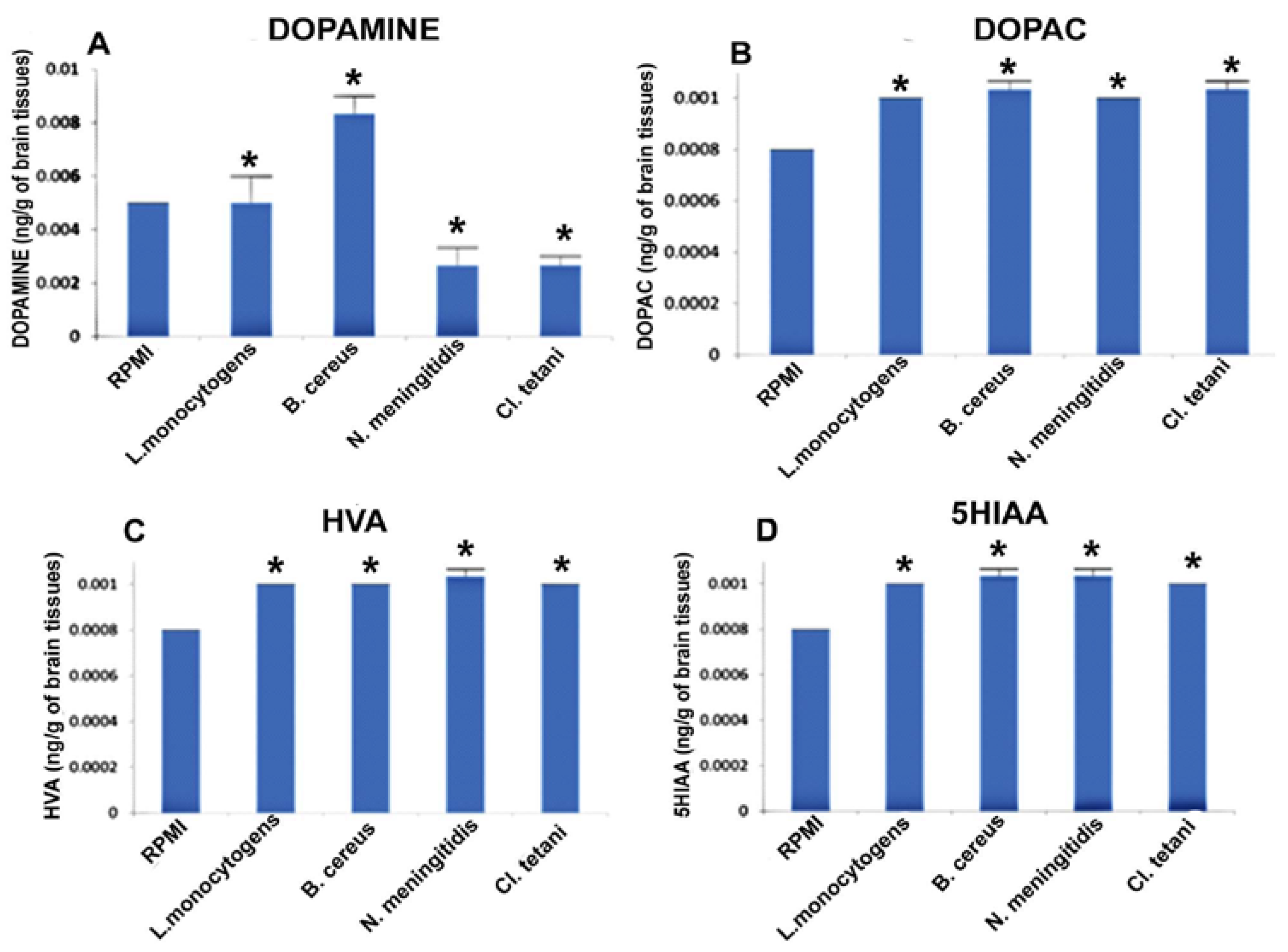
2.6. Quantification of Neurotransmitters in Response to Neuropathogenic Bacterial Peptide
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation of Etiological Agents fromCerebrospinalFluid (CSF) Samples
4.3. Filter-Sterilized Cell Free Cultural Broth (SCFB) Preparation
4.4. Animals
4.5. Experimental Procedure and Challenge
4.6. Behavioural Study
4.7. Extraction of Biogenic Amines
4.8. HPLC-EC
4.9. Peptide Extraction
4.10. Sterility Check
4.11. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| BBB | Blood Brain Barrier |
| Bc | B. cereus |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| Ct | Cl. tetani |
| DOPAC | Dihydroxyphenyl acetic acid |
| DA | Dopamine |
| HPLC-EC | High-performance liquid chromatography with electrochemical detection |
| LPS | Lipopolysaccharide |
| Lm | L. monocytogenes |
| Nm | N. meningitidis |
| PG | Peptidoglycan |
| 5HT | Serotonin |
| SD | Sprague Dawley |
| SCFB | Filter sterilized cell free cultural broth |
| SCFBs | Filter sterilized cell free cultural broths |
References
- Disson, O.; Lecuit, M. Targeting of the central nervous system by Listeria monocytogenes. Virulence 2012, 3, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Collodel, A.; Moreira, A.P.; Almeida, S.M.D. Pathophysiology of acute meningitis caused by Streptococcus pneumoniae and adjunctive therapy approaches. Arq. Neuro-Psiquiatr. 2012, 70, 366–372. [Google Scholar] [CrossRef]
- Drevets, D.A.; Leenen, P.J.; Greenfield, R.A. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 2004, 17, 323–347. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Lécuyer, H.; Join-Lambert, O.; Bourdoulous, S.; Marullo, S.; Nassif, X.; Coureuil, M. Neisseria meningitidis colonization of the brain endothelium and cerebrospinal fluid invasion. Cell. Microbiol. 2013, 15, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Dupin, N.; Lecuyer, H.; Carlotti, A.; Poyart, C.; Coureuil, M.; Chanal, J.; Morand, P.C. Chronic meningococcemia cutaneous lesions involve meningococcal perivascular invasion through the remodeling of endothelial barriers. Clin. Infect. Dis. 2012, 54, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Corbett, D.; Goldrick, M.; Roberts, I.S.; Brough, D. Inhibition of calpain blocks the phagosomal escape of Listeria monocytogenes. PLoS ONE 2012, 7, e35936. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Microbial translocation of the blood-brain barrier. Int. J. Parasitol. 2006, 36, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Pulzova, L.; Bhide, M.R.; Andrej, K. Pathogen translocation across the blood-brain barrier. FEMS Immunol. Med. Microbiol. 2009, 57, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Tuomanen, E. Molecular and cellular mechanisms for microbial entry into the CNS. J. Neurovirol. 1999, 5, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Coureuil, M.; Join-Lambert, O.; Lécuyer, H.; Bourdoulous, S.; Marullo, S.; Nassif, X. Mechanism of meningeal invasion by Neisseria meningitidis. Virulence 2012, 3, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wang, S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav. Brain Res. 2014, 259, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Heppel, N.; Jägerhuber, R.; Kim, K.S.; Frosch, M.; Eigenthaler, M.; Schubert-Unkmeir, A. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: Role of MAP-and tyrosine kinases in invasion and inflammatory cytokine release. Cell. Microbiol. 2004, 6, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Scheld, W.M.; Koedel, U.; Nathan, B.; Pfister, H.W. Pathophysiology of bacterial meningitis: Mechanism (s) of neuronal injury. J. Infect. Dis. 2002, 186 (Suppl. 2), S225–S233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Schurr, M.J.; LeBlanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of bacterial pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.G.; Hanaa-Mansour, A.; Hasan, W.A.; Wageh, S.A. Neurotransmitters’ Changes in Bacterial Infected Treated and Untreated Rats. Glob. J. Pharmacol. 2015, 9, 121–129. [Google Scholar]
- Adham, K.G.; Al-Humaidhi, E.M.; Daghestani, M.H.; Aleisa, N.A.; Farhood, M.H. Protective role of indomethacin on lipopolysaccharide-stimulated fever induction and cerebral catecholamine biosynthesis in Wistar rat. Neuroendocrinol. Lett. 2012, 33, 713–721. [Google Scholar] [PubMed]
- Francis, J.; MohanKumar, P.S.; MohanKumar, S.M. Lipopolysaccharide stimulates norepinephrine efflux from the rat hypothalamus in vitro: Blockade by soluble IL-1 receptor. Neurosci. Lett. 2001, 308, 71–74. [Google Scholar] [CrossRef]
- MohanKumar, S.M.; MohanKumar, P.S.; Quadri, S.K. Lipopolysaccharide-induced changes in monoamines in specific areas of the brain: Blockade by interleukin-1 receptor antagonist. Brain Res. 1999, 824, 232–237. [Google Scholar] [CrossRef]
- MohanKumar, S.M.; MohanKumar, P.S.; Quadri, S.K. Effects of bacterial lipopolysaccharide on central monoamines and fever in the rat: Involvement of the vagus. Neurosci. Lett. 2000, 284, 159–162. [Google Scholar] [CrossRef]
- Schrijver, I.A.; van Meurs, M.; Melief, M.J.; Ang, C.W.; Buljevac, D.; Ravid, R.; Laman, J.D. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 2001, 124, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.; Mattsson, E. The role of cytokines in gram-positive bacterial shock. Trends Microbiol. 1995, 3, 136–140. [Google Scholar] [CrossRef]
- Blum, F.C.; Chen, C.; Kroken, A.R.; Barbieri, J.T. Tetanus toxin and botulinum toxin a utilize unique mechanisms to enter neurons of the central nervous system. Infect. Immun. 2012, 80, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Pathan, N.; Faust, S.N.; Levin, M. Pathophysiology of meningococcal meningitis and septicaemia. Arch. Dis. Child. 2003, 88, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004, 12, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Stins, M.F.; Kim, K.S. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2000, 2, 1237–1244. [Google Scholar] [CrossRef]
- Linthorst, A.C.; Reul, J.M. Brain neurotransmission during peripheral inflammation. Ann. N. Y. Acad. Sci. 1998, 840, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, J.Y.; Lo, Y.K.; Carvey, P.M.; Ling, Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009, 1265, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Zhukovsky, D.S. Pathophysiology and management of fever. J. Support Oncol. 2006, 4, 9–16. [Google Scholar] [PubMed]
- Linthorst, A.C.; Flachskamm, C.; Muller-Preuss, P.; Holsboer, F.; Reul, J.M. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: An in vivo microdialysis study. J. Neurosci. 1995, 15, 2920–2934. [Google Scholar] [PubMed]
- Tavares, E.; Miñano, F.J. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemia: Neuroendocrine and systemic studies. Clin. Sci. 2010, 119, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M. Tetanus. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 101–104. [Google Scholar] [CrossRef]
- Somand, D.; Meurer, W. Central nervous system infections. Emerg. Med. Clin. N. Am. 2009, 27, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kaizaki, A.; Tien, L.T.; Pang, Y.; Cai, Z.; Tanaka, S.; Numazawa, S.; Fan, L.W. Celecoxib reduces brain dopaminergic neuronal dysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. J. Neuroinflamm. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Prado, C.E.; Barrientos, M.J.; Bernales, S. Role of dopamine in the physiology of T-cells and dendritic cells. J. Neuroimmunol. 2009, 216, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.E.; Vecsernyés, M.; Zelles, T.; Kádár, K.; Nagy, G.M. Role of peripheral and brain-derived dopamine (DA) in immune regulation. Adv. Neuro-immune Biol. 2012, 3, 111–155. [Google Scholar]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Elam, K.; Bearman, G. Meningitis due to Bacillus cereus: A case report and review of the literature. Can. J. Infect. Dis. Med. Microbiol. 2012, 23, e16–e19. [Google Scholar] [PubMed]
- Moniuszko, A.; Zajkowska, A.; Tumiel, E.; Rutkowski, K.; Czupryna, P.; Pancewicz, S.; Rutkowski, R.; Zdrodowska, A.; Zajkowska, J. Meningitis, Clinical Presentation of Tetanus. Case Rep. Infect. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]


| S. No | Challenge | SD Rat Model Detail |
|---|---|---|
| 01 | SCFBs | Group 1→Bc(SCFB) |
| Group 2→Ct(SCFB) | ||
| Group 3→Lm(SCFB) | ||
| Group 4→Nm(SCFB) | ||
| Group 5→Control(SCFB) | ||
| Group 6→Saline | ||
| 02 | Bacterial peptides purified from spent nutrient broth | Group 1→Bc(NB) |
| Group 2→Ct(NB) | ||
| Group 3→Lm(NB) | ||
| Group 4→Nm(NB) | ||
| Group 5→Control(NB) | ||
| 03 | Bacterial peptides purified from spent RPMI 1640 supplemented with human serum | Group 1→Bc(RPMI) |
| Group 2→Ct(RPMI) | ||
| Group 3→Lm(RPMI) | ||
| Group 4→Nm(RPMI) | ||
| Group 5→Control(RPMI) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taj, A.; Jamil, N. Implications of Neuroinvasive Bacterial Peptides on Rodents Behaviour and Neurotransmission. Pathogens 2017, 6, 27. https://doi.org/10.3390/pathogens6030027
Taj A, Jamil N. Implications of Neuroinvasive Bacterial Peptides on Rodents Behaviour and Neurotransmission. Pathogens. 2017; 6(3):27. https://doi.org/10.3390/pathogens6030027
Chicago/Turabian StyleTaj, Aneela, and Nusrat Jamil. 2017. "Implications of Neuroinvasive Bacterial Peptides on Rodents Behaviour and Neurotransmission" Pathogens 6, no. 3: 27. https://doi.org/10.3390/pathogens6030027





