Morpho-Functional Traits Reveal Differences in Size Fractionated Phytoplankton Communities but Do Not Significantly Affect Zooplankton Grazing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Incubation Experiment
2.2. Trait Analyses
2.3. Statistical Analysis
3. Results
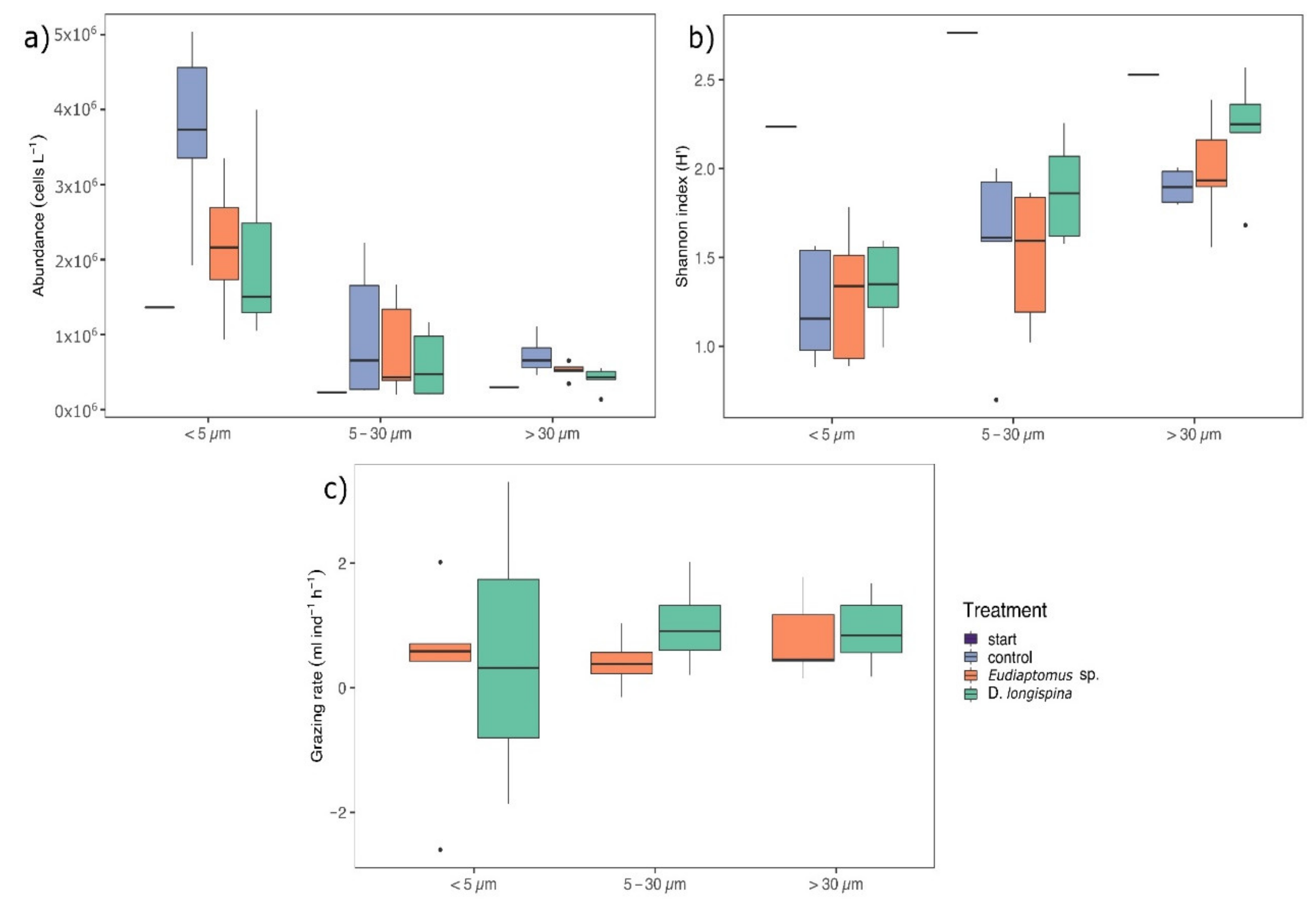
3.1. Phytoplankton Abundance, Diversity and Grazing Rates
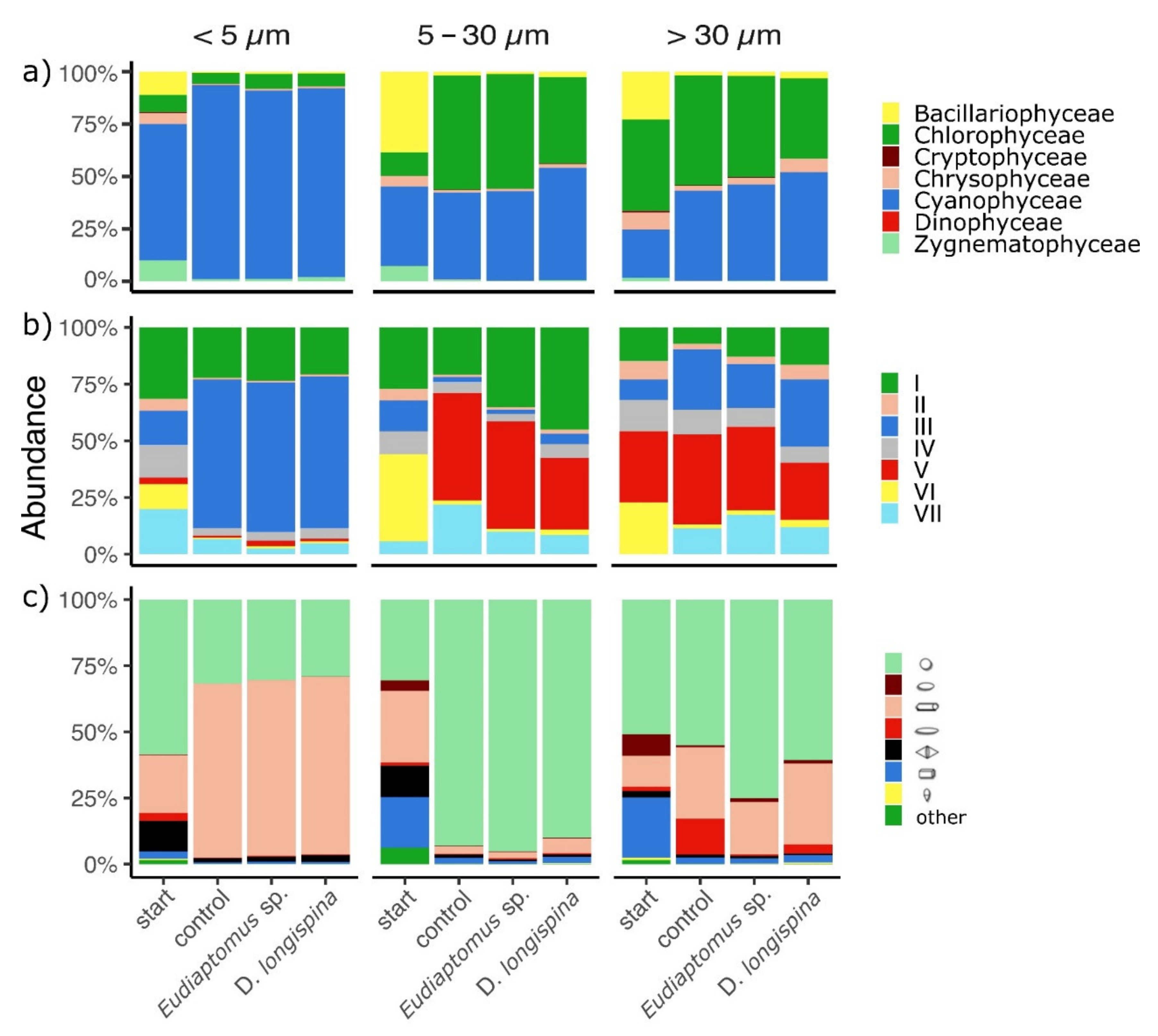
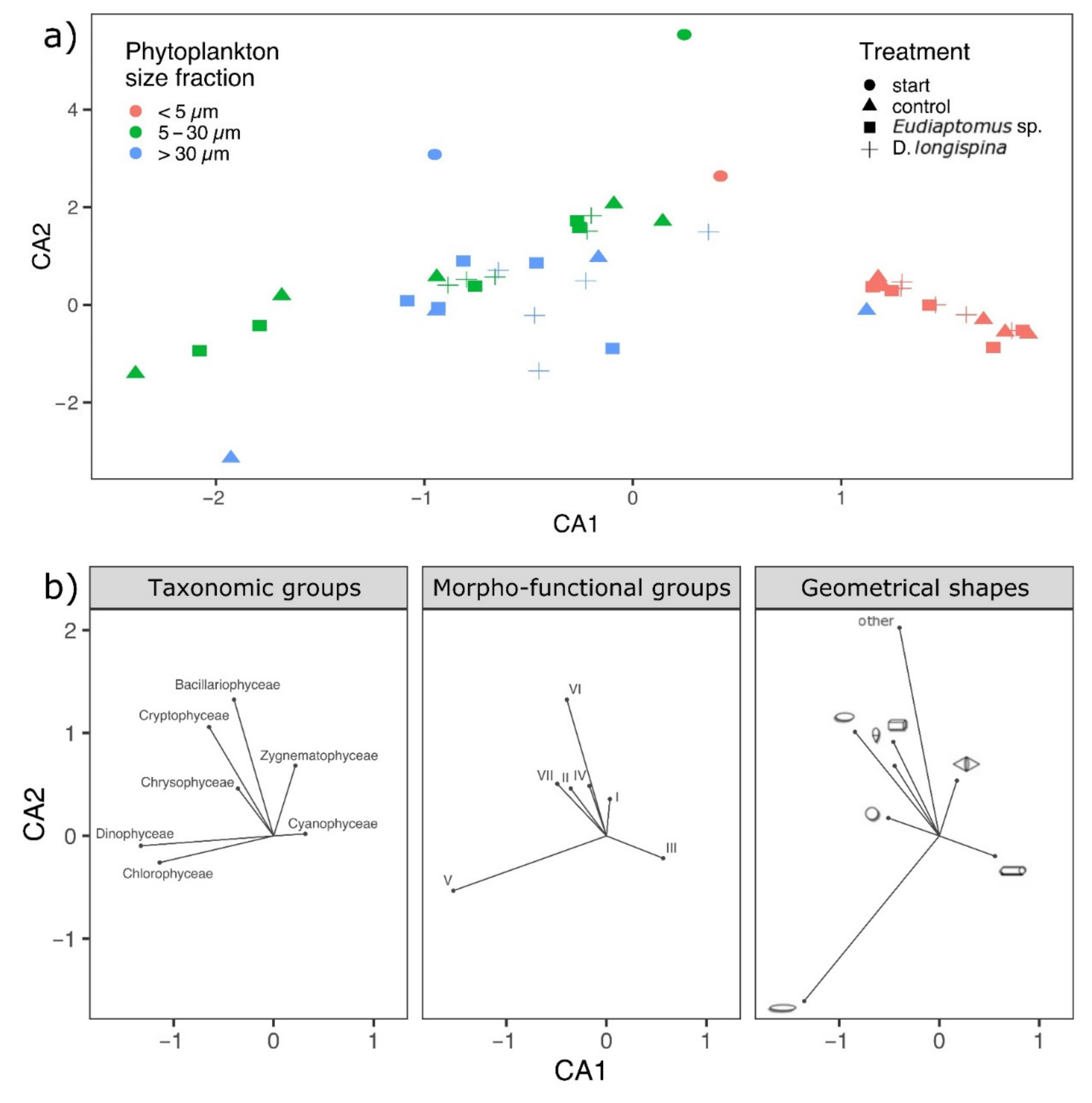
3.2. Phytoplankton Trait Analyses
4. Discussion
4.1. Grazing Phase and Food Selectivity
4.2. Phytoplankton Size-Fractionated Composition and Structure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Princeton University Press: Princeton, NJ, USA, 2007; ISBN 9781400849727. [Google Scholar]
- Weithoff, G. The concepts of ‘plant functional types’ and ‘functional diversity’ in lake phytoplankton—A new understanding of phytoplankton ecology? Freshw. Biol. 2003, 48, 1669–1675. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-Based Community Ecology of Phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef] [Green Version]
- Borics, G.; Tóthmérész, B.; Lukács, B.A.; Várbíró, G. Functional groups of phytoplankton shaping diversity of shallow lake ecosystems. Hydrobiologia 2012, 698, 251–262. [Google Scholar] [CrossRef]
- Vallina, S.M.; Cermeno, P.; Dutkiewicz, S.; Loreau, M.; Montoya, J.M. Phytoplankton functional diversity increases ecosystem productivity and stability. Ecol. Modell. 2017, 361, 184–196. [Google Scholar] [CrossRef]
- Ye, L.; Chang, C.; Matsuzaki, S.S.; Takamura, N.; Widdicombe, C.E.; Hsieh, C. Functional diversity promotes phytoplankton resource use efficiency. J. Ecol. 2019, 107, 2353–2363. [Google Scholar] [CrossRef]
- Reynolds, C.S. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshw. Biol. 1984, 14, 111–142. [Google Scholar] [CrossRef]
- Sipaúba-Tavares, L.H.; Bachion, M.A.; Braga, F.M.D.S. Effects of food quality on growth and biochemical composition of a calanoid copepod, Argyrodiaptomus furcatus, and its importance as a natural food source for larvae of two tropical fishes. Hydrobiologia 2001, 453–454, 393–401. [Google Scholar] [CrossRef]
- Zeng, H.; Song, L.; Yu, Z.; Chen, H. Distribution of phytoplankton in the Three-Gorge Reservoir during rainy and dry seasons. Sci. Total Environ. 2006, 367, 999–1009. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Landry, M.R.; Chiang, K.-P.; Wang, L.; Huang, B. Responses of Phytoplankton Communities to Environmental Variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- DeMott, W.R. The role of taste in food selection by freshwater zooplankton. Oecologia 1986, 69, 334–340. [Google Scholar] [CrossRef]
- Brendelberger, H.; Herbeck, M.; Lang, H.; Lampert, W. Daphnia’s filters are not solid walls. Arch. Hydrobiol. 1986, 107, 197–202. [Google Scholar]
- Bundy, M.H.; Gross, T.F.; Vanderploeg, H.A.; Strickler, J.R. Perception of inert particles by calanoid copepods: Behavioral observations and a numerical model. J. Plankton Res. 1998, 20, 2129–2152. [Google Scholar] [CrossRef] [Green Version]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, England, 1991; 468p, ISBN 0903874210. [Google Scholar]
- Ventelä, A.M.; Wiackowski, K.; Moilanen, M.; Saarikari, V.; Vuorio, K.; Sarvala, J. The effect of small zooplankton on the microbial loop and edible algae during a cyanobacterial bloom. Freshw. Biol. 2002, 47, 1807–1819. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A.; Strickler, J.R.; Alcaraz, M. Suspension-feeding by herbivorous calanoid copepods: A cinematographic study. Mar. Biol. 1982, 67, 193–199. [Google Scholar] [CrossRef]
- Price, H.J.; Paffenhöfer, G.A.; Strickler, J.R. Modes of cell capture in calanoid copepods. Limnol. Oceanogr. 1983, 28, 116–123. [Google Scholar] [CrossRef]
- Légier-Visser, M.F.; Mitchell, J.G.; Okubo, A.; Fuhrman, J.A. Mechanoreception in calanoid copepods. Mar. Biol. 1986, 90, 529–535. [Google Scholar] [CrossRef]
- Paffenhöfer, G.-A. On the relation of structure, perception and activity in marine planktonic copepods. J. Mar. Syst. 1998, 15, 457–473. [Google Scholar] [CrossRef]
- Alcaraz, M.; Paffenhöfer, G.A.; Strickler, J.R. Catching the algae: A first account of visual observations on filter-feeding calanoids. In Evolution and Ecology of Zooplankton Communities; Kerfoot, W.C., Ed.; University Press of New England: Lebanon, NH, USA, 1980; pp. 241–248. [Google Scholar]
- Landry, M.R. Detection of prey by Calanus pacificus: Implications of the first antennae. Limnol. Oceanogr. 1980, 25, 545–549. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Invited review Fight on plankton! Or, phytoplankton shape and size as adaptive tools to get ahead in the struggle for life. Cryptogam. Algol. 2011, 32, 157–204. [Google Scholar] [CrossRef]
- Pančić, M.; Kiørboe, T. Phytoplankton defence mechanisms: Traits and trade-offs. Biol. Rev. 2018, 93, 1269–1303. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M. Grazing resistance in phytoplankton. Hydrobiologia 2021, 848, 237–249. [Google Scholar] [CrossRef]
- Smetacek, V. A watery arms race. Nature 2001, 411, 745. [Google Scholar] [CrossRef] [PubMed]
- Morabito, G.; Oggioni, A.; Caravati, E.; Panzani, P. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 2007, 578, 47–57. [Google Scholar] [CrossRef]
- Sieburth, J.M.N.; Smetacek, V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions 1. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Beardall, J.; Allen, D.; Bragg, J.; Finkel, Z.V.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Richardson, A.; Raven, J.A. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol. 2009, 181, 295–309. [Google Scholar] [CrossRef]
- Lewis, W.M. Surface/Volume Ratio: Implications for Phytoplankton Morphology. Science 1976, 192, 885–887. [Google Scholar] [CrossRef]
- Lafond, M.; Pinel-Alloul, B.; Ross, P. Biomass and photosynthesis of size-fractionated phytoplankton in Canadian Shield lakes. Hydrobiologia 1990, 196, 25–38. [Google Scholar] [CrossRef]
- Bruno, S.F.; Staker, R.D.; Sharma, G.M.; Turner, J.T. Primary productivity and phytoplankton size fraction dominance in a temperate North Atlantic estuary. Estuaries 1983, 6, 200–211. [Google Scholar] [CrossRef]
- Zafar, A.R. Seasonality of phytoplankton in some South Indian lakes. Hydrobiologia 1986, 138, 177–187. [Google Scholar] [CrossRef]
- Waite, A.M.; Thompson, P.A.; Harrison, P.J. Does energy control the sinking rates of marine diatoms? Limnol. Oceanogr. 1992, 37, 468–477. [Google Scholar] [CrossRef]
- Tremblay, J.É.; Klein, B.; Legendre, L.; Rivkin, R.B.; Therriault, J.C. Estimation of f-ratios in oceans based on phytoplankton size structure. Limnol. Oceanogr. 1997, 42, 595–601. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnoecology the Ecology of Lakes and Streams, 2nd ed.; Oxford University Press: Oxford, UK, 2007; ISBN 9780199213931. [Google Scholar]
- Von Rückert, G.; Giani, A. Biological interactions in the plankton community of a tropical eutrophic reservoir: Is the phytoplankton controlled by zooplankton? J. Plankton Res. 2008, 30, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Ryabov, A.; Kerimoglu, O.; Litchman, E.; Olenina, I.; Roselli, L.; Basset, A.; Stanca, E.; Blasius, B. Shape matters: The relationship between cell geometry and diversity in phytoplankton. Ecol. Lett. 2021, 24, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Böing, W.J.; Wagner, A.; Voigt, H.; Deppe, T.; Benndorf, J. Phytoplankton responses to grazing by Daphnia galeata in the biomanipulated Bautzen reservoir. Hydrobiologia 1998, 389, 101–114. [Google Scholar] [CrossRef]
- Van Donk, E. Defenses in phytoplankton against grazing induced by nutrient limitation, UV-B stress and infochemicals. Aquat. Ecol. 1997, 31, 53–58. [Google Scholar] [CrossRef]
- Kruk, C.; Huszar, V.L.M.; Peeters, E.T.H.M.; Bonilla, S.; Costa, L.; LüRling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Colina, M.; Calliari, D.; Carballo, C.; Kruk, C. A trait-based approach to summarize zooplankton–phytoplankton interactions in freshwaters. Hydrobiologia 2016, 767, 221–233. [Google Scholar] [CrossRef]
- Sarnelle, O.; Gustafsson, S.; Hansson, L.A. Effects of cyanobacteria on fitness components of the herbivore Daphnia. J. Plankton Res. 2010, 32, 471–477. [Google Scholar] [CrossRef]
- Blaxter, J.H.S.; Douglas, B.; Tyler, P.A.; Mauchline, J. The Biology of Calanoid Copepods: The Biology of Calanoid Copepods; Academic Press: San Diego, CA, USA, 1998; Volume 33, ISBN 9780080579566. [Google Scholar]
- Barnett, A.; Beisner, B.E. Zooplankton biodiversity and lake trophic state: Explanations invoking resource abundance and distribution. Ecology 2007, 88, 1675–1686. [Google Scholar] [CrossRef]
- Mauchline, J. The biology of calanoid copepods. In Advances in Marine Biology; Elsevier Academic Press: New York, NY, USA, 1998; ISBN 1-281-71129-2. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int. Ver Limnol. 1958, 9, 38. [Google Scholar] [CrossRef]
- Rott, E. Some results from phytoplankton counting intercalibrations. Schweiz. Z. Hydrol. 1981, 43, 34–62. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W.W. The Mathematical Theory of Communications.; University of Illinois Press: Urbana, IL, USA, 1963; ISBN 0252725484 9780252725487. [Google Scholar]
- Bamstedt, U.; Gifford, D.J.; Irigoien, X.; Atkinson, A.; Roman, M. Feeding. In ICES Zooplankton Methodology Manual; Academic Press: San Diego, CA, USA, 2000; pp. 297–399. ISBN 9780123276452. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Chevene, F.; Doleadec, S.; Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package 2014. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 13 December 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 35, ISBN 9780387981406. [Google Scholar]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: The cause of contrasting top–down controls on freshwater and marine phytoplankton. Oecologia 2006, 147, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kruk, C. Morphology Captures Function in Phytoplankton A Large-Scale Analysis of Phytoplankton Communities in Relation to their Environment. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2010. [Google Scholar]
- Harbison, G.R.; McAlister, V.L. Fact and artifact in copepod feeding experiments1. Limnol. Oceanogr. 1980, 25, 971–981. [Google Scholar] [CrossRef]
- Mullin, M.M. Size Fractionation of Particulate Organic Carbon in the Surface Waters of the Western Indian OCEAN. Limnol. Oceanogr. 1965, 10, 459–462. [Google Scholar] [CrossRef]
- Runge, J.A.; Ohman, M.D. Size fractionation of phytoplankton as an estimate of food available to herbivores. Limnol. Oceanogr. 1982, 27, 570–576. [Google Scholar] [CrossRef]
- Cermeño, P.; Marañón, E.; Rodríguez, J.; Fernández, E. Size dependence of coastal phytoplankton photosynthesis under vertical mixing conditions. J. Plankton Res. 2005, 27, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Marañón, E.; Cermeño, P.; Latasa, M.; Tadonléké, R.D. Temperature, resources, and phytoplankton size structure in the ocean. Limnol. Oceanogr. 2012, 57, 1266–1278. [Google Scholar] [CrossRef]
- Sin, Y.; Wetzel, R.L.; Anderson, I.C. Seasonal variations of size-fractionated phytoplankton along the salinity gradient in the York River estuary, Virginia (USA). J. Plankton Res. 2000, 22, 1945–1960. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Taylor, W.R.; Loftus, M.E. Significance of nanoplankton in the Chesapeake Bay estuary and problems associated with the measurement of nanoplankton productivity. Mar. Biol. 1974, 24, 7–16. [Google Scholar] [CrossRef]
- Durbin, E.G.; Krawiec, R.W.; Smayda, T.J. Seasonal studies on the relative importance of different size fractions of phytoplankton in Narragansett Bay (USA). Mar. Biol. 1975, 32, 271–287. [Google Scholar] [CrossRef]
- Sommer, U.; Charalampous, E.; Genitsaris, S.; Moustaka-Gouni, M. Benefits, costs and taxonomic distribution of marine phytoplankton body size. J. Plankton Res. 2017, 39, 494–508. [Google Scholar] [CrossRef]
| Mucilage Presence/Absence | Df | Sum of Squares | Mean Square | F Value | R2 | p-Value |
| Treatment | 3 | 0.363 | 0.121 | 1.436 | 0.055 | 0.219 |
| Fraction | 2 | 2.889 | 1.444 | 17.115 | 0.439 | 0.001 *** |
| Treatment x Fraction | 6 | 0.371 | 0.061 | 0.734 | 0.056 | 0.752 |
| Residuals | 35 | 2.954 | 0.084 | 0.449 | ||
| Total | 46 | 6.580 | 1 | |||
| Flagella Presence/Absence | Df | Sum of Squares | Mean Square | F Value | R2 | p-Value |
| Treatment | 3 | 0.382 | 0.127 | 1.420 | 0.048 | 0.199 |
| Fraction | 2 | 3.880 | 1.940 | 21.61 | 0.495 | 0.001 *** |
| Treatment x Fraction | 6 | 0.419 | 0.069 | 0.779 | 0.053 | 0.716 |
| Residuals | 35 | 3.141 | 0.089 | 0.401 | ||
| Total | 46 | 7.823 | 1 | |||
| Aerotopes Presence/Absence | Df | Sum of Squares | Mean Square | F Value | R2 | p-Value |
| Treatment | 3 | 0.328 | 0.109 | 1.285 | 0.046 | 0.263 |
| Fraction | 2 | 3.291 | 1.645 | 19.32 | 0.468 | 0.001 *** |
| Treatment x Fraction | 6 | 0.421 | 0.070 | 0.824 | 0.059 | 0.624 |
| Residuals | 35 | 2.981 | 0.085 | 0.424 | ||
| Total | 46 | 7.022 | 1 | |||
| Unicellularity/Coloniality | Df | Sum of Squares | Mean Square | F Value | R2 | p-Value |
| Treatment | 3 | 0.331 | 0.110 | 1.339 | 0.049 | 0.229 |
| Fraction | 2 | 3.165 | 1.582 | 19.186 | 0.469 | 0.001 *** |
| Treatment x Fraction | 6 | 0.351 | 0.058 | 0.710 | 0.052 | 0.749 |
| Residuals | 35 | 2.887 | 0.082 | 0.428 | ||
| Total | 46 | 6.736 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titocci, J.; Bon, M.; Fink, P. Morpho-Functional Traits Reveal Differences in Size Fractionated Phytoplankton Communities but Do Not Significantly Affect Zooplankton Grazing. Microorganisms 2022, 10, 182. https://doi.org/10.3390/microorganisms10010182
Titocci J, Bon M, Fink P. Morpho-Functional Traits Reveal Differences in Size Fractionated Phytoplankton Communities but Do Not Significantly Affect Zooplankton Grazing. Microorganisms. 2022; 10(1):182. https://doi.org/10.3390/microorganisms10010182
Chicago/Turabian StyleTitocci, Jessica, Melanie Bon, and Patrick Fink. 2022. "Morpho-Functional Traits Reveal Differences in Size Fractionated Phytoplankton Communities but Do Not Significantly Affect Zooplankton Grazing" Microorganisms 10, no. 1: 182. https://doi.org/10.3390/microorganisms10010182






