Roles of Aerotolerance, Biofilm Formation, and Viable but Non-Culturable State in the Survival of Campylobacter jejuni in Poultry Processing Environments
Abstract
:1. Introduction
2. Physiology of Campylobacter
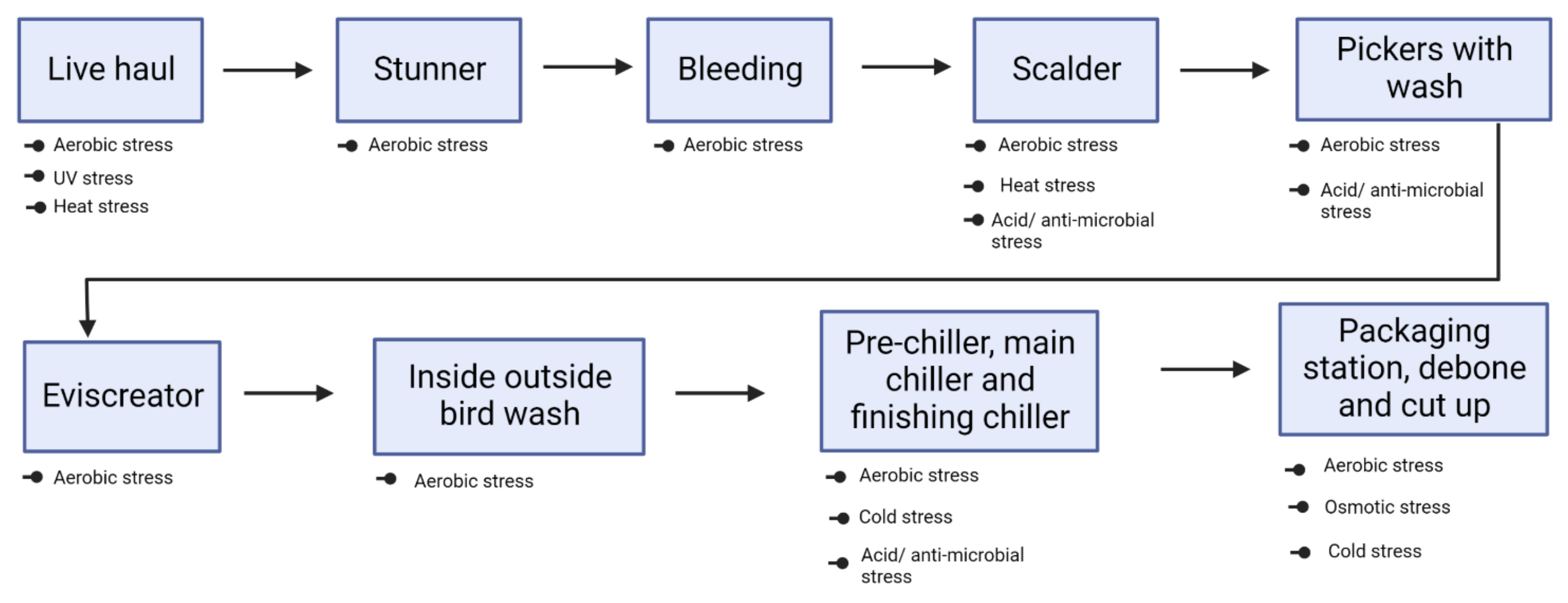
3. Incidence of Campylobacter in Poultry Processing Facilities
4. Stress Encountered by Campylobacter inside Poultry Processing Facilities
4.1. Aerobic Stress
4.2. Heat Stress
4.3. Refrigeration and Freezing Stress
4.4. Osmotic Stress
4.5. UV Stress
4.6. Acid Stress
5. Potential Survival Mechanisms
5.1. Aerotolerance Development
5.2. Biofilm Formation
Genes Involved in Biofilm Formation by Campylobacter
5.3. Viable but non Culturable (VBNC) State Formation
6. Future Research to Fill Current Knowledge Gaps
7. Conclusions
| Source of Contamination | Country of Occurrence | Incidence of C. jejuni | References |
|---|---|---|---|
| Birds with feathers, birds at rehang, birds after evisceration, immediately after entering chiller, after exiting chlorinated chill tank | USA—Georgia | 87.27%, (n = 55) | [123] |
| Rehang and post chill whole carcass rinse | USA—Alabama, Arkansas, California, Delaware, Georgia, Indiana, Missouri, North Carolina, South Carolina, Tennessee, Texas, Virginia, and West Virginia | Rehang—74.5%, (n = 800) Post-chill—34.90%, (n = 800) | [124] |
| Feces, pasture soil, whole carcass rinse directly after processing (WCR-P), final product whole carcass rinse after chilling and storage time (WCR-F), and ceca samples collected during processing from each farm | Southeastern USA from March 2014 to November 2017 | 39.08%, (n = 2305) | [125] |
| Air, feces-litter, feed pans and water lines | USA—Virginia | 26.66%, (n = 120) | [126] |
| Fecal and environmental samples | USA—North Carolina | Fecal: 29.50%; (n = 400) Environmental sample: 1%, (n = 500) | [127] |
| Pre-scald (feather and skin) | USA—Delmarva Peninsula | 77%, (n = 48) | [128] |
| Evisceration | USA | 96–100%, (n = 48) | [129] |
| Slaughterhouse | Brazil- Parana, santa Catarina and Rio Grande do Sul | Campylobacter spp.—35.84% (n = 816) C. jejuni—78.47% (n = 144) | [130] |
| Defeathering, Evisceration, Shackles, Converyor belt | North of Spain | Defeathering—80% (n = 30) Eviseration—100% (n = 39) Shackles—100% (n = 23) Converyor belt—96.6% (n = 29) | [131] |
| Defeathering machine, evisceration machine, conveyor belts, scald tank, water | France | 87% (34/39) | [45] |
| Stressors | Gene Involved | Gene Function | References |
|---|---|---|---|
| Heat stress |
| Express DnaJ chaperone protein-protein folding and heat shock response | [51] |
| Component of RacR-RacS temperature-responsive signal transduction system | [53] | |
| High temperature requirement A (HtrA)-like protease and chaperones | [91] | |
| Express iron sequestration ferritin protein (Dps) | [132] | |
| Express DnaK chaperonin- protein folding | ||
| Refrigeration and freezing stress |
| Superoxide dismutase | [59] |
| S-ribosylhomocysteinase-catalyzes the formation of autoinducer-2 (AI-2) molecules and homocysteine | [133] | |
| Osmotic stress |
| Capsule export gene-encodes capsule export apparatus | [65] |
| Acid stress |
| ATP-dependent Protease-heat shock gene | [79] |
| Thioredoxin-disulfide reductase-protein folding | [87] | |
| Ferric uptake regulator gene | [88] | |
| UV stress | recA | Express recA protein involved in DNA repair | [78] |
| Gene | Gene Product or Function | Involved Function in Aerotolerance | References |
|---|---|---|---|
| ahpC | Alkyl hydroperoxide reductase-Antioxidant | Antioxidant | [40,90] |
| katA | Catalase | Peroxide detoxification | [90] |
| sodB | Iron co-factored superoxide dismutase- | Antioxidant | [90] |
| fdxA | Ferredoxin A-cyclophilin gene | Antioxidant | [93] |
| htrA | High temperature requirement-A protease | Removal of misfolded proteins as a result of oxygen stress | [92] |
| tpx | Thiol peroxidase- | Scavenges molecular oxygen | [96] |
| bcpb | Express Bacterioferritin comigratory protein | Regulate oxidative stress by attacking molecular oxygen | [96] |
| ctb | Truncated hemoglobin | Oxygen-protective physiological role by increasing oxygen uptake rates | [94] |
| Cj1556 | MarR family transcriptional regulator | Oxidative stress response | [95] |
| Gene | Gene Product or Function | Involved Function in Biofilm | References |
|---|---|---|---|
| flaA | Glycosylated structural flagellins-A | Involved in cell associated with motility | [106] |
| luxS | S-ribosylhomocysteianse | Quorum sensing | [17] |
| cadF | Campylobacter adhesion to fibronectin | Adhesion | [17] |
| dnaJ | Chaperone DnaJ | Stress response | [107] |
| cbrA | Campylobacter bile resistance regulator | Stress response | [107] |
| htrA | High temperature requirement A | Stress response | [107] |
| sodB | Superoxide dismutase | Stress response | [107] |
| ahpC | Alkyl hydroperoxide reductase | Involved in oxidative stress response | [107] |
| peb4 | encode homolog of cluster 3 binding protein | adhesion | [109] |
| trxA | Thioredoxin A | Involved in oxidative stress response | [18] |
| trxB | Thioredoxin B | Involved in oxidative stress response | [18] |
| ilvE | Branched chain amino transferases for leucine, isoleucine, and valine | Involved in oxidative stress response | [18] |
| nuoC | NuoC- subunit of complex I (ubiquinone oxidoreductase) | Assembly or the stability of ubiquinone oxidoreductase | [18] |
| Gene | Gene Product or Function | Involved Function in VBNC | References |
|---|---|---|---|
| ppk1 | Poly phosphate kinase 1-codes for PPK1 enzyme mediates the synthesis of poly P | Mutant of ppk1 decreased the accumulation of poly P-decreased stress response | [134] |
| cadF | Code 37 kDa adhesin-bind to fibronectin and mediates bacteria-host interaction | Retain ability to adhere to host cells | [116] |
| flaA, flab | Flagellin-Involved bacteria internalization | Decreased expression- conserve energy for metabolism | [29] |
| cdtA, cdtB and cdtC | Cytolethal distending toxins-arrest G2/M phase of cell cycle causing cell death | Decreased expression-conserve energy for metabolism | [29] |
| ciaB | Campylobacter invasion antigen B | Decreased expression-conserve energy for metabolism | [29] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Campylobacter (Campylobacteriosis). (Final Update). Available online: https://www.cdc.gov/campylobacter/index.html (accessed on 10 September 2022).
- Tam, C.C.; Rodrigues, L.C.; Viviani, L.; Dodds, J.P.; Evans, M.R.; Hunter, P.R.; Gray, J.J.; Letley, L.H.; Rait, G.; Tompkins, D.S.; et al. Longitudinal Study of Infectious Intestinal Disease in the UK (IID2 Study): Incidence in the Community and Presenting to General Practice. Gut 2012, 61, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Mongodin, E.F.; Mandrell, R.E.; Miller, W.G.; Rasko, D.A.; Ravel, J.; Brinkac, L.M.; Deboy, R.T.; Parker, C.T.; Daugherty, S.C.; et al. Major Structural Differences and Novel Potential Virulence Mechanisms from the Genomes of Multiple Campylobacter Species. PLoS Biol. 2005, 3, e15. [Google Scholar] [CrossRef] [PubMed]
- Dogan, O.B.; Clarke, J.; Mattos, F.; Wang, B. A Quantitative Microbial Risk Assessment Model of Campylobacter in Broiler Chickens: Evaluating Processing Interventions. Food Control 2019, 100, 97–110. [Google Scholar] [CrossRef]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter Species and Guillain-Barre Syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Shane, S.M. The Significance of Campylobacter Jejuni Infection in Poultry: A Review. Avian Pathol. 1992, 21, 189–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, K.; Cunningham, R.; Jones, K. Isolation of Campylobacter Jejuni from Groundwater. J. Appl. Microbiol. 1998, 85, 187–191. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of Environmental Survival in Transmission of Campylobacter Jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Havelaar, A.H.; Mangen, M.J.J.; De Koeijer, A.A.; Bogaardt, M.J.; Evers, E.G.; Jacobs-Reitsma, W.F.; Van Pelt, W.; Wagenaar, J.A.; De Wit, G.A.; Van Der Zee, H.; et al. Effectiveness and Efficiency of Controlling Campylobacter on Broiler Chicken Meat. Risk Anal. 2007, 27, 831–844. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.; Jeon, B. Impact of Oxidative Stress Defense on Bacterial Survival and Morphological Change in Campylobacter Jejuni under Aerobic Conditions. Front. Microbiol. 2015, 6, 295. [Google Scholar] [CrossRef]
- Uzunović-Kamberović, S.; Zorman, T.; Heyndrickx, M.; Možina, S.S. Role of Poultry Meat in Sporadic Campylobacter Infections in Bosnia and Herzegovina: Laboratory-Based Study. Croat. Med. J. 2007, 48, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.; Chui, L.; Bae, J.; Li, V.; Ma, A.; Mutschall, S.K.; Taboada, E.N.; McMullen, L.M.; Jeon, B. Frequent Implication of Multistress-Tolerant Campylobacter Jejuni in Human Infections. Emerg. Infect. Dis. 2018, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouftah, S.F.; Cobo-Díaz, J.F.; Álvarez-Ordóñez, A.; Mousa, A.; Calland, J.K.; Pascoe, B.; Sheppard, S.K.; Elhadidy, M. Stress Resistance Associated with Multi-Host Transmission and Enhanced Biofilm Formation at 42 °C among Hyper-Aerotolerant Generalist Campylobacter Jejuni. Food Microbiol. 2021, 95, 103706. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, H.P.; Parijs, I.; Foster, K.R.; Vanderleyden, J. Experimental Evolution in Biofilm Populations. FEMS Microbiol. Rev. 2016, 40, 373–397. [Google Scholar] [CrossRef] [Green Version]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Reeser, R.J.; Medler, R.T.; Billington, S.J.; Jost, B.H.; Joens, L.A. Characterization of Campylobacter Jejuni Biofilms under Defined Growth Conditions. Appl. Environ. Microbiol. 2007, 73, 1908–1913. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, B.; Méric, G.; Murray, S.; Yahara, K.; Mageiros, L.; Bowen, R.; Jones, N.H.; Jeeves, R.E.; Lappin-Scott, H.M.; Asakura, H.; et al. Enhanced Biofilm Formation and Multi-Host Transmission Evolve from Divergent Genetic Backgrounds in Campylobacter Jejuni. Environ. Microbiol. 2015, 17, 4779–4789. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken Juice Enhances Surface Attachment and Biofilm Formation of Campylobacter Jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef] [Green Version]
- Thames, H.T.; Sukumaran, A.T. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Teh, A.H.T.; Lee, S.M.; Dykes, G.A. Does Campylobacter Jejuni Form Biofilms in Food-Related Environments? Appl. Environ. Microbiol. 2014, 80, 5154–5160. [Google Scholar] [CrossRef]
- Dykes, G.A.; Sampathkumar, B.; Korber, D.R. Planktonic or Biofilm Growth Affects Survival, Hydrophobicity and Protein Expression Patterns of a Pathogenic Campylobacter Jejuni Strain. Int. J. Food Microbiol. 2003, 89, 1–10. [Google Scholar] [CrossRef]
- Chaveerach, P.; Huurne, A.A.H.M.T.; Lipman, L.J.A.; van Knapen, F. Survival and Resuscitation of Ten Strains Of Campylobacter Jejuni and Campylobacter coli under Acid Conditions. Society 2003, 69, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baffone, W.; Casaroli, A.; Citterio, B.; Pierfelici, L.; Campana, R.; Vittoria, E.; Guaglianone, E.; Donelli, G. Campylobacter Jejuni Loss of Culturability in Aqueous Microcosms and Ability to Resuscitate in a Mouse Model. Int. J. Food Microbiol. 2006, 107, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Guzej, B.; Jamnik, P.; Vučković, D.; Abram, M.; Možina, S.S. Stress Response and Pathogenic Potential of Campylobacter Jejuni Cells Exposed to Starvation. Res. Microbiol. 2009, 160, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.N.; Davis, B.; Tirado, S.M.; Duggal, M.; Van Frankenhuyzen, J.K.; Deaville, D.; Wijesinghe, M.A.K.; Tessaro, M.; Trevors, J.T. Survival Mechanisms and Culturability of Campylobacter Jejuni under Stress Conditions. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2009, 96, 377–394. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The Importance of the Viable but Non-Culturable State in Human Bacterial Pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Rollins, D.M.; Colwell, R.R. Viable but Nonculturable Stage of Campylobacter Jejuni and Its Role in Survival in the Natural Aquatic Environment. Appl. Environ. Microbiol. 1986, 52, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Chaisowwong, W.; Kusumoto, A.; Hashimoto, M.; Harada, T.; Maklon, K.; Kawamoto, K. Physiological Characterization of Campylobacter Jejuni under Cold Stresses Conditions: Its Potential for Public Threat. J. Vet. Med. Sci. 2012, 74, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.U.H.; Hill, S.; Nowak, E.; Edge, T.A. Effect of Incubation Temperature on the Detection of Thermophilic Campylobacter Species from Freshwater Beaches, Nearby Wastewater Effluents, and Bird Fecal Droppings. Appl. Environ. Microbiol. 2013, 79, 7639–7645. [Google Scholar] [CrossRef] [Green Version]
- Oyarzabal, O.A.; Carrillo, C.D. Isolation, Identification, and Typing of Campylobacter Strains from Food Samples; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Esson, D.; Mather, A.E.; Scanlan, E.; Gupta, S.; De Vries, S.P.W.; Bailey, D.; Harris, S.R.; McKinley, T.J.; Méric, G.; Berry, S.K.; et al. Genomic Variations Leading to Alterations in Cell Morphology of Campylobacter spp. Sci. Rep. 2016, 6, 38303. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.K.; Sherburne, R.; Taylor, D.E.; Stiles, M.E. Morphological Forms and Viability of Campylobacter Species Studied by Electron Microscopy. J. Bacteriol. 1985, 164, 338–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangwatcharin, P.; Chanthachum, S.; Khopaibool, P.; Griffiths, M.W. Morphological and Physiological Responses of Campylobacter Jejuni to Stress. J. Food Prot. 2006, 69, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Buck, G.E.; Parshall, K.A.; Davis, C.P. Electron Microscopy of the Coccoid Form of Campylobacter Jejuni. J. Clin. Microbiol. 1983, 18, 420–421. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, P.L. Morphological Changes of Campylobacter Jejuni Growing in Liquid Culture. Lett. Appl. Microbiol. 1993, 17, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Frirdich, E.; Biboy, J.; Adams, C.; Lee, J.; Ellermeier, J.; Gileda, L.D.; DiRita, V.J.; Girardin, S.E.; Vollmer, W.; Gaynor, E.C. Peptidoglycan-Modifying Enzyme Pgp1 Is Required ForHelical Cell Shape and Pathogenicity Traits In Campylobacter Jejuni. PLoS Pathog. 2012, 8, e1002602. [Google Scholar] [CrossRef]
- Parkhill, J.; Wren, B.W.; Mungall, K.; Ketley, J.M.; Churcher, C.; Basham, D.; Chillingworth, T.; Davies, R.M.; Feltwell, T.; Holroyd, S.; et al. The Genome Sequence of C. Jejuni. Nature 2000, 403, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesci, E.C.; Cottle, D.L.; Pickett, C.L. Genetic, Enzymatic, and Pathogenic Studies of the Iron Superoxide Dismutase of Campylobacter Jejuni. Infect. Immun. 1994, 62, 2687–2694. [Google Scholar] [CrossRef] [Green Version]
- Baillon, M.L.A.; Van Vliet, A.H.M.; Ketley, J.M.; Constantinidou, C.; Penn, C.W. An Iron-Regulated Alkyl Hydroperoxide Reductase (AhpC) Confers Aerotolerance and Oxidative Stress Resistance to the Microaerophilic Pathogen Campylobacter Jejuni. J. Bacteriol. 1999, 181, 4798–4804. [Google Scholar] [CrossRef] [Green Version]
- Hue, O.; Allain, V.; Laisney, M.J.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.Y.; Picherot, M.; et al. Campylobacter Contamination of Broiler Caeca and Carcasses at the Slaughterhouse and Correlation with Salmonella Contamination. Food Microbiol. 2011, 28, 862–868. [Google Scholar] [CrossRef]
- Berrang, M.E.; Buhr, R.J.; Cason, J.A.; Dickens, J.A. Broiler Carcass Contamination with Campylobacter from Feces during Defeathering. J. Food Prot. 2001, 64, 2063–2066. [Google Scholar] [CrossRef]
- Borges, K.A.; Cisco, I.C.; Furian, T.Q.; Tedesco, D.C.; Rodrigues, L.B.; do Nascimento, V.P.; dos Santos, L.R. Detection and Quantification of Campylobacter spp. In Brazilian Poultry Processing Plants. J. Infect. Dev. Ctries. 2020, 14, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Perez-Arnedo, I.; Gonzalez-Fandos, E. Prevalence of Campylobacter spp. In Poultry in Three Spanish Farms, a Slaughterhouse and a Further Processing Plant. Foods 2020, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrat, M.B.; Soumet, C.; Maris, P.; Sanders, P. Recovery of Campylobacter Jejuni from Surfaces of Poultry Slaughterhouses after Cleaning and Disinfection Procedures: Analysis of a Potential Source of Carcass Contamination. Int. J. Food Microbiol. 2008, 124, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Pocheron, A.L.; Hernould, M.; Haddad, N.; Tresse, O.; Cappelier, J.M. Description of Campylobacter Jejuni Bf, an Atypical Aero-Tolerant Strain. Gut Pathog. 2015, 7, 30. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative Analysis of Aerotolerance, Antibiotic Resistance, and Virulence Gene Prevalence in Campylobacter Jejuni Isolates from Retail Raw Chicken and Duck Meat in South Korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef] [Green Version]
- Hilbert, F.; Scherwitzel, M.; Paulsen, P.; Szostak, M.P. Survival of Campylobacter Jejuni under Conditions of Atmospheric Oxygen Tension with the Support of Pseudomonas spp. Appl. Environ. Microbiol. 2010, 76, 5911–5917. [Google Scholar] [CrossRef] [Green Version]
- Habib, I.; Uyttendaele, M.; De Zutter, L. Survival of Poultry-Derived Campylobacter Jejuni of Multilocus Sequence Type Clonal Complexes 21 and 45 under Freeze, Chill, Oxidative, Acid and Heat Stresses. Food Microbiol. 2010, 27, 829–834. [Google Scholar] [CrossRef]
- Lindqvist, R.; Upadhyay, A.; Överby, A.K. Tick-Borne Flaviviruses and the Type I Interferon Response. Viruses 2018, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Konkel, M.E.; Kim, B.J.; Klena, J.D.; Young, C.R.; Ziprin, R. Characterization of the Thermal Stress Response of Campylobacter Jejuni. Infect. Immun. 1998, 66, 3666–3672. [Google Scholar] [CrossRef]
- Stewart, G.R.; Robertson, B.D.; Young, D.B. Analysis of the Function of Mycobacterial DnaJ Proteins by Overexpression and Microarray Profiling. Tuberculosis 2004, 84, 180–187. [Google Scholar] [CrossRef]
- Brás, A.M.; Chatterjee, S.; Wren, B.W.; Newell, D.G.; Ketley, J.M. A Novel Campylobacter Jejuni Two-Component Regulatory System Important for Temperature-Dependent Growth and Colonization. J. Bacteriol. 1999, 181, 3298–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental Survival Mechanisms of the Foodborne Pathogen Campylobacter Jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Snith, S.C.; Coloe, P.J. Survival and Growth of Campylobacter Jejuni after Artificial Inoculation onto Chicken Skin as a Function of Temperature and Packaging Conditions. J. Food Prot. 1998, 61, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Tran, H.L.; Kanenaka, R.Y.; Kathariou, S. Survival of Clinical and Poultry-Derived Isolates of Campylobacter Jejuni at a Low Temperature (4 °C). Appl. Environ. Microbiol. 2001, 67, 4186–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelana, L.C.; Griffiths, M.W. Use of an Autobioluminescent Campylobacter Jejuni to Monitor Cell Survival as a Function of Temperature, PH, and Sodium Chloride. J. Food Prot. 2003, 66, 2032–2037. [Google Scholar] [CrossRef]
- Moen, B.; Oust, A.; Langsrud, Ø.; Dorrell, N.; Marsden, G.L.; Hinds, J.; Kohler, A.; Wren, B.W.; Rudi, K. Explorative Multifactor Approach for Investigating Global Survival Mechanisms of Campylobacter Jejuni under Environmental Conditions. Appl. Environ. Microbiol. 2005, 71, 2086–2094. [Google Scholar] [CrossRef] [Green Version]
- Stead, D.; Park, S.F. Roles of Fe Superoxide Dismutase and Catalase in Resistance of Campylobacter Coli to Freeze-Thaw Stress. Appl. Environ. Microbiol. 2000, 66, 3110–3112. [Google Scholar] [CrossRef] [Green Version]
- White, D.; Drummond, J.; Fuqua, C. The Physiology and Biochemistry of the Prokaryotes; Oxford University Press: New York, NY, USA, 2011; p. 656. [Google Scholar]
- Doyle, M.P.; Roman, D.J. Response of Campylobacter Jejuni to Sodium Chloride. Appl. Environ. Microbiol. 1982, 43, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Sampers, I.; Habib, I.; De Zutter, L.; Dumoulin, A.; Uyttendaele, M. Survival of Campylobacter spp. in Poultry Meat Preparations Subjected to Freezing, Refrigeration, Minor Salt Concentration, and Heat Treatment. Int. J. Food Microbiol. 2010, 137, 147–153. [Google Scholar] [CrossRef]
- Reezal, A.; Mcneil, B.; Anderson, J.G. Effect of Low-Osmolality Nutrient Media on Growth and Culturability of Campylobacter Species. Appl. Environ. Microbiol. 1998, 64, 4643–4649. [Google Scholar] [CrossRef] [Green Version]
- Strom, A.R.; Kaasen, I. Trehalose Metabolism in Escherichia Coli: Stress Protection and Stress Regulation of Gene Expression. Mol. Microbiol. 1993, 8, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Frirdich, E.; Huynh, S.; Parker, C.T.; Gaynor, E.C. Hyperosmotic Stress Response of Campylobacter Jejuni. J. Bacteriol. 2012, 194, 6116–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempf, B.; Bremer, E. Uptake and Synthesis of Compatible Solutes as Microbial Stress Responses to High-Osmolality Environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 21348 Definitions of Solar Irradiance Spectral Categories. Environment 2007, 5, 6–7. [Google Scholar]
- Zajac, A. Irradiation in The Pro-Duction, Processing and Handling of Food; Food and Drug Administration: Silver Spring, MD, USA, 2007; Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? (accessed on 10 September 2022).
- Sinton, L.; Hall, C.; Braithwaite, R. Sunlight Inactivation of Campylobacter Jejuni and Salmonella Enterica, Compared with Escherichia Coli, in Seawater and River Water. J. Water Health 2007, 5, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Butler, R.C.; Lund, V.; Carlson, D.A. Susceptibility of Campylobacter Jejuni and Yersinia Enterocolitica to UV Radiation. Appl. Environ. Microbiol. 1987, 53, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, L.E.; Maclean, M.; MacGregor, S.J.; Anderson, J.G. Inactivation of Campylobacter Jejuni by Exposure to High-Intensity 405-Nm Visible Light. Foodborne Pathog. Dis. 2010, 7, 1211–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Modelling the Effect of UV Light at Different Wavelengths and Treatment Combinations on the Inactivation of Campylobacter Jejuni. Innov. Food Sci. Emerg. Technol. 2021, 69, 102626. [Google Scholar] [CrossRef]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J.; Fanning, S.; Whyte, P. Efficacy of UV Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J. Food Prot. 2011, 74, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Isohanni, P.M.I.; Lyhs, U. Use of Ultraviolet Irradiation to Reduce Campylobacter Jejuni on Broiler Meat. Poult. Sci. 2009, 88, 661–668. [Google Scholar] [CrossRef]
- Obiri-Danso, K.; Paul, N.; Jones, K. The Effects of UVB and Temperature on the Survival of Natural Populations and Pure Cultures of Campylobacter Jejuni, Camp. Coli, Camp. Lari and Urease-Positive Thermophilic Campylobacters (UPTC) in Surface Waters. J. Appl. Microbiol. 2001, 90, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Ye, C.; Cui, L.; Wan, K.; Chen, S.; Zhang, S.; Yu, X. Population and Single Cell Metabolic Activity of UV-Induced VBNC Bacteria Determined by CTC-FCM and D2O-Labeled Raman Spectroscopy. Environ. Int. 2019, 130, 104883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, C.; Lin, H.; Lv, L.; Yu, X. UV Disinfection Induces a Vbnc State in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015, 49, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Guerry, P.; Pope, P.M.; Burr, D.H.; Leifer, J.; Joseph, S.W.; Bourgeois, A.L. Development and Characterization of RecA Mutants of Campylobacter Jejuni for Inclusion in Attenuated Vaccines. Infect. Immun. 1994, 62, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Reid, A.N.; Pandey, R.; Palyada, K.; Naikare, H.; Stintzi, A. Identification of Campylobacter Jejuni Genes Involved in the Response to Acidic PH and Stomach Transit. Appl. Environ. Microbiol. 2008, 74, 1583–1597. [Google Scholar] [CrossRef] [Green Version]
- Stern, N.J.; Rothenberg, P.J.; Stone, J.M. Enumeration and Reduction of Campylobacter Jejuni in Poultry and Red Meats. J. Food Prot. 1985, 48, 606–610. [Google Scholar] [CrossRef]
- Birk, T.; Grønlund, A.C.; Christensen, B.B.; Knøchel, S.; Lohse, K.; Rosenquist, H. Effect of Organic Acids and Marination Ingredients on the Survival of Campylobacter Jejuni on Meat. J. Food Prot. 2010, 73, 258–265. [Google Scholar] [CrossRef]
- Waterman, S.R.; Small, P.L.C. Acid-Sensitive Enteric Pathogens Are Protected from Killing under Extremely Acidic Conditions of PH 2.5 When They Are Inoculated onto Certain Solid Food Sources. Appl. Environ. Microbiol. 1998, 64, 3882–3886. [Google Scholar] [CrossRef] [Green Version]
- Goodson, M.; Rowbury, R.J. Habituation to Normally Lethal Acidity by Prior Growth of Escherichia Coli at a Sub-Lethal Acid PH Value. Lett. Appl. Microbiol. 1989, 8, 77–79. [Google Scholar] [CrossRef]
- Murphy, C.; Carroll, C.; Jordan, K.N. Induction of an Adaptive Tolerance Response in the Foodborne Pathogen, Campylobacter Jejuni. FEMS Microbiol. Lett. 2003, 223, 89–93. [Google Scholar] [CrossRef]
- winiecka-krusnell, J.; Wreiber, K.; von Euler, A.; Engstrand, L.; Linder, E. Free-Living Amoebae Promote Growth and Survival of Helicobacter Pylori. Scand. J. Infect. Dis. 2002, 34, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Axelsson-Olsson, D.; Svensson, L.; Olofsson, J.; Salomon, P.; Waldenström, J.; Ellström, P.; Olsen, B. Increase in Acid Tolerance of Campylobacter Jejuni through Coincubation with Amoebae. Appl. Environ. Microbiol. 2010, 76, 4194–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birk, T.; Wik, M.T.; Lametsch, R.; Knøchel, S. Acid Stress Response and Protein Induction in Campylobacter Jejuni Isolates with Different Acid Tolerance. BMC Microbiol. 2012, 12, 174. [Google Scholar] [CrossRef] [Green Version]
- Askoura, M.; Sarvan, S.; Couture, J.F.; Stintzi, A. The Campylobacter Jejuni Ferric Uptake Regulator Promotes Acid Survival and Cross-Protection against Oxidative Stress. Infect. Immun. 2016, 84, 1287–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.; Andrews, K.J.; McMullen, L.M.; Jeon, B. Tolerance to Stress Conditions Associated with Food Safety in Campylobacter Jejuni Strains Isolated from Retail Raw Chicken. Sci. Rep. 2019, 9, 11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.C.; Oh, E.; Hwang, S.; Ryu, S.; Jeon, B. Non-Selective Regulation of Peroxide and Superoxide Resistance Genes by PerR in Campylobacter Jejuni. Front. Microbiol. 2015, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Kaakoush, N.O.; Miller, W.G.; De Reuse, H.; Mendz, G.L. Oxygen Requirement and Tolerance of Campylobacter Jejuni. Res. Microbiol. 2007, 158, 644–650. [Google Scholar] [CrossRef]
- Brøndsted, L.; Andersen, M.T.; Parker, M.; Jørgensen, K.; Ingmer, H. The HtrA Protease of Campylobacter Jejuni Is Required for Heat and Oxygen Tolerance and for Optimal Interaction with Human Epithelial Cells. Appl. Environ. Microbiol. 2005, 71, 3205–3212. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, A.H.M.; Baillon, M.L.A.; Penn, C.W.; Ketley, J.M. The Iron-Induced Ferredoxin FdxA of Campylobacter Jejuni Is Involved in Aerotolerance. FEMS Microbiol. Lett. 2001, 196, 189–193. [Google Scholar] [CrossRef]
- Wainwright, L.M.; Elvers, K.T.; Park, S.F.; Poole, R.K. A Truncated Haemoglobin Implicated in Oxygen Metabolism by the Microaerophilic Food-Borne Pathogen Campylobacter Jejuni. Microbiology 2005, 151, 4079–4091. [Google Scholar] [CrossRef]
- Gundogdu, O.; Mills, D.C.; Elmi, A.; Martin, M.J.; Wren, B.W.; Dorrell, N. The Campylobacter Jejuni Transcriptional Regulator Cj1556 Plays a Role in the Oxidative and Aerobic Stress Response and Is Important for Bacterial Survival In Vivo. J. Bacteriol. 2011, 193, 4238–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atack, J.M.; Harvey, P.; Jones, M.A.; Kelly, D.J. The Campylobacter Jejuni Thiol Peroxidases Tpx and Bcp Both Contribute to Aerotolerance and Peroxide-Mediated Stress Resistance but Have Distinct Substrate Specificities. J. Bacteriol. 2008, 190, 5279–5290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Buswell, C.M.; Herlihy, Y.M.; Lawrence, L.M.; McGuiggan, J.T.M.; Marsh, P.D.; Keevil, C.W.; Leach, S.A. Extended Survival and Persistence of Campylobacter spp. Water and Aquatic Biofilms and Their Detection by Immunofluorescent-Antibody and -RRNA Staining. Appl. Environ. Microbiol. 1998, 64, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron through Oxidative Stress in Campylobacter Jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.-L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic Analysis of Campylobacter Jejuni 11168 Biofilms Reveals a Role for the Motility Complex in Biofilm Formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Wu, Q.; Zhang, J.; Ma, Z.; Wang, J.; Nie, X.; Ding, Y.; Xue, L.; Chen, M.; Wu, S.; et al. Campylobacter Jejuni Biofilm Formation Under Aerobic Conditions and Inhibition by ZnO Nanoparticles. Front. Microbiol. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Van de Giessen, A.W.; Heuvelman, C.J.; Abee, T.; Hazeleger, W.C. Experimental Studies on the Infectivity of Non-Culturable Forms of Campylobacter spp. in Chicks and Mice. Epidemiol. Infect. 1996, 117, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Hazeleger, W.C.; Janse, J.D.; Koenraad, P.M.F.J.; Beumer, R.R.; Rombouts, F.M.; Abee, T. Temperature-Dependent Membrane Fatty Acid and Cell Physiology Changes in Coccoid Forms of Campylobacter Jejuni. Appl. Environ. Microbiol. 1995, 61, 2713–2719. [Google Scholar] [CrossRef] [Green Version]
- Asakura, H.; Brüggemann, H.; Sheppard, S.K.; Ekawa, T.; Meyer, T.F.; Yamamoto, S.; Igimi, S. Molecular Evidence for the Thriving of Campylobacter Jejuni ST-4526 in Japan. PLoS ONE 2012, 7, e48394. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; Strachan, N.J.C.; MacRae, M.; McCarthy, N.D.; Wilson, D.J.; Gormley, F.J.; Falush, D.; Ogden, L.D.; Maiden, M.C.J.; et al. Campylobacter Genotyping to Determine the Source of Human Infection. Clin. Infect. Dis. 2009, 48, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Park, C.; Kim, Y.J. Role of FlgA for Flagellar Biosynthesis and Biofilm Formation of Campylobacter Jejuni NCTC11168. J. Microbiol. Biotechnol. 2015, 25, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jeon, B. Role of Alkyl Hydroperoxide Reductase (AhpC) in the Biofilm Formation of Campylobacter Jejuni. PLoS ONE 2014, 9, e87312. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Burucoa, C.; Grignon, B.; Baqar, S.; Huang, X.-Z.; Kopecko, D.J.; Bourgeois, A.; Fauchere, J.-L.; Blaser, M.J. Mutation in the Peb1A Locus Of Campylobacter Jejuni Reduces Interactions with Epithelial Cells and Intestinal Colonization of Mice. Infect. Immun. 1998, 66, 938–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakura, H.; Yamasaki, M.; Yamamoto, S.; Igimi, S. Deletion of Peb4 Gene Impairs Cell Adhesion and Biofilm Formation in Campylobacter Jejuni. FEMS Microbiol. Lett. 2007, 275, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.L.; Hanman, K.; Reuter, M.; Betts, R.P.; van Vliet, A.H.M. Campylobacter Jejuni Biofilms Contain Extracellular DNA and Are Sensitive to DNase I Treatment. Front. Microbiol. 2015, 6, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Park, C.; Lee, E.J.; Bang, W.S.; Kim, Y.J.; Kim, J.S. Biofilm Formation of Campylobacter Strains Isolated from Raw Chickens and Its Reduction with DNase I Treatment. Food Control 2017, 71, 94–100. [Google Scholar] [CrossRef]
- Wagle, B.R.; Donoghue, A.M.; Shrestha, S.; Upadhyaya, I.; Arsi, K.; Gupta, A.; Liyanage, R.; Rath, N.C.; Donoghue, D.J.; Upadhyay, A. Carvacrol Attenuates Campylobacter Jejuni Colonization Factors and Proteome Critical for Persistence in the Chicken Gut. Poult. Sci. 2020, 99, 4566–4577. [Google Scholar] [CrossRef]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus Extracts as Inhibitors of Quorum Sensing, Biofilm Formation and Motility of Campylobacter Jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Yu, H.H.; Song, Y.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Investigating the Antimicrobial and Antibiofilm Effects of Cinnamaldehyde against Campylobacter spp. Using Cell Surface Characteristics. J. Food Sci. 2020, 85, 157–164. [Google Scholar] [CrossRef]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S. Trans-Cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter Jejuni Biofilms and Modulate Expression of Select Genes and Proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrone, V.; Campana, R.; Vallorani, L.; Dominici, S.; Federici, S.; Casadei, L.; Gioacchini, A.M.; Stocchi, V.; Baffone, W. CadF Expression in Campylobacter Jejuni Strains Incubated under Low-Temperature Water Microcosm Conditions Which Induce the Viable but Non-Culturable (VBNC) State. Antonie Leeuwenhoek 2013, 103, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Sutcliffe, E.M.; Curry, A. Recovery of Viable but Non-Culturable Campylobacter Jejuni. J. Gen. Microbiol. 1991, 137, 2477–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Saha, S.; Sanyal, S.C. Recovery of Injured Campylobacter Jejuni Cells after Animal Passage. Appl. Environ. Microbiol. 1991, 57, 3388–3389. [Google Scholar] [CrossRef] [Green Version]
- Cappelier, J.M.; Magras, C.; Jouve, J.L.; Federighi, M. Recovery of Viable but Non-Culturable Campylobacter Jejuni Cells in Two Animal Models. Food Microbiol. 1999, 16, 375–383. [Google Scholar] [CrossRef]
- Pearson, A.D.; Greenwood, M.; Healing, T.D.; Rollins, D.; Shahamat, M.; Donaldson, J.; Colwell, R.R. Colonization of Broiler Chickens by Waterborne Campylobacter Jejuni. Appl. Environ. Microbiol. 1993, 59, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Beumer, R.R.; de Vries, J.; Rombouts, F.M. Campylobacter Jejuni Non-Culturable Coccoid Cells. Int. J. Food Microbiol. 1991, 15, 153–163. [Google Scholar] [CrossRef]
- Lázaro, B.; Cárcamo, J.; Audícana, A.; Perales, I.; Fernández-Astorga, A. Viability and DNA Maintenance in Nonculturable Spiral Campylobacter Jejuni Cells after Long-Term Exposure to Low Temperatures. Appl. Environ. Microbiol. 1999, 65, 4677–4681. [Google Scholar] [CrossRef] [Green Version]
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter spp. in Environmental Farm Samples and Processing Plant Carcass Rinses from Commercial Broiler Chicken Flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef] [Green Version]
- Berrang, M.E.; Bailey, J.S.; Altekruse, S.F.; Patel, B.; Shaw, W.K.; Meinersmann, R.J.; Fedorka-Cray, P.J. Prevalence and Numbers of Campylobacter on Broiler Carcasses Collected at Rehang and Postchill in 20 U.S. Processing Plants. J. Food Prot. 2007, 70, 1556–1560. [Google Scholar] [CrossRef]
- Xu, X.; Rothrock, M.J.; Mohan, A.; Kumar, G.D.; Mishra, A. Using Farm Management Practices to Predict Campylobacter Prevalence in Pastured Poultry Farms. Poult. Sci. 2021, 100, 101122. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.W.; Eifert, J.D.; Ponder, M.A.; Schmale, D.G. Association of Campylobacter spp. Levels between Chicken Grow-out Environmental Samples and Processed Carcasses. Poult. Sci. 2014, 93, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Brake, J.; Keelara, S.; Zou, M.; Susick, E. Farm and Environmental Distribution of Campylobacter and Salmonella in Broiler Flocks. Res. Vet. Sci. 2013, 94, 33–42. [Google Scholar] [CrossRef]
- Kotula, K.L.; Pandya, Y. Bacterial Contamination of Broiler Chickens before Scalding. J. Food Prot. 1995, 58, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, J.K.; Berrang, M.E.; Dickens, J.A.; Fletcher, D.L.; Cox, N.A. Effect of Broiler Age, Feed Withdrawal, and Transportation on Levels of Coliforms, Campylobacter, Escherichia Coli and Salmonella on Carcasses before and after Immersion Chilling. Poult. Sci. 2003, 82, 169–173. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Armendaris, P.M.; de Sá, C.V.G.C.; Haddad, J.P.A.; de Melo, C.B. Prevalence of Campylobacter spp. in Chicken Carcasses in Slaughterhouses from South of Brazil. Curr. Microbiol. 2021, 78, 2242–2250. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Hänninen, M.L.; Rossi, M.; Rovira, J. Campylobacter Jejuni Survival in a Poultry Processing Plant Environment. Food Microbiol. 2017, 65, 185–192. [Google Scholar] [CrossRef]
- Kim, J.C.; Oh, E.; Kim, J.; Jeon, B. Regulation of Oxidative Stress Resistance in Campylobacter Jejuni, a Microaerophilic Foodborne Pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef] [Green Version]
- Ligowska, M.; Cohn, M.T.; Stabler, R.A.; Wren, B.W.; Brøndsted, L. Effect of Chicken Meat Environment on Gene Expression of Campylobacter Jejuni and Its Relevance to Survival in Food. Int. J. Food Microbiol. 2011, 145 (Suppl. 1), S111–S115. [Google Scholar] [CrossRef]
- Gangaiah, D.; Kassem, I.I.; Liu, Z.; Rajashekara, G. Importance of Polyphosphate Kinase 1 for Campylobacter Jejuni Viable-but-Nonculturable Cell Formation, Natural Transformation, and Antimicrobial Resistance. Appl. Environ. Microbiol. 2009, 75, 7838–7849. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhrel, D.; Thames, H.T.; Zhang, L.; Dinh, T.T.N.; Schilling, W.; White, S.B.; Ramachandran, R.; Theradiyil Sukumaran, A. Roles of Aerotolerance, Biofilm Formation, and Viable but Non-Culturable State in the Survival of Campylobacter jejuni in Poultry Processing Environments. Microorganisms 2022, 10, 2165. https://doi.org/10.3390/microorganisms10112165
Pokhrel D, Thames HT, Zhang L, Dinh TTN, Schilling W, White SB, Ramachandran R, Theradiyil Sukumaran A. Roles of Aerotolerance, Biofilm Formation, and Viable but Non-Culturable State in the Survival of Campylobacter jejuni in Poultry Processing Environments. Microorganisms. 2022; 10(11):2165. https://doi.org/10.3390/microorganisms10112165
Chicago/Turabian StylePokhrel, Diksha, Hudson T. Thames, Li Zhang, Thu T. N. Dinh, Wes Schilling, Shecoya B. White, Reshma Ramachandran, and Anuraj Theradiyil Sukumaran. 2022. "Roles of Aerotolerance, Biofilm Formation, and Viable but Non-Culturable State in the Survival of Campylobacter jejuni in Poultry Processing Environments" Microorganisms 10, no. 11: 2165. https://doi.org/10.3390/microorganisms10112165





