Impact of TR34/L98H, TR46/Y121F/T289A and TR53 Alterations in Azole-Resistant Aspergillus fumigatus on Sterol Composition and Modifications after In Vitro Exposure to Itraconazole and Voriconazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates of A. fumigatus
2.2. Culture Conditions
2.3. Total Sterol Extraction
2.4. Sterol Derivatization
2.5. Sterol Content Analysis
2.6. Statistical Analysis
3. Results and Discussion
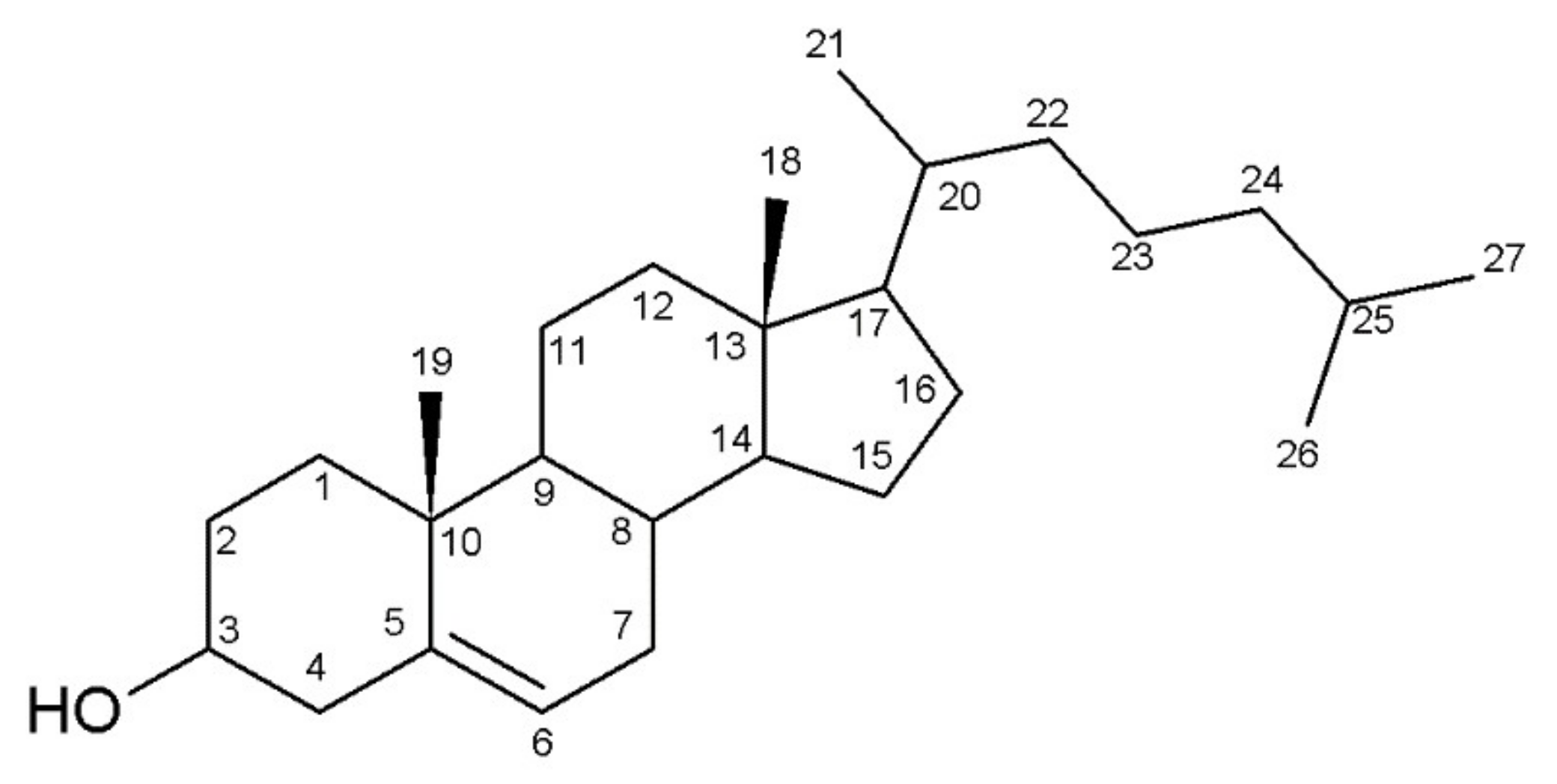
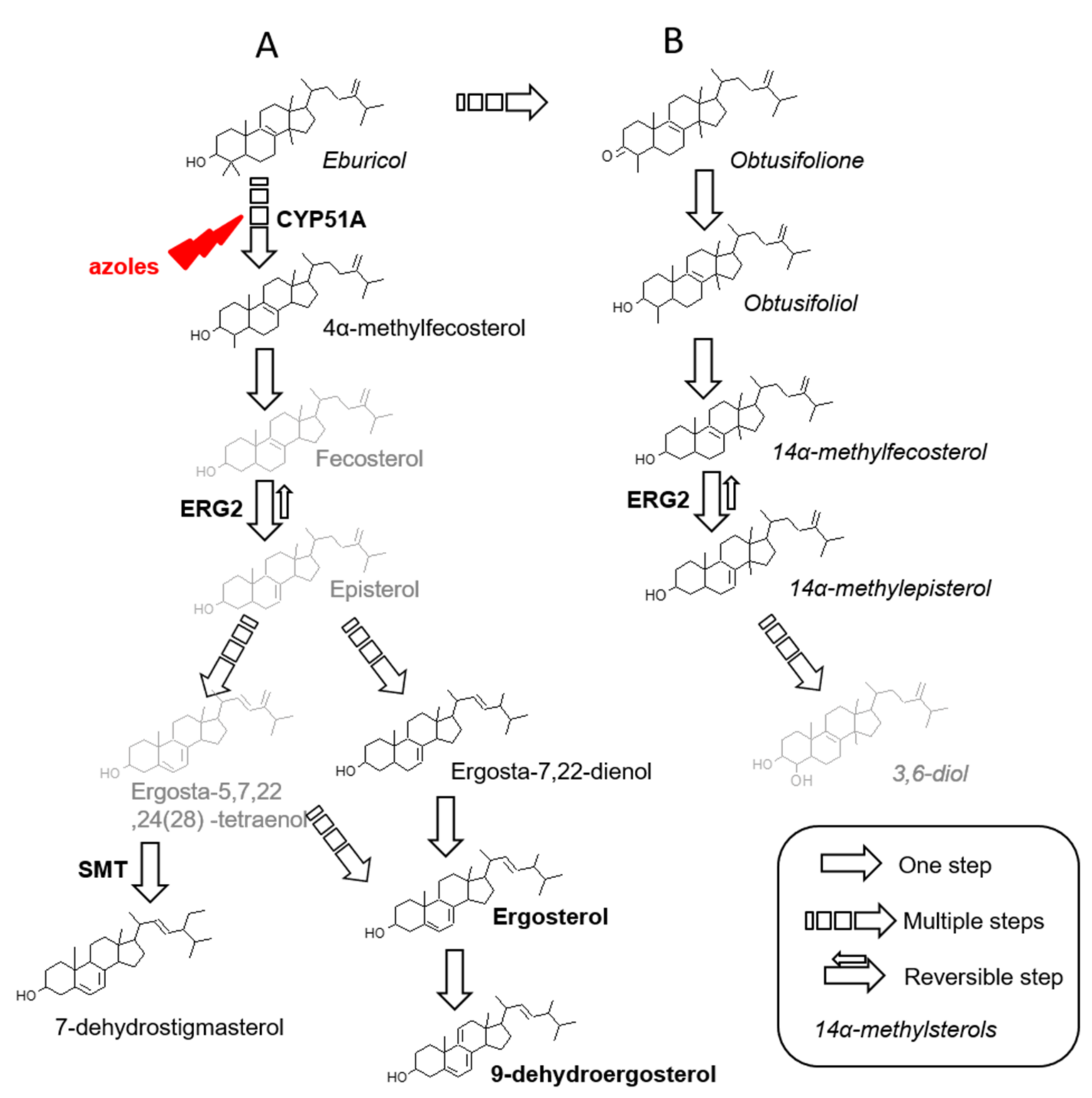
3.1. Identification of Sterols
3.2. Qualitative Composition and Relative Amount of Sterols
3.2.1. Under Basal Conditions
3.2.2. Under Itraconazole or Voriconazole Exposure
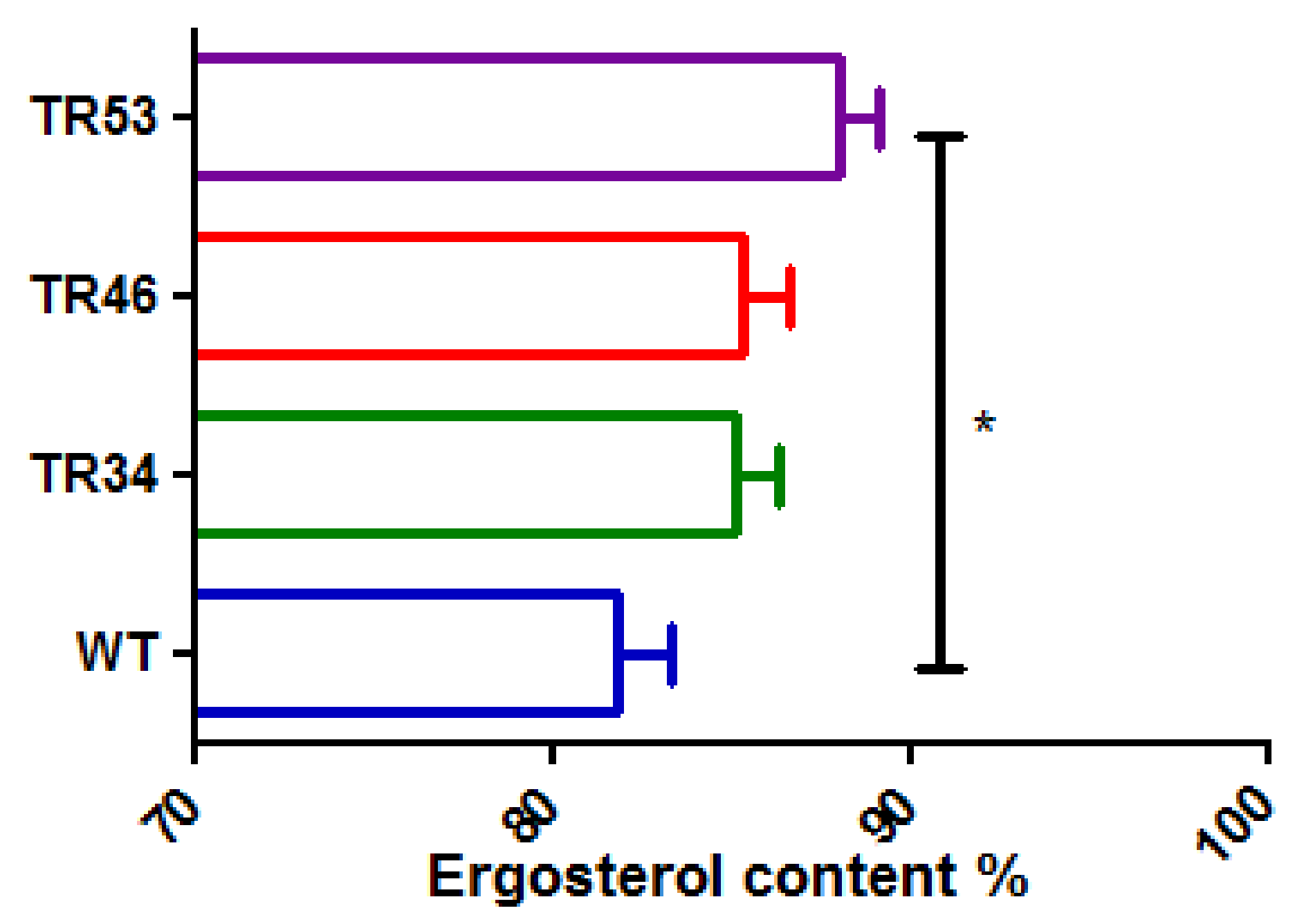
3.3. Statistical Comparison of Ergosterol Content
3.4. Statistical Comparison of 14α-Methylsterols Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.E.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R.; Denning, D.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Lass-Flörl, C.; Garcia-Rubio, R.; Daneshnia, F.F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. J. Fungi 2020, 6, 138. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2013, 3, 439. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Mellado, E.; Melchers, W. Multiple-Triazole–Resistant Aspergillosis. New Engl. J. Med. 2007, 356, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- Mellado, E.; Garcia-Effron, G.; Alcázar-Fuoli, L.; Melchers, W.J.G.; Verweij, P.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L. A New Aspergillus fumigatus Resistance Mechanism Conferring In Vitro Cross-Resistance to Azole Antifungals Involves a Combination of cyp51A Alterations. Antimicrob. Agents Chemother. 2007, 51, 1897–1904. [Google Scholar] [CrossRef] [Green Version]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole resistance in Aspergillus fumigatus: Recent insights and challenges for patient management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef]
- Nywening, A.V.; Rybak, J.M.; Rogers, P.D.; Fortwendel, J.R. Mechanisms of triazole resistance in Aspergillus fumigatus. Environ. Microbiol. 2020, 22, 4934–4952. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Cuenca-Estrella, M.; Mellado, E. Triazole Resistance in Aspergillus Species: An Emerging Problem. Drugs 2017, 77, 599–613. [Google Scholar] [CrossRef]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. A Clinical Isolate of Candida albicans with Mutations in ERG11 (Encoding Sterol 14α-Demethylase) and ERG5 (Encoding C22 Desaturase) Is Cross Resistant to Azoles and Amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, M.A.; Spellberg, B.J.; Ibrahim, A.S.; Ritchie, J.A.; Currie, B.; Spitzer, E.D.; Edwards, J.E.; Casadevall, A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob. Agents Chemother. 1994, 38, 2029–2033. [Google Scholar] [CrossRef] [Green Version]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Lopez, J.F.; Grimalt, J.O.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 2008, 73, 339–347. [Google Scholar] [CrossRef]
- Müller, C.; Neugebauer, T.; Zill, P.; Lass-Flörl, C.; Bracher, F.; Binder, U. Sterol Composition of Clinically Relevant Mucorales and Changes Resulting from Posaconazole Treatment. Mol. 2018, 23, 1218. [Google Scholar] [CrossRef] [Green Version]
- Dannaoui, E.; Persat, F.; Borel, E.; Piens, M.A.; Picot, S. Sterol composition of itraconazole-resistant and itraconazole-susceptible isolates of Aspergillus fumigatus. Can. J. Microbiol. 2001, 47, 706–710. [Google Scholar] [CrossRef]
- Hagiwara, D.; Arai, T.; Takahashi, H.; Kusuya, Y.; Watanabe, A.; Kamei, K. Non-cyp51A Azole-Resistant Aspergillus fumigatus Isolates with Mutation in HMG-CoA Reductase. Emerg. Infect. Dis. 2018, 24, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Buitrago, M.J.; López, J.; Grimalt, J.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Aspergillus fumigatus C-5 Sterol Desaturases Erg3A and Erg3B: Role in Sterol Biosynthesis and Antifungal Drug Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Rybak, J.M.; Ge, W.; Wiederhold, N.P.; Parker, J.E.; Kelly, S.L.; Rogers, P.D.; Fortwendel, J.R. Mutations in hmg1, Challenging the Paradigm of Clinical Triazole Resistance in Aspergillus fumigatus. mBio 2019, 10, e00437-19. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Van Rhijn, N.; Fraczek, M.; Gsaller, F.; Davies, E.; Carr, P.; Gago, S.; Fortune-Grant, R.; Rahman, S.; Gilsenan, J.M.; et al. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat. Commun. 2020, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Warrilow, A.; Parker, J.; Price, C.L.; Rolley, N.J.; Nes, W.D.; Kelly, D.E.; Kelly, S.L. Isavuconazole and voriconazole inhibition of sterol 14α-demethylases (CYP51) from Aspergillus fumigatus and Homo sapiens. Int. J. Antimicrob. Agents 2019, 54, 449–455. [Google Scholar] [CrossRef]
- Arendrup, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Definitive Document E.Def 9.3.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/ast_of_moulds/ (accessed on 10 November 2021).
- Müller, C.; Junker, J.; Bracher, F.; Giera, M. A gas chromatography–mass spectrometry-based whole-cell screening assay for target identification in distal cholesterol biosynthesis. Nat. Protoc. 2019, 14, 2546–2570. [Google Scholar] [CrossRef]
- The Lipid Web. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=index.html (accessed on 1 November 2021).
- Akihisa, T.; Goad, J. Analyse des Sterols; Blackie Academic and Professional: London, UK, 1997; ISBN 0751402303. [Google Scholar]
- Müller, C.; Binder, U.; Bracher, F.; Giera, M. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography–mass spectrometry. Nat. Protoc. 2017, 12, 947–963. [Google Scholar] [CrossRef]
- Müller, C.; Staudacher, V.; Krauss, J.; Giera, M.; Bracher, F. A convenient cellular assay for the identification of the molecular target of ergosterol biosynthesis inhibitors and quantification of their effects on total ergosterol biosynthesis. Steroids 2013, 78, 483–493. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Horning, E.C.; Young, J.S. Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids 1968, 3, 391–402. [Google Scholar] [CrossRef]
- Weete, J.D.; Gandhi, S.R. Sterols of the Phylum Zygomycota: Phylogenetic Implications. Lipids 1997, 32, 1309–1316. [Google Scholar] [CrossRef]
- Quail, M.A.; Arnold, A.; Moore, D.J.; Goosey, M.W.; Kelley, S.L. Ketoconazole-mediated growth inhibition in Botrytis cinerea and Saccharomyces cerevisiae. Phytochemistry 1993, 32, 273–280. [Google Scholar] [CrossRef]
- Shirane, N.; Murabayashi, A.; Masuko, M.; Uomori, A.; Yoshimura, Y.; Seo, S.; Uchida, K.; Takeda, K. Effect on ergosterol biosynthesis of a fungicide, SSF-109, in Botrytis cinerea. Phytochemistry 1990, 29, 2513–2520. [Google Scholar] [CrossRef]
- Moss, G.P. Nomenclature of steroids (Recommendations 1989). Pure Appl. Chem. 1989, 61, 1783–1822. [Google Scholar] [CrossRef]
- Bouvier-Nave, P.; Husselstein, T.; Benveniste, P. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. JBIC J. Biol. Inorg. Chem. 1998, 256, 88–96. [Google Scholar] [CrossRef]
- Nes, W. Sterol methyl transferase: Enzymology and inhibition. Biochim. et Biophys. Acta BBA Bioenerg. 2000, 1529, 63–88. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr. Top. Med. Chem. 2011, 11, 2060–2071. [Google Scholar] [CrossRef]
- Nes, W.; Xu, S.; Haddon, W.F. Evidence for similarities and differences in the biosynthesis of fungal sterols. Steroids 1989, 53, 533–558. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Qin, Y.-L.; Yan, L.; Wang, L.; Zhang, C.; Jiang, Y.-Y. NSG2 (ORF19.273) Encoding Protein Controls Sensitivity of Candida albicans to Azoles through Regulating the Synthesis of C14-Methylated Sterols. Front. Microbiol. 2018, 9, 218. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Walsh, T.J. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 1996, 40, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Rolley, N.; Kelly, D.E.; Kelly, S.L. Identification and Characterization of Four Azole-Resistant erg3 Mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [Google Scholar] [CrossRef] [Green Version]
- Marichal, P.; Gorrens, J.; Laurijssens, L.; Vermuyten, K.; Van Hove, C.; Le Jeune, L.; Verhasselt, P.; Sanglard, D.; Borgers, M.; Ramaekers, F.C.S.; et al. Accumulation of 3-Ketosteroids Induced by Itraconazole in Azole-Resistant Clinical Candida albicans Isolates. Antimicrob. Agents Chemother. 1999, 43, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Venkateswarlu, K.; Kelly, S.L. Biochemical characterisation of ketoconazole inhibitory action on Aspergillus fumigatus. FEMS Immunol. Med Microbiol. 1996, 16, 11–20. [Google Scholar] [CrossRef]
- Sherald, J.L.; Sisler, H.D. Antifungal mode of action of triforine. Pestic. Biochem. Physiol. 1975, 5, 477–488. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of Cholesterol and Other Sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Snelders, E.; Karawajczyk, A.; Verhoeven, R.J.; Venselaar, H.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J. The structure–function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: The mechanism of L98H azole resistance. Fungal Genet. Biol. 2011, 48, 1062–1070. [Google Scholar] [CrossRef]
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Muller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol Biosynthesis and Azole Tolerance Is Governed by the Opposing Actions of SrbA and the CCAAT Binding Complex. PLoS Pathog. 2016, 12, e1005775. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.; Karawajczyk, A.; Rijs, A.J.; Zoll, J.; Verweij, P.; Melchers, W.J. Genotype–phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet. Biol. 2015, 82, 129–135. [Google Scholar] [CrossRef]
- Watson, P.; Rose, M.; Ellis, S.; England, H.; Kelly, S. Defective sterol C5-6 desaturation and azole resistance: A new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 1989, 164, 1170–1175. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6 -desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef] [Green Version]
| Name | Origin | CYP51A Alteration | MIC Itraconazole mg/L | MIC Voriconazole mg/L |
|---|---|---|---|---|
| ATCC 204305 | NA | 0.25 (S) | 0.5 (S) | |
| AF1861 | C | 0.125 (S) | 1 (S) | |
| AF1799 | C | 0.5 (S) | 1 (S) | |
| AF899 | C | TR34/L98H | >32 (R) | 8 (R) |
| AF1897 | C | TR34/L98H | >32 (R) | 16 (R) |
| AF23 | E | TR34/L98H | >32 (R) | 16 (R) |
| AF1168 | C | TR46/Y121F/T289A | 1 (S) | >32 (R) |
| AF2226 | C | TR46/Y121F/T289A | 8 (R) | >32 (R) |
| AF1468 | C | TR46/Y121F/T289A | 8 (R) | >32 (R) |
| AF84 | E | TR53 | >32 (R) | 3 (R) |
| AF112 | E | TR53 | >32 (R) | 16 (R) |
| AF124 | E | TR53 | 6 (R) | 2 (R) |
| Identified Sterols | IUPAC Name | MW(TMS) | RRT | Reference |
|---|---|---|---|---|
| 9-dehydroergosterol | 24-methylcholesta-5,7,9(11),22-tetraen-3ß-ol or ergosta -5,7,9(11),22-tetraen-3ß-ol | 466 | 1.05 | [24,25] |
| Unknown 1 | 480 | 1.06 | ||
| Ergosterol Z Ergosterol E | 24-methylcholesta-5,7,22-trien-3ß-ol or ergosta-5,7,22Z-trien-3ß-ol or ergosta-5,7,22E-trien-3ß-ol | 468 | 1.06 1.10 | [26] |
| Ergosta-7,22-dienol | 24-methylcholesta-7,22-dien-3ß-ol or ergosta-7,22-dien-3ß-ol | 470 | 1.13 | [24] |
| 14α-methylepisterol | 14α,24- dimethylcholesta-7,24(28)-dien-3ß-ol or 14-methylergosta-7,24(28)-dien-3ß-ol | 484 | 1.14 | |
| Unknown 2 | 470 | 1.18 | ||
| 7-dehydrostigmasterol | 24 ethyl-cholesta-5,7,22-trienol | 482 | 1.19–1.20 | [27] |
| 14α-methylfecosterol | 14α,24- dimethylcholesta-8,24(28)-dien-3ß-ol or 14α-methylergosta -8,24(28)-dien-3ß-ol | 484 | 1.20 | [28] |
| Obtusifolione | 4α,14α,24-trimethylcholesta-8,24(28)-dien-3ß-one or 4α,14α-dimethylergosta-8,24(28)-dien-3ß-one | 424 | 1.22 | [29] |
| Obtusifoliol | 4α,14α,24-trimethylcholesta-8,24(28)-dien-3ß-ol or 4α,14α-dimethylergosta-8,24(28)-dien-3ß-ol | 498 | 1.23 | [24] |
| 4-methylfecosterol | 4α,24- dimethylcholesta-8,24(28)-dien-3ß-ol or 4α- methylergosta-8,24(28)-dien-3ß-ol | 484 | 1.25 | [24] |
| Eburicol | 4α,4ß,14α,24-tetramethylcholesta-8,24(28)-dien-3ß-ol or 4α,4ß,14α-trimethylergosta-8,24(28)-dien-3ß-ol | 512 | 1.31 | [28] |
| ATCC 204305 | AF1861 | AF1799 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | ITC | VRC | Basal | ITC | VRC | Basal | ITC | VRC | |
| ergosterol Z et E | 80.0 (±0.4) | 81.1 (±5.3) | 70.4 (±2.9) | 79.5 (±6.1) | 78.8 (±4.1) | 68.5 (±5.6) | 85.9 (±2.7) | 68.3 (±56.9) | 65.3 (±2.0) |
| Unknown 1 | 1.3 (±1.5) | ||||||||
| 14α-methylepisterol | 1.6 (±0.2) | 1.8 (±0.4) | 2.4 (±0.5) | 3.0 (±0.8) | |||||
| 7-dehydrostigmasterol | 20.0 (±0.4) | 13.9 (±7.0) | 9.9 (±1.1) | 18.9 (±5.5) | 17.0 (±4.0) | 2.1 (±1.0) | 14.2 (±2.6) | 1.0 (±1.7) | 0.7 (±1.2) |
| obtusifoliol + obtusifolione | 2.4 (±2.5) | 8.8 (±1.0) | 0.8 (±1.4) | 1.5 (±0.1) | 13.3 (±3.3) | 12.6 (±3.1) | 14.1 (±0.8) | ||
| eburicol | 2.5 (±1.3) | 8.1 (±1.2) | 0.8 (±1.3) | 2.8 (±0.5) | 14.4 (±1.3) | 15.8 (±6.4) | 16.9 (±2.1) | ||
| AF23 | AF0899 | AF1897 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | ITC | VRC | Basal | ITC | VRC | Basal | ITC | VRC | |
| 9-dehydroergosterol | 0.4 (±0.7) | ||||||||
| ergosterol Z et E | 82.9 (±2.7) | 74.0 (±5.5) | 68.8 (±3.4) | 88.0 (±1.0) | 72.8 (±3.3) | 69.5 (±3.1) | 84.6 (±4.3) | 69.5 (±5.5) | 44.4 (±9.7) |
| ergosta-7,22-dienol | 0.3 (±0.6) | 0.4 (±0.7) | |||||||
| 14α-methylepisterol | 1.1 (±0.2) | 1.9 (±0.2) | 1.6 (±1.6) | ||||||
| unknown 2 | 0.5 (±0.9) | ||||||||
| 7-dehydrostigmasterol | 16.7 (±2.1) | 16.4 (±6.8) | 5.7 (±1.7) | 12.0 (±0.9) | 12.8 (±5.0) | 4.9 (±3.9) | 15.0 (±3.6) | 14.6 (±4.4) | 0.7 (±1.2) |
| obtusifoliol + obtusifolione | 2.0 (±0.6) | 10.4 (±2.8) | 4.4 (±0.4) | 10.0 (±2.1) | 4.4 (±0.6) | 20.3 (±4.3) | |||
| 4-methyl-fecosterol | 0.3 (±0.6) | ||||||||
| eburicol | 8.0 (±0.4) | 16.3 (±5.4) | 10.0 (±2.3) | 13.2 (±4.6) | 11.1 (±2.6) | 33.0 (±7.9) | |||
| AF1168 | AF2226 | AF1468 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | ITC | VRC | Basal | ITC | VRC | Basal | ITC | VRC | |
| ergosterol Z et E | 82.8 (±2.5) | 79.1 (±3.5) | 62.5 (±3.3) | 83.7 (±4.1) | 68.3 (±3.7) | 67.0 (±3.7) | 89.5 (±0.9) | 76.9 (±2.0) | 70.2 (±3.2) |
| ergosta-7,22-dienol | 0.7 (±0.6) | ||||||||
| 14α-methylepisterol | 0.4 (±0.8) | 1.7 (±1.0) | 0.4 (±0.8) | * | |||||
| 7-dehydrostigmasterol | 17.2 (±2.5) | 11.7 (±3.8) | 0.8 (±1.3) | 16.3 (±4.1) | 18.3v (±1.1) | 7.3 (±1.2) | 10.5 (±0.9) | 9.2 (±1.7) | 7.3 (±2.9) |
| obtusifoliol + obtusifolione | 3.5 (±1.9) | 13.6 (±3.4) | 1.7 (±0.8) | 6.9 (±0.8) | 2.5 (±0.7) | 5.4 (±0.6) | |||
| eburicol | 5.2 (±3.5) | 21.5 (±4.3) | 11.1 (±3.0) | 18.5 (±1.4) | 11.5 (±3.4) | 17.0 (±4.2) | |||
| AF84 | AF112 | AF124 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | ITC | VRC | Basal | ITC | VRC | Basal | ITC | VRC | |
| ergosterol Z et E | 89.1 (±2.9) | 67.2 (±3.6) | 74.2 (±5.1) | 85.9 (±4.1) | 70.3 (±3.6) | 53.7 (±3.9) | 89.1 (±2.9) | 61.8 (±12.8) | 58.9 (±6.6) |
| ergosta-7,22-dienol | 1.1 (±1.0) | ||||||||
| 14α-methylepisterol | ** | ** | 0.5 (±0.9) | 2.5 (±2.4) | 2.3 (±0.6) | ||||
| Unknown 2 | 1.2 (±0.1) | ||||||||
| 7-dehydrostigmasterol | 10.9 (±2.9) | 12.6 (±5.9) | 8.6 (±4.9) | 14.1 (±4.0) | 15.7 (±3.1) | 1.1 (±1.5) | 10.9 (±2.9) | 2.5 (±4.3) | 1.5 (±0.1) |
| 14α-methylfecosterol | *** | ||||||||
| obtusifoliol + obtusifolione | 4.6 (±0.4) | 5.6 (±0.5) | 3.3 (±0.3) | 13.0 (±1.2) | 14.5 (±6.8) | 15.2 (±2.8) | |||
| eburicol | 14.5 (±1.5) | 11.6 (±10.2) | 10.7 (±2.1) | 32.0 (±2.7) | 17.7 (±8.9) | 22.5 (±3.4) | |||
| Reference | Isolates Name | Condition of Growth | Relative Composition of Sterols | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | T °C | Liquid Medium | 1 | 2 | 3 | 4 | 5 | 6 ** | ||
| [12,16] | CM237 | 18 h | 37 °C | MM | 73.5–75.8 | 4.7–6.1 | 1.9–2.7 | 1.3–1.6 | 1.1 | 13.8–17.0 |
| [13] | ATCC46645 | Overnight | 37 °C | RPMI 1640 | 95.0 | 5.0 | ||||
| [14] | ASFU1112 | 24 h | 35 °C | Sabouraud | 89.9 | 0.9 | 0.3 | 8.9 | ||
| ASFU1119 | 87.1 | 0.3 | 12.6 | |||||||
| ASFU1463 * | 75.5 | 19.4 | 5.1 | |||||||
| [19] | ATCC MYA-4609 | 48 h | 37 °C | Sabouraud | 90.8 | 0.6 | 8.6 | |||
| This study | 48 h | 37 °C | YPD | 79.5–89.5 | 10.5–20.0 | <1.0 | <1.0 | <1.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavergne, R.-A.; Albassier, M.; Hardouin, J.-B.; Alvarez-Moreno, C.; Pagniez, F.; Morio, F.; Le Pape, P.; Ourliac-Garnier, I. Impact of TR34/L98H, TR46/Y121F/T289A and TR53 Alterations in Azole-Resistant Aspergillus fumigatus on Sterol Composition and Modifications after In Vitro Exposure to Itraconazole and Voriconazole. Microorganisms 2022, 10, 104. https://doi.org/10.3390/microorganisms10010104
Lavergne R-A, Albassier M, Hardouin J-B, Alvarez-Moreno C, Pagniez F, Morio F, Le Pape P, Ourliac-Garnier I. Impact of TR34/L98H, TR46/Y121F/T289A and TR53 Alterations in Azole-Resistant Aspergillus fumigatus on Sterol Composition and Modifications after In Vitro Exposure to Itraconazole and Voriconazole. Microorganisms. 2022; 10(1):104. https://doi.org/10.3390/microorganisms10010104
Chicago/Turabian StyleLavergne, Rose-Anne, Marjorie Albassier, Jean-Benoît Hardouin, Carlos Alvarez-Moreno, Fabrice Pagniez, Florent Morio, Patrice Le Pape, and Isabelle Ourliac-Garnier. 2022. "Impact of TR34/L98H, TR46/Y121F/T289A and TR53 Alterations in Azole-Resistant Aspergillus fumigatus on Sterol Composition and Modifications after In Vitro Exposure to Itraconazole and Voriconazole" Microorganisms 10, no. 1: 104. https://doi.org/10.3390/microorganisms10010104







