Co-Fermentations of Kveik with Non-Conventional Yeasts for Targeted Aroma Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Media
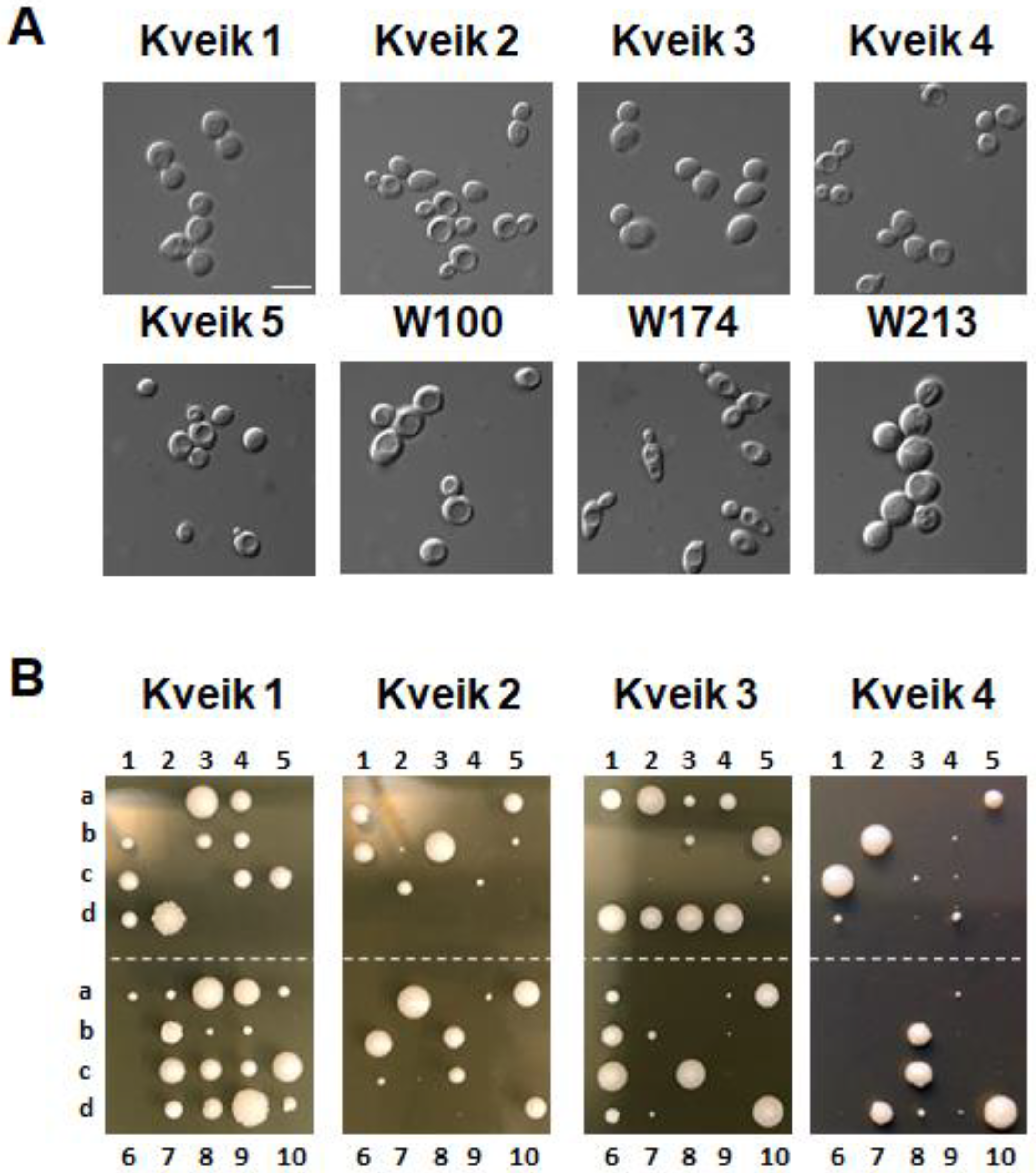
2.2. Microscopy and Micromanipulation
2.3. Fermentation Conditions
2.4. Analytical Methods
2.5. Statistical Analyses
3. Results
3.1. Kveiks Differ from Ale Yeasts in Their Germination Efficiency of Spores
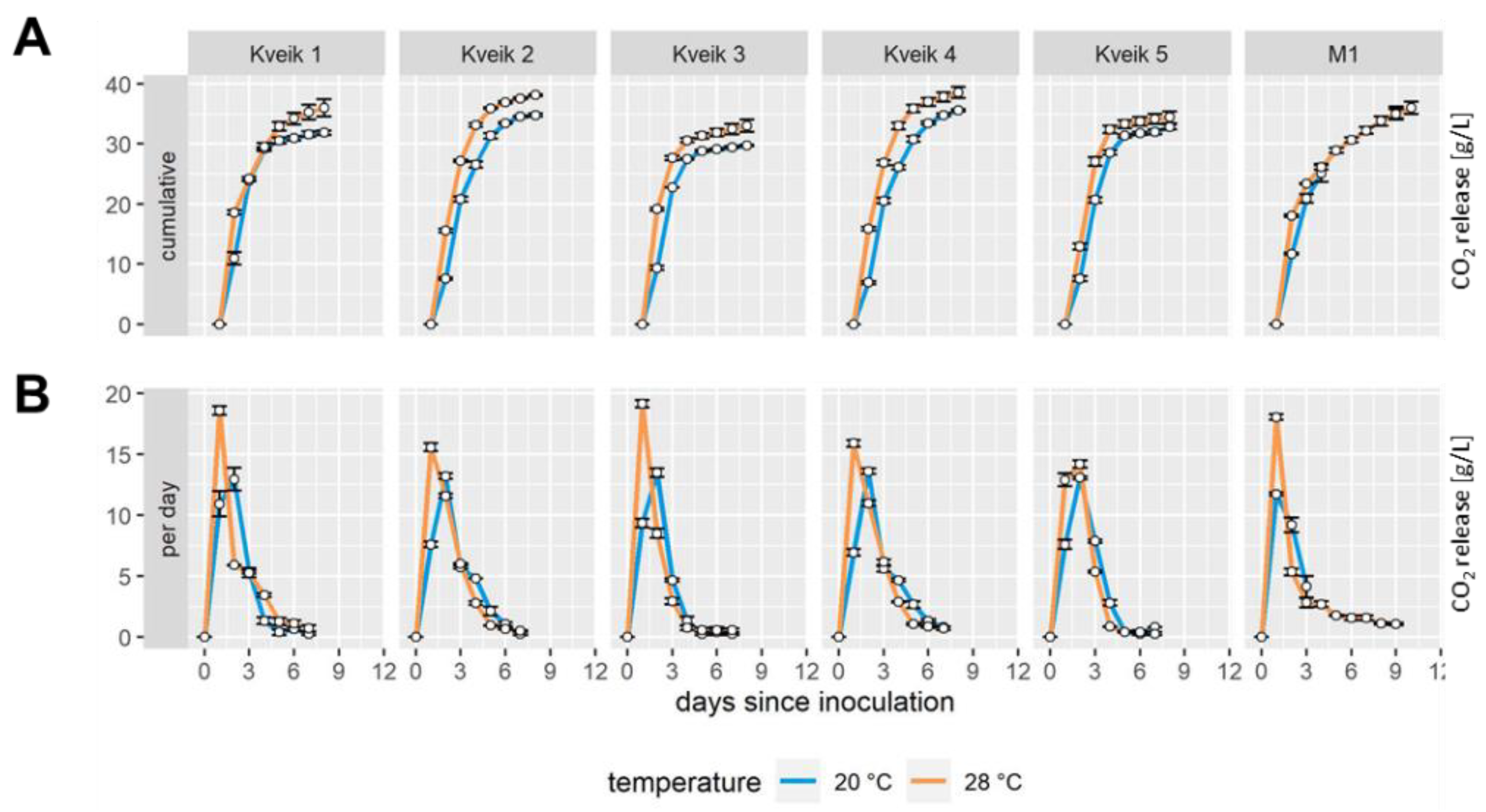
3.2. Fermentations with Kveiks Are Faster at Higher Temperature and Show Differential Maltotriose Utilization
3.3. Volatile Compound Formation of Kveiks at Different Fermentation Temperatures
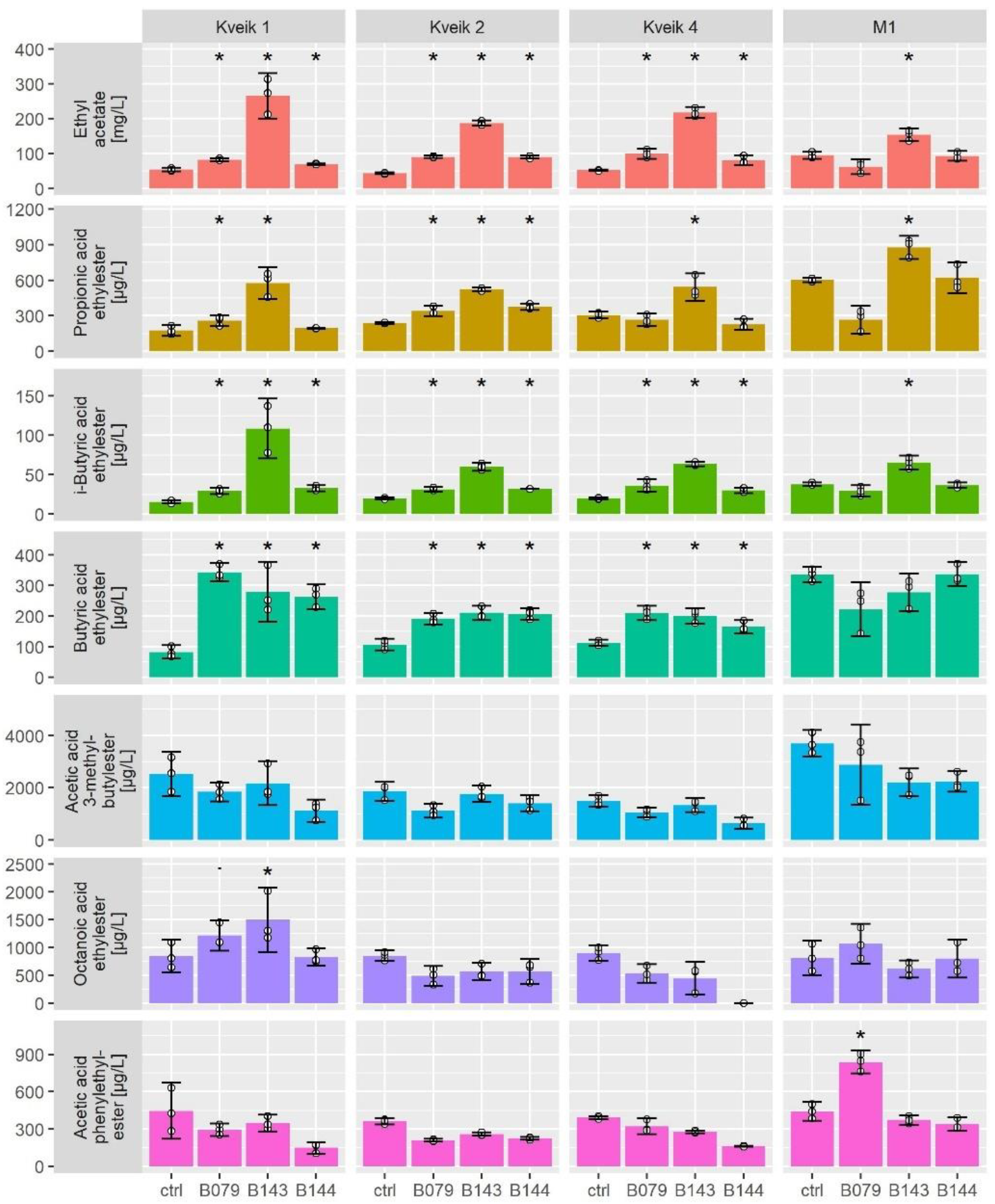
3.4. Co-Fermentations of Kveiks with Selected Non-Conventional Yeasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahue, C.; Madden, A.A.; Dunn, R.R.; Smukowski Heil, C. History and domestication of Saccharomyces cerevisiae in bread baking. Front. Genet. 2020, 11, 584718. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Maro, A. Human evolution and dietary ethanol. Nutrients 2021, 13, 2419. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J. Lager yeast comes of age. Eukaryot. Cell 2014, 13, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.N.; Gorter de Vries, A.R.; van den Broek, M.; Brouwers, N.; de la Torre Cortes, P.; Kuijpers, N.G.A.; Daran, J.G.; Abeel, T. Chromosome level assembly and comparative genome analysis confirm lager-brewing yeasts originated from a single hybridization. BMC Genom. 2019, 20, 916. [Google Scholar] [CrossRef]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome sequence of Saccharomyces carlsbergensis, the world’s first pure culture lager yeast. G3 (Bethesda) 2014, 4, 783–793. [Google Scholar] [CrossRef]
- van den Broek, M.; Bolat, I.; Nijkamp, J.F.; Ramos, E.; Luttik, M.A.; Koopman, F.; Geertman, J.M.; de Ridder, D.; Pronk, J.T.; Daran, J.M. Chromosomal Copy Number Variation in Saccharomyces pastorianus Is Evidence for Extensive Genome Dynamics in Industrial Lager Brewing Strains. Appl. Environ. Microbiol. 2015, 81, 6253–6267. [Google Scholar] [CrossRef]
- Turgeon, Z.; Sierocinski, T.; Brimacombe, C.A.; Jin, Y.; Goldhawke, B.; Swanson, J.M.; Husnik, J.I.; Dahabieh, M.S. Industrially applicable de novo lager yeast hybrids with a unique genomic architecture: Creation and characterization. Appl. Environ. Microbiol. 2021, 87, e02434-20. [Google Scholar] [CrossRef]
- Vidgren, V.; Multanen, J.P.; Ruohonen, L.; Londesborough, J. The temperature dependence of maltose transport in ale and lager strains of brewer’s yeast. FEMS Yeast Res. 2010, 10, 402–411. [Google Scholar] [CrossRef]
- Preiss, R.; Tyrawa, C.; Krogerus, K.; Garshol, L.M.; van der Merwe, G. Traditional Norwegian kveik are a genetically distinct group of domesticated Saccharomyces cerevisiae brewing yeasts. Front. Microbiol. 2018, 9, 2137. [Google Scholar] [CrossRef]
- Foster, B.; Tyrawa, C.; Ozsahin, E.; Lubberts, M.; Krogerus, K.; Preiss, R.; van der Merwe, G. Kveik brewing yeasts demonstrate wide flexibility in beer fermentation temperature tolerance and exhibit enhanced trehalose accumulation. Front. Microbiol. 2022, 13, 747546. [Google Scholar] [CrossRef]
- Krogerus, K.; Preiss, R.; Gibson, B. A unique Saccharomyces cerevisiae x Saccharomyces uvarum hybrid isolated from Norwegian farmhouse beer: Characterization and reconstruction. Front. Microbiol. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Pietrzak, W.; Paszkot, J.; Glowacki, A.; Gasinski, A. Characteristics of new england india pale ale beer produced with the use of Norwegian kveik yeast. Molecules 2022, 27, 2291. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Pietrzak, W.; Paszkot, J.; Glowacki, A.; Gasinski, A.; Leszczynski, P. The potential of traditional Norwegian kveik yeast for brewing novel beer on the example of foreign extra stout. Biomolecules 2021, 11, 1778. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.J.; Speers, R.A.; Willoughby, N.; Lumsden, W.B.; Maskell, D.L. Pre-fermentation of malt whisky wort using Lactobacillus plantarum and its influence on new-make spirit character. Food Chem. 2020, 320, 126605. [Google Scholar] [CrossRef] [PubMed]
- Tsapou, E.A.; Drosou, F.; Koussissi, E.; Dimopoulou, M.; Dourtoglou, T.; Dourtoglou, V. Addition of yogurt to wort for the production of spirits: Evaluation of the spirit aroma over a two-year period. J. Food Sci. 2020, 85, 2069–2079. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genoves, S.; Valles, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Ianieva, O.; Podgorsky, V. Enological potential of non-Saccharomyces yeast strains of enological and brewery origin from Ukrainian collection of microorganisms. Mycology 2021, 12, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding flavor to beverages with non-conventional yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Guang, J.; Ge, F.; Yan, S. Development of Chinese chestnut whiskey: Yeast strains isolation, fermentation system optimization, and scale-up fermentation. AMB Express 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Akan, M.; Michling, F.; Matti, K.; Krause, S.; Muno-Bender, J.; Wendland, J. Snails as taxis for a large yeast biodiversity. Fermentation 2020, 6, 90. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Camara, J.S.; Alves, M.A.; Marques, J.C. Development of headspace solid-phase microextraction-gas chromatography–mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar] [CrossRef]
- Scansani, S.; van Wyk, N.; Bou Nader, K.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pasch, L.; Pretorius, I.S.; von Wallbrunn, C.; et al. The film-forming Pichia spp. in a winemaker’s toolbox: A simple isolation procedure and their performance in a mixed-culture fermentation of Vitis vinifera L. cv. Gewürztraminer must. Int. J. Food Microbiol. 2022, 365, 109549. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Fukuda, N. Crossbreeding of yeasts domesticated for fermentation: Infertility challenges. Int. J. Mol. Sci. 2020, 21, 7985. [Google Scholar] [CrossRef]
- Magalhaes, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeast Saccharomyces pastorianus. FEMS Yeast Res. 2016, 16, fow053. [Google Scholar] [CrossRef] [PubMed]
- Barbour, E.A.; Priest, F.G. Some effects of Lactobacillus contamination in Scotch whisky fermentations. J. Inst. Brew. 1988, 94, 89–92. [Google Scholar] [CrossRef]
- van Beek, S.; Priest, F.G. Evolution of the lactic acid bacterial community during malt whisky fermentation: A polyphasic study. Appl. Environ. Microbiol. 2002, 68, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, E.; Linforth, R.S.T.; Jack, F.; Cook, D.J. Origins of the perceived nutty character of new-make malt whisky spirit. J. Inst. Brew. 2014, 120, 16–22. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef]
- Henriques, D.; Alonso-Del-Real, J.; Querol, A.; Balsa-Canto, E. Saccharomyces cerevisiae and S. kudriavzevii synthetic wine fermentation performance dissected by predictive modeling. Front. Microbiol. 2018, 9, 88. [Google Scholar] [CrossRef]
- Oliveira, I.; Ferreira, V. Modulating fermentative, varietal and aging aromas of wine using non-Saccharomyces yeasts in a sequential inoculation approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef]
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Non-conventional yeasts as tools for innovation and differentiation in brewing. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of non-Saccharomyces on wine chemistry: A focus on aroma-related compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione production by non-Saccharomyces yeasts and its impact on winemaking: A review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef]
- Lin, C.L.; Garcia-Caro, R.C.; Zhang, P.; Carlin, S.; Gottlieb, A.; Petersen, M.A.; Vrhovsek, U.; Bond, U. Packing a punch: Understanding how flavours are produced in lager fermentations. FEMS Yeast Res. 2021, 21, foab040. [Google Scholar] [CrossRef] [PubMed]
- Geijer, C.; Ledesma-Amaro, R.; Tomas-Pejo, E. Unraveling the potential of non-conventional yeasts in biotechnology. FEMS Yeast Res. 2022, 22, foab071. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef]
- Zhu, L.X.; Wang, G.Q.; Aihaiti, A. Combined indigenous yeast strains produced local wine from over ripen Cabernet Sauvignon grape in Xinjiang. World J. Microbiol. Biotechnol. 2020, 36, 122. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef]
- Bendoni, B.; Cavalieri, D.; Casalone, E.; Polsinelli, M.; Barberio, C. Trifluoroleucine resistance as a dominant molecular marker in transformation of strains of Saccharomyces cerevisiae isolated from wine. FEMS Microbiol. Lett. 1999, 180, 229–233. [Google Scholar] [CrossRef]
- Oba, T.; Nomiyama, S.; Hirakawa, H.; Tashiro, K.; Kuhara, S. Asp578 in Leu4p is one of the key residues for leucine feedback inhibition release in sake yeast. Biosci. Biotechnol. Biochem. 2005, 69, 1270–1273. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologica 2001, 29, 217–280. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. R Package Version 2.5 (2019). R Package Version. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 18 August 2022).

| Glucose [g/L] | Fructose [g/L] | Maltose [g/L] | Sucrose [g/L] | Maltotriose [g/L] | Total Sugars [g/L] | |
|---|---|---|---|---|---|---|
| Wort | 18.7 | 11.8 | 43.7 | 29.1 | 14.2 | 117.5 |
| GYBC * | Strain + | Description | Source |
|---|---|---|---|
| 145 | Kveik No 1 | Framgarden | Aurora Spirit Distillery, Lyngseidet, Norway |
| 146 | Kveik No 2 | Strada | |
| 147 | Kveik No 3 | Tormodgarden | |
| 148 | Kveik No 4 | Ebbegarden | |
| 149 | Kveik No 5 | Hornindal | |
| SafSpirit M1 | Distillery yeast | Fermentis, Marcq-en-Barœul, France | |
| 214 | Hanseniaspora uvarum B079 | [24] | |
| 215 | Meyerozyma guilliermondii B143 | [24] | |
| 216 | Pichia kudriavzevii B144 | [24] | |
| W100 | Ale yeast | Hefebank Weihenstephan GmbH, Hallertau, Germany | |
| W174 | Ale yeast | ||
| W213 | Ale yeast |
| Strain | Sporulation * | Germination + |
|---|---|---|
| Kveik 1 | 78% | 64% |
| Kveik 2 | 68% | 66% |
| Kveik 3 | 31% | 62% |
| Kveik 4 | 57% | 36% |
| Kveik 5 | 43% | 9% |
| W100 | 20% | 8% |
| W174 | 90% | 4% |
| W213 | 30% | 3% |
| Strain | Glucose [g/L] | Fructose [g/L] | Maltose [g/L] | Sucrose [g/L] | Maltotriose [g/L] | Total Sugars [g/L] | Ethanol [g/L] | Ethanol [%] |
|---|---|---|---|---|---|---|---|---|
| Fermentations at 20 °C | ||||||||
| Kveik 1 | <1 | <1 | <1 | <1 | 2.0 ± 0.7 | 2.0 ± 0.7 | 50.7 ± 1.1 | 6.4 ± 0.1 |
| Kveik 2 | <1 | <1 | <1 | <1 | 1.3 | 1.3 | 56.4 ± 0.9 | 7.1 ± 0.1 |
| Kveik 3 | <1 | <1 | <1 | <1 | 10.0 ± 0.2 | 10 ± 0.2 | 47.3 ± 0.3 | 6.0 |
| Kveik 4 | <1 | <1 | 1.0 ± 0.1 | <1 | 1.2 | 1.9 ± 0.6 | 55.4 ± 0.3 | 7.0 |
| Kveik 5 | <1 | <1 | 2.7 | <1 | 2.7 ± 0.2 | 3.5 ± 1.7 | 50.7 ± 0.4 | 6.4 |
| M1 | <1 | <1 | <1 | <1 | <1 | <1 | 58.5 ± 1.8 | 7.4 ± 0.2 |
| Fermentations at 28 °C | ||||||||
| Kveik 1 | <1 | <1 | <1 | <1 | 2.6 ± 0.8 | 2.6 ± 0.8 | 42.4 ± 1.4 | 5.4 ± 0.2 |
| Kveik 2 | <1 | <1 | 1.0 ± 0.1 | <1 | 1.2 | 2.2 ± 0.1 | 47.1 ± 0.4 | 6.0 ± 0.1 |
| Kveik 3 | <1 | <1 | <1 | <1 | 10.6 ± 0.6 | 10.6 ± 0.6 | 39.5 ± 0.9 | 5.0 ± 0.1 |
| Kveik 4 | <1 | <1 | <1 | <1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 46.4 ± 0.9 | 5.9 ± 0.1 |
| Kveik 5 | <1 | <1 | <1 | <1 | 2.1 ± 0.4 | 2.1 ± 0.4 | 44.1 ± 0.1 | 5.6 |
| M1 | <1 | <1 | 5.3 ± 0.9 | <1 | <1 | 6.3 ± 0.9 | 53.4 ± 1.8 | 6.8 ± 0.2 |
| Single | B079 | B143 | B144 | |
|---|---|---|---|---|
| K1 | 6.4 ± 0.1 | 7.7 ± 0.3 * | 7.7 ± 0.1 * | 7.0 ± 0.1 * |
| K2 | 7.1 ± 0.1 | 7.3 ± 0.1 + | 7.3 ± 0.1 + | 7.1 ± 0.2 |
| K3 | 6.0 ± 0.0 | 5.5 ± 0.8 | 6.0 ± 0.4 | 5.7 ± 0.1 |
| K4 | 7.0 ± 0.1 | 7.8 ± 0.1 * | 7.4 ± 0.3 + | 7.0 ± 0.1 |
| K5 | 6.4 ± 0.1 | 6.3 ± 0.4 | 6.9 ± 0.1 * | 6.5 ± 0.1 + |
| M1 | 7.4 ± 0.2 | 7.0 ± 0.0 | 7.7 ± 0.1 | 7.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippel, K.; Matti, K.; Muno-Bender, J.; Michling, F.; Brezina, S.; Semmler, H.; Rauhut, D.; Wendland, J. Co-Fermentations of Kveik with Non-Conventional Yeasts for Targeted Aroma Modulation. Microorganisms 2022, 10, 1922. https://doi.org/10.3390/microorganisms10101922
Dippel K, Matti K, Muno-Bender J, Michling F, Brezina S, Semmler H, Rauhut D, Wendland J. Co-Fermentations of Kveik with Non-Conventional Yeasts for Targeted Aroma Modulation. Microorganisms. 2022; 10(10):1922. https://doi.org/10.3390/microorganisms10101922
Chicago/Turabian StyleDippel, Kevin, Katrin Matti, Judith Muno-Bender, Florian Michling, Silvia Brezina, Heike Semmler, Doris Rauhut, and Jürgen Wendland. 2022. "Co-Fermentations of Kveik with Non-Conventional Yeasts for Targeted Aroma Modulation" Microorganisms 10, no. 10: 1922. https://doi.org/10.3390/microorganisms10101922





