Lipoproteins Are Potent Activators of Nuclear Factor Kappa B in Mammary Epithelial Cells and Virulence Factors in Mycoplasma bovis Mastitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. M. bovis Strain, Cell Lines, and Culture Conditions
2.3. Inactivation of M. bovis by UV-Irradiation
2.4. Activation of EpH4 Cells
2.5. M. bovis Intramammary Challenge of Lactating Mice
2.6. Bioluminescence and Fluorescence Imaging
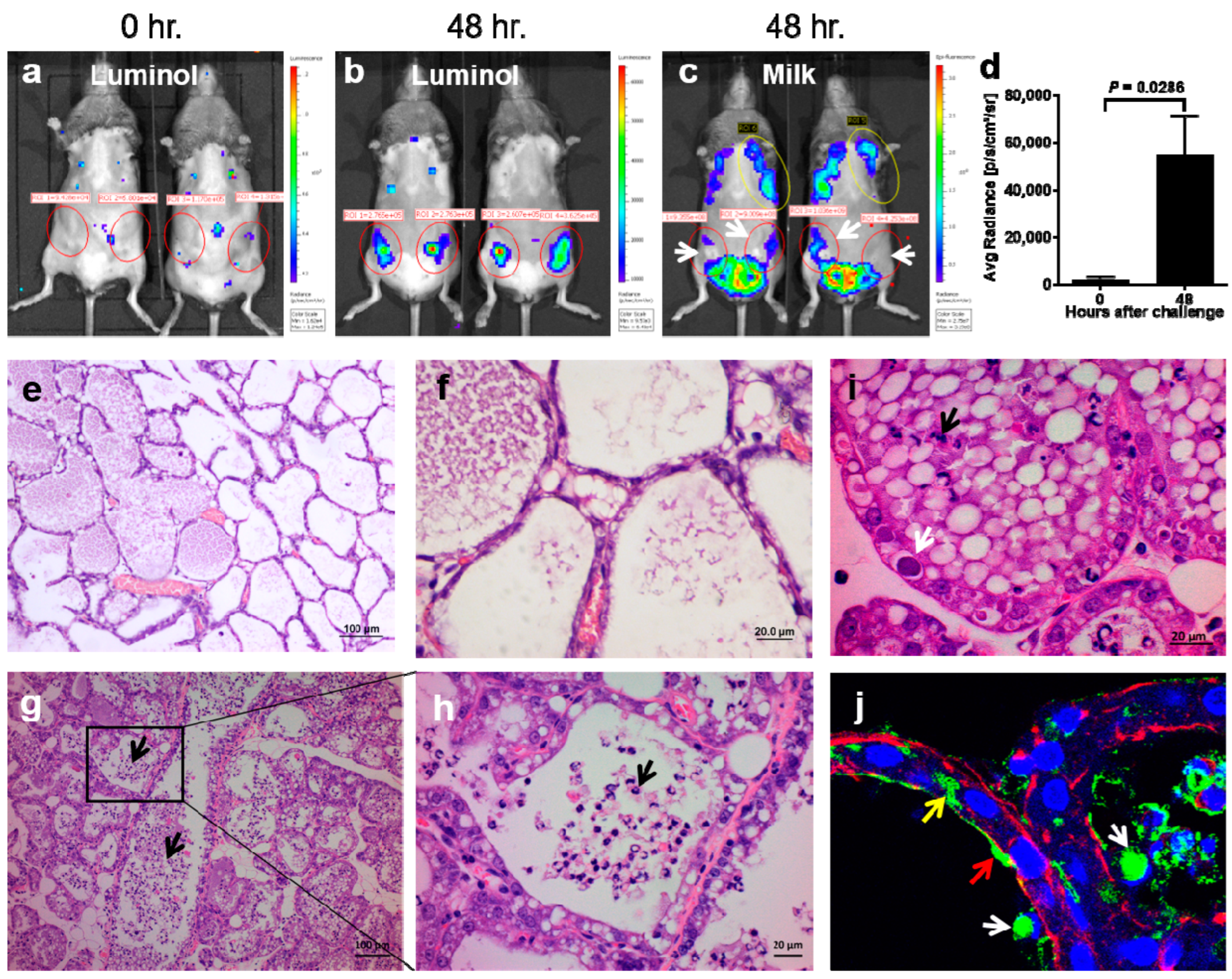
2.7. Histopathological Analysis
2.8. Mammary Gene Expression Analysis
2.9. Statistical Analysis
3. Results
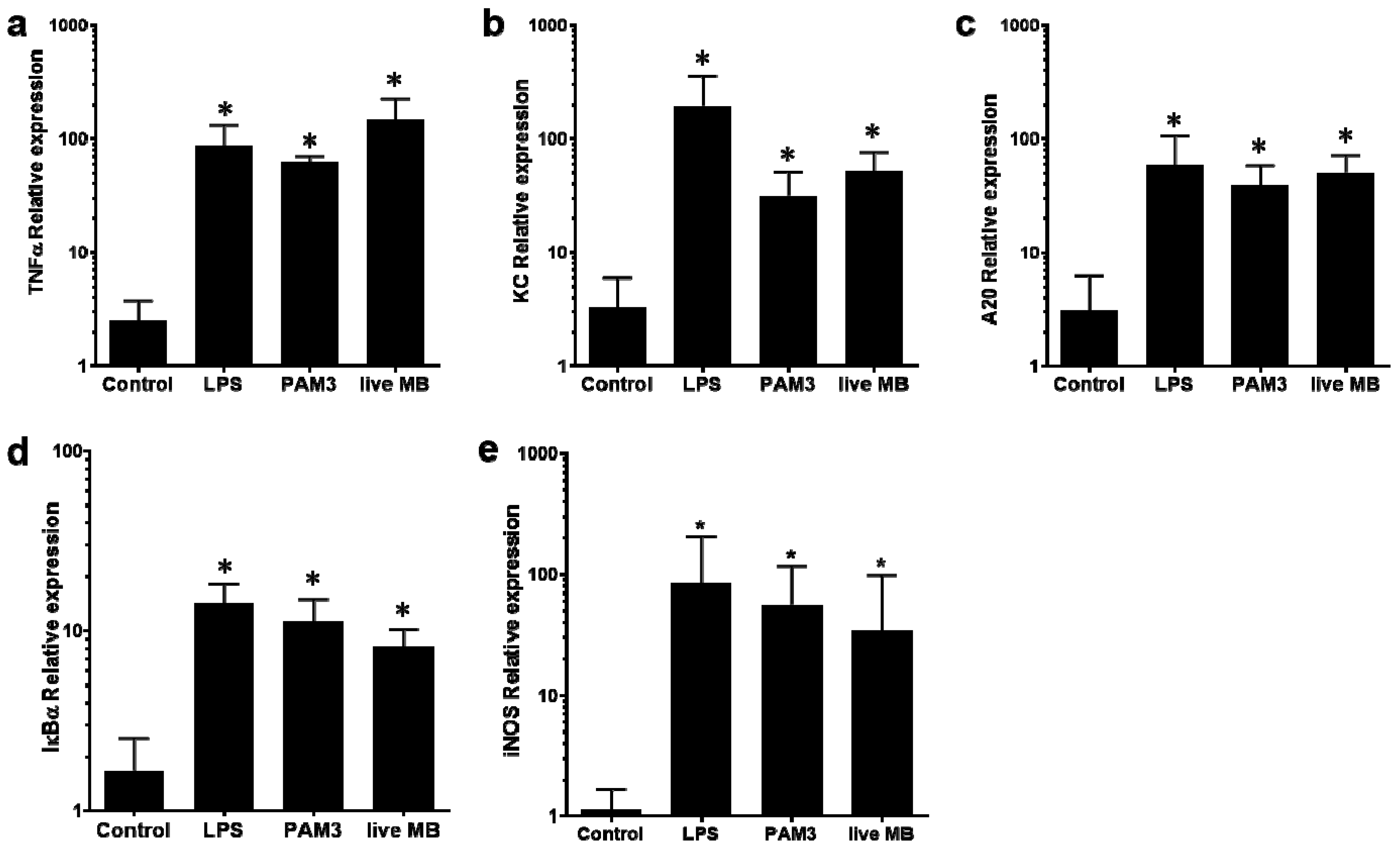
3.1. Differential Activation of NF-kB in mMECs
3.2. The Establishment and Characterization of M. bovis Murine Mastitis Model
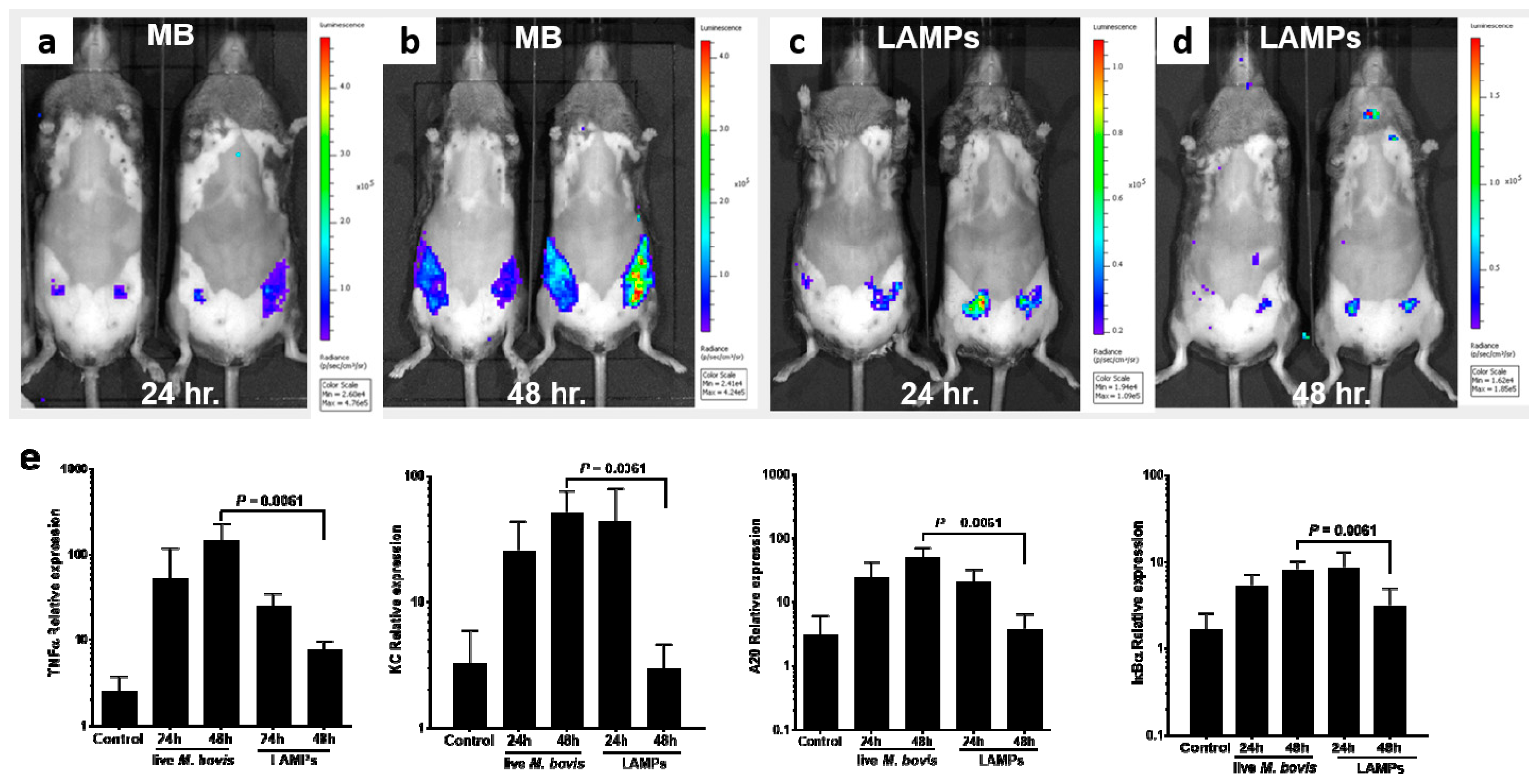
3.3. Induction of Mammary Inflammation by LAMPs and M. bovis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, R.N.; Wilson, D.J. Mycoplasmal mastitis in dairy herds. Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 199–221. [Google Scholar] [CrossRef]
- Nicholas, R.A.; Fox, L.K.; Lysnyansky, I. Mycoplasma mastitis in cattle: To cull or not to cull. Vet. J. 2016, 216, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, M.J.; Lysnyansky, I.; Sachse, K.; Fox, L.K.; Nicholas, R.A.J.; Ayling, R.D. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 91–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, L.K. Mycoplasma mastitis: Causes, transmission, and control. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Barkema, H.W.; Green, M.J.; Bradley, A.J.; Zadoks, R.N. Invited review: The role of contagious disease in udder health. J. Dairy Sci. 2009, 92, 4717–4729. [Google Scholar] [CrossRef] [Green Version]
- Schukken, Y.H.; Gunther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.J.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Chase, C. Mycoplasma bovis: Interactions with the immune system and failure to generate an effective immune response. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 471–483. [Google Scholar] [CrossRef]
- Perez-Casal, J. Pathogenesis and virulence of Mycoplasma bovis. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 269–278. [Google Scholar] [CrossRef]
- Goto, S.; Konnai, S.; Okagawa, T.; Nishimori, A.; Maekawa, N.; Gondaira, S.; Higuchi, H.; Koiwa, M.; Tajima, M.; Kohara, J.; et al. Increase of cells expressing PD-1 and PD-L1 and enhancement of IFN-gamma production via PD-1/PD-L1 blockade in bovine mycoplasmosis. Immun. Inflamm. Dis. 2017, 5, 355–363. [Google Scholar] [CrossRef]
- Goto, S.; Konnai, S.; Hirano, Y.; Kohara, J.; Okagawa, T.; Maekawa, N.; Sajiki, Y.; Watari, K.; Minato, E.; Kobayashi, A.; et al. Upregulation of PD-L1 expression by prostaglandin E2 and the enhancement of IFN-gamma by anti-PD-L1 antibody combined with a COX-2 inhibitor in Mycoplasma bovis infection. Front. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Gondaira, S.; Nishi, K.; Tanaka, T.; Yamamoto, T.; Nebu, T.; Watanabe, R.; Konnai, S.; Hayashi, T.; Kiku, Y.; Okamoto, M.; et al. Immunosuppression in cows following intramammary infusion of Mycoplasma bovis. Infect. Immun. 2020, 88, e00521-19. [Google Scholar] [CrossRef] [PubMed]
- Sajiki, Y.; Konnai, S.; Goto, S.; Okagawa, T.; Ohira, K.; Shimakura, H.; Maekawa, N.; Gondaira, S.; Higuchi, H.; Tajima, M.; et al. The suppression of Th1 response by inducing TGF-beta1 from regulatory T cells in bovine mycoplasmosis. Front. Vet. Sci. 2020, 7, 609443. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bürki, S.; Gaschen, V.; Stoffel, M.H.; Stojiljkovic, A.; Frey, J.; Kuehni-Boghenbor, K.; Pilo, P. Invasion and persistence of Mycoplasma bovis in embryonic calf turbinate cells. Vet. Res. 2015, 46, 53. [Google Scholar] [CrossRef] [Green Version]
- Josi, C.; Burki, S.; Stojiljkovic, A.; Wellnitz, O.; Stoffel, M.H.; Pilo, P. Bovine epithelial in vitro infection models for Mycoplasma bovis. Front. Cell. Infect. Microbiol. 2018, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- van der Merwe, J.; Prysliak, T.; Perez-Casal, J. Invasion of bovine peripheral-blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect. Immun. 2010, 78, 4570–4578. [Google Scholar] [CrossRef] [Green Version]
- Suleman, M.; Prysliak, T.; Clarke, K.; Burrage, P.; Windeyer, C.; Perez-Casal, J. Mycoplasma bovis isolates recovered from cattle and bison (Bison bison) show differential in vitro effects on PBMC proliferation, alveolar macrophage apoptosis and invasion of epithelial and immune cells. Vet. Microbiol. 2016, 186, 28–36. [Google Scholar] [CrossRef]
- Bannerman, D.D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Underhill, D.M. Toll-like receptors: Networking for success. Eur. J. Immunol. 2003, 33, 1767–1775. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Lien, E.; Ingalls, R.R.; Tuomanen, E.; Dziarski, R.; Golenbock, D. Cutting edge: Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J. Immunol. 1999, 163, 1–5. [Google Scholar] [PubMed]
- Morath, S.; Stadelmaier, A.; Geyer, A.; Schmidt, R.R.; Hartung, T. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 2002, 195, 1635–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, N.W.J.; Morath, S.; Alexander, C.; Hamann, L.; Hartung, T.; Zahringer, U.; Gobel, U.B.; Weber, J.R.; Schumann, R.R. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 2003, 278, 15587–15594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiguchi, M.; Matsumoto, M.; Takao, T.; Hoshino, M.; Shimonishi, Y.; Tsuji, S.; Begum, N.A.; Takeuchi, O.; Akira, S.; Toyoshima, K.; et al. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: Role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 2001, 166, 2610–2616. [Google Scholar] [CrossRef] [Green Version]
- Seya, T.; Matsumoto, M. A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2. Int. J. Biochem. Cell. Biol. 2002, 34, 901–906. [Google Scholar] [CrossRef]
- He, J.; You, X.; Zeng, Y.; Yu, M.; Zuo, L.; Wu, Y. Mycoplasma genitalium-derived lipid-associated membrane proteins activate NF-κB through toll-Like receptors 1, 2, and 6 and CD14 in a MyD88-dependent pathway. Clin. Vaccine Immunol. 2009, 16, 1750–1757. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Li, Y.; Wang, Q.; Shao, J.; Chen, Y.; Xin, J. Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1beta production through the NF-kappaB pathway via toll-like receptor 2 and MyD88. Dev. Comp. Immunol. 2016, 55, 111–118. [Google Scholar] [CrossRef]
- Shimizu, T.; Kida, Y.; Kuwano, K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-κB through TLR1, TLR2, and TLR6. J. Immunol. 2005, 175, 4641–4646. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Kida, Y.; Kuwano, K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology 2007, 121, 473–483. [Google Scholar] [CrossRef]
- Shimizu, T.; Kida, Y.; Kuwano, K. A triacylated lipoprotein from Mycoplasma genitalium activates NF-κB through TLR1 and TLR2. Infect. Immun. 2008, 76, 3672–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Kida, Y.; Kuwano, K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 2008, 76, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Hirschfeld, M.; Ma, Y.; Weis, J.H.; Vogel, S.N.; Weis, J.J. Cutting edge: Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 2000, 165, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Gondaira, S.; Higuchi, H.; Iwano, H.; Nishi, K.; Nebu, T.; Nakajima, K.; Nagahata, H. Innate immune response of bovine mammary epithelial cells to Mycoplasma bovis. J. Vet. Sci. 2018, 19, 79–87. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Lin, C.; Yan, R.; Li, Z.; Chen, Q.; Zhang, H.; Xu, H.; Chen, X.; Chen, Y.; et al. Regularity of Toll-like receptors in bovine mammary epithelial cells Induced by Mycoplasma bovis. Front. Vet. Sci. 2022, 9, 846700. [Google Scholar] [CrossRef]
- Satoh, T.; Akira, S. Toll-like receptor signaling and its inducible proteins. Microbiol. Spectr. 2016, 4, 4–6. [Google Scholar] [CrossRef]
- Brantley, D.M.; Yull, F.E.; Muraoka, R.S.; Hicks, D.J.; Cook, C.M.; Kerr, L.D. Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech. Dev. 2000, 97, 149–155. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Heeley, J.L.; Chapman, R.; Aillet, F.; Hay, R.T.; Wyllie, A.; Watson, C.J. NF-kappaB inhibits apoptosis in murine mammary epithelia. J. Biol. Chem. 2000, 275, 12737–12742. [Google Scholar] [CrossRef] [Green Version]
- Mulongo, M.; Prysliak, T.; Scruten, E.; Napper, S.; Perez-Casal, J. In vitro infection of bovine monocytes with Mycoplasma bovis delays apoptosis and suppresses production of gamma interferon and tumor necrosis factor alpha but not interleukin-10. Infect. Immun. 2014, 82, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, S.; Altun, S. Genome-wide analysis of mRNAs and lncRNAs in Mycoplasma bovis infected and non-infected bovine mammary gland tissues. Mol. Cell. Probes 2020, 50, 101512. [Google Scholar] [CrossRef]
- Elazar, S.; Gonen, E.; Livneh-Kol, A.; Rosenshine, I.; Shpigel, N.Y. Neutrophil recruitment in endotoxin-induced murine mastitis is strictly dependent on mammary alveolar macrophages. Vet. Res. 2010, 41, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elazar, S.; Gonen, E.; Livneh-Kol, A.; Rosenshine, I.; Shpigel, N.Y. Essential role of neutrophils but not mammary alveolar macrophages in a murine model of acute Escherichia coli mastitis. Vet. Res. 2010, 41, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefois, T.; Vernerey, M.S.; Rodrigues, V.; Totte, P.; Puech, C.; Ripoll, C.; Thiaucourt, F.; Manso-Silvan, L. Development of fluorescence expression tools to study host-mycoplasma interactions and validation in two distant mycoplasma clades. J. Biotechnol. 2016, 236, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chopra-Dewasthaly, R.; Marenda, M.; Rosengarten, R.; Jechlinger, W.; Citti, C. Construction of the first shuttle vectors for gene cloning and homologous recombination in Mycoplasma agalactiae. FEMS Microbiol. Lett. 2005, 253, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Chopra-Dewasthaly, R.; Zimmermann, M.; Rosengarten, R.; Citti, C. First steps towards the genetic manipulation of Mycoplasma agalactiae and Mycoplasma bovis using the transposon Tn4001mod. Int. J. Med. Microbiol. 2005, 294, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Talenton, V.; Baby, V.; Gourgues, G.; Mouden, C.; Claverol, S.; Vashee, S.; Blanchard, A.; Labroussaa, F.; Jores, J.; Arfi, Y.; et al. Genome engineering of the fast-growing Mycoplasma feriruminatoris toward a live vaccine chassis. ACS Synth. Biol. 2022, 11, 1919–1930. [Google Scholar] [CrossRef]
- Ipoutcha, T.; Rideau, F.; Gourgues, G.; Arfi, Y.; Lartigue, C.; Blanchard, A.; Sirand-Pugnet, P. Genome editing of veterinary relevant mycoplasmas using a CRISPR-Cas base editor system. Appl. Environ. Microbiol. 2022, 88, e0099622. [Google Scholar] [CrossRef]
- Yair, Y.; Borovok, I.; Mikula, I.; Falk, R.; Fox, L.K.; Gophna, U.; Lysnyansky, I. Genomics-based epidemiology of bovine Mycoplasma bovis strains in Israel. BMC Genom. 2020, 21, 70. [Google Scholar] [CrossRef]
- Register, K.B.; Lysnyansky, I.; Jelinski, M.D.; Boatwright, W.D.; Waldner, M.; Bayles, D.O.; Pilo, P.; Alt, D.P. Comparison of two multilocus sequence typing schemes for Mycoplasma bovis and revision of the PubMLST reference method. J. Clin. Microbiol. 2020, 58, e00283-20. [Google Scholar] [CrossRef]
- Friis, N.F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare. Nord. Vet. 1975, 27, 333–339. [Google Scholar]
- Rodwell, A.W.; Whitcomb, R.F. Methods for direct and indirect measurement of mycoplasma growth. In Methods in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: New York, NY, USA, 1983; Volume 1, pp. 185–196. [Google Scholar]
- Mintz, D.; Salamon, H.; Mintz, M.; Rosenshine, I.; Shpigel, N.Y. Intraepithelial neutrophils in mammary, urinary and gall bladder infections. Vet. Res. 2019, 50, 56. [Google Scholar] [CrossRef] [PubMed]
- Salamon, H.; Nissim-Eliraz, E.; Ardronai, O.; Nissan, I.; Shpigel, N.Y. The role of O-polysaccharide chain and complement resistance of Escherichia coli in mammary virulence. Vet. Res. 2020, 51, 77. [Google Scholar] [CrossRef] [PubMed]
- Lauerman, L.H. Nucleic Acid Amplification Assays for Diagnosis of Animal Diseases; American Association of Veterinary Laboratory Diagnosticians: Turlock, CA, USA, 1998. [Google Scholar]
- Gonen, E.; Vallon-Eberhard, A.; Elazar, S.; Harmelin, A.; Brenner, O.; Rosenshine, I.; Jung, S.; Shpigel, N.Y. Toll-like receptor 4 is needed to restrict the invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine model of acute mastitis. Cell. Microbiol. 2007, 9, 2826–2838. [Google Scholar] [CrossRef]
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissim-Eliraz, E.; Nir, E.; Marsiano, N.; Yagel, S.; Shpigel, N.Y. NF-kappa-B activation unveils the presence of inflammatory hotspots in human gut xenografts. PLoS ONE 2021, 16, e0243010. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Christodoulides, A.; Gupta, N.; Yacoubian, V.; Maithel, N.; Parker, J.; Kelesidis, T. The role of lipoproteins in mycoplasma-mediated immunomodulation. Front. Microbiol. 2018, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- Browning, G.F.; Marenda, M.S.; Noormohammadi, A.H.; Markham, P.F. The central role of lipoproteins in the pathogenesis of mycoplasmoses. Vet. Microbiol. 2011, 153, 44–50. [Google Scholar] [CrossRef]
- Rainard, P.; Gilbert, F.B.; Germon, P. Immune defenses of the mammary gland epithelium of dairy ruminants. Front. Immunol. 2022, 13, 6294. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Maina, T.; Prysliak, T.; Perez-Casal, J. Mycoplasma bovis delay in apoptosis of macrophages is accompanied by increased expression of anti-apoptotic genes, reduced cytochrome C translocation and inhibition of DNA fragmentation. Vet. Immunol. Immunopathol. 2019, 208, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, C.; Pilo, P.; Frey, J.; Bruckmaier, R.M.; Wellnitz, O. The immune response of bovine mammary epithelial cells to live or heat-inactivated Mycoplasma bovis. Vet. Microbiol. 2015, 179, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 years of NF-kappaB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V. Neutrophils stepping through (to the other side). Immunity 2018, 49, 992–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürki, S.; Frey, J.; Pilo, P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 2015, 179, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, C.J.; Anderson, J.C.; Gourlay, R.N.; Taylor-Robinson, D. Production of mastitis in mice with human and bovine ureaplasmas (T-mycoplasmas). J. Med. Microbiol. 1975, 8, 523–529. [Google Scholar] [CrossRef]
- Anderson, J.C.; Howard, C.J.; Gourlay, R.N. Experimental mycoplasma mastitis in mice. Infect. Immun. 1976, 13, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Kauf, A.C.W.; Rosenbusch, R.F.; Paape, M.J.; Bannerman, D.D. Innate immune response to intramammary Mycoplasma bovis infection. J. Dairy Sci. 2007, 90, 3336–3348. [Google Scholar] [CrossRef] [Green Version]
- Jasper, D.E.; Jain, N.C.; Brazil, L.H. Clinical and laboratory observations on bovine mastitis due to mycoplasma. J. Am. Vet. Med. Assoc. 1966, 148, 1017–1029. [Google Scholar]
- Parker, A.M.; House, J.K.; Hazelton, M.S.; Bosward, K.L.; Mohler, V.L.; Maunsell, F.P.; Sheehy, P.A. Milk acidification to control the growth of Mycoplasma bovis and Salmonella Dublin in contaminated milk. J. Dairy Sci. 2016, 99, 9875–9884. [Google Scholar] [CrossRef] [Green Version]
- Byrne, W.; Markey, B.; McCormack, R.; Egan, J.; Ball, H.; Sachse, K. Persistence of Mycoplasma bovis infection in the mammary glands of lactating cows inoculated experimentally. Vet. Rec. 2005, 156, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Gondaira, S.; Fujiki, J.; Katagata, M.; Sawada, C.; Eguchi, A.; Iwasaki, T.; Iwano, H.; Higuchi, H. Invasion of Mycoplasma bovis into bovine synovial cells utilizing the clathrin-dependent endocytosis pathway. Vet. Microbiol. 2021, 253, 108956. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, F.; Ogawa, M.; Nakura, Y.; Hamada, Y.; Nakayama, M.; Mitobe, J.; Hiraide, A.; Sakai, N.; Takeuchi, M.; Yoshimori, T.; et al. Intracellular fate of Ureaplasma parvum entrapped by host cellular autophagy. Microbiologyopen 2017, 6, e00441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, B.B.A.; Turnbull, L.; Jenkins, C.; Madhkoor, R.; Schleicher, I.; Uphoff, C.C.; Whitchurch, C.B.; Rohde, M.; Djordjevic, S.P. Mycoplasma hyopneumoniae resides intracellularly within porcine epithelial cells. Sci. Rep. 2018, 8, 17697. [Google Scholar] [CrossRef] [Green Version]
- Brouillette, E.; Malouin, F. The pathogenesis and control of Staphylococcus aureus-induced mastitis: Study models in the mouse. Microbes Infect. 2005, 7, 560–568. [Google Scholar] [CrossRef]
- Notebaert, S.; Meyer, E. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Q. 2006, 28, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Ingman, W.V.; Glynn, D.J.; Hutchinson, M.R. Mouse models of mastitis—How physiological are they? Int. Breastfeed. J. 2015, 10, 12. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, P.; Brill, R.; Schouten, I.; Nissim-Eliraz, E.; Lysnyansky, I.; Shpigel, N.Y. Lipoproteins Are Potent Activators of Nuclear Factor Kappa B in Mammary Epithelial Cells and Virulence Factors in Mycoplasma bovis Mastitis. Microorganisms 2022, 10, 2209. https://doi.org/10.3390/microorganisms10112209
Schneider P, Brill R, Schouten I, Nissim-Eliraz E, Lysnyansky I, Shpigel NY. Lipoproteins Are Potent Activators of Nuclear Factor Kappa B in Mammary Epithelial Cells and Virulence Factors in Mycoplasma bovis Mastitis. Microorganisms. 2022; 10(11):2209. https://doi.org/10.3390/microorganisms10112209
Chicago/Turabian StyleSchneider, Peleg, Re’ella Brill, Iftach Schouten, Einat Nissim-Eliraz, Inna Lysnyansky, and Nahum Yehuda Shpigel. 2022. "Lipoproteins Are Potent Activators of Nuclear Factor Kappa B in Mammary Epithelial Cells and Virulence Factors in Mycoplasma bovis Mastitis" Microorganisms 10, no. 11: 2209. https://doi.org/10.3390/microorganisms10112209




