Non-Dairy Fermented Beverages Produced with Functional Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Safety Evaluation of the Bacterial Strains Used as Inoculum
Detection of Virulence Genes
2.3. Genetic Screening of LAB Functional Properties
2.4. Fermentation of Wheat Bran and Root Vegetables with Selected LAB Strains
2.5. Microbiological Analyses
2.6. Physical, Chemical, and Nutritional Characteristics
2.7. Functional Properties
2.8. Statistical Analysis
3. Results
3.1. Safety Evaluation of the Bacterial Strains Used as Inoculum
3.2. Genetic Screening of LAB Functional Properties
3.3. Fermentation of Wheat Bran and Root Vegetables with Selected LAB Strains
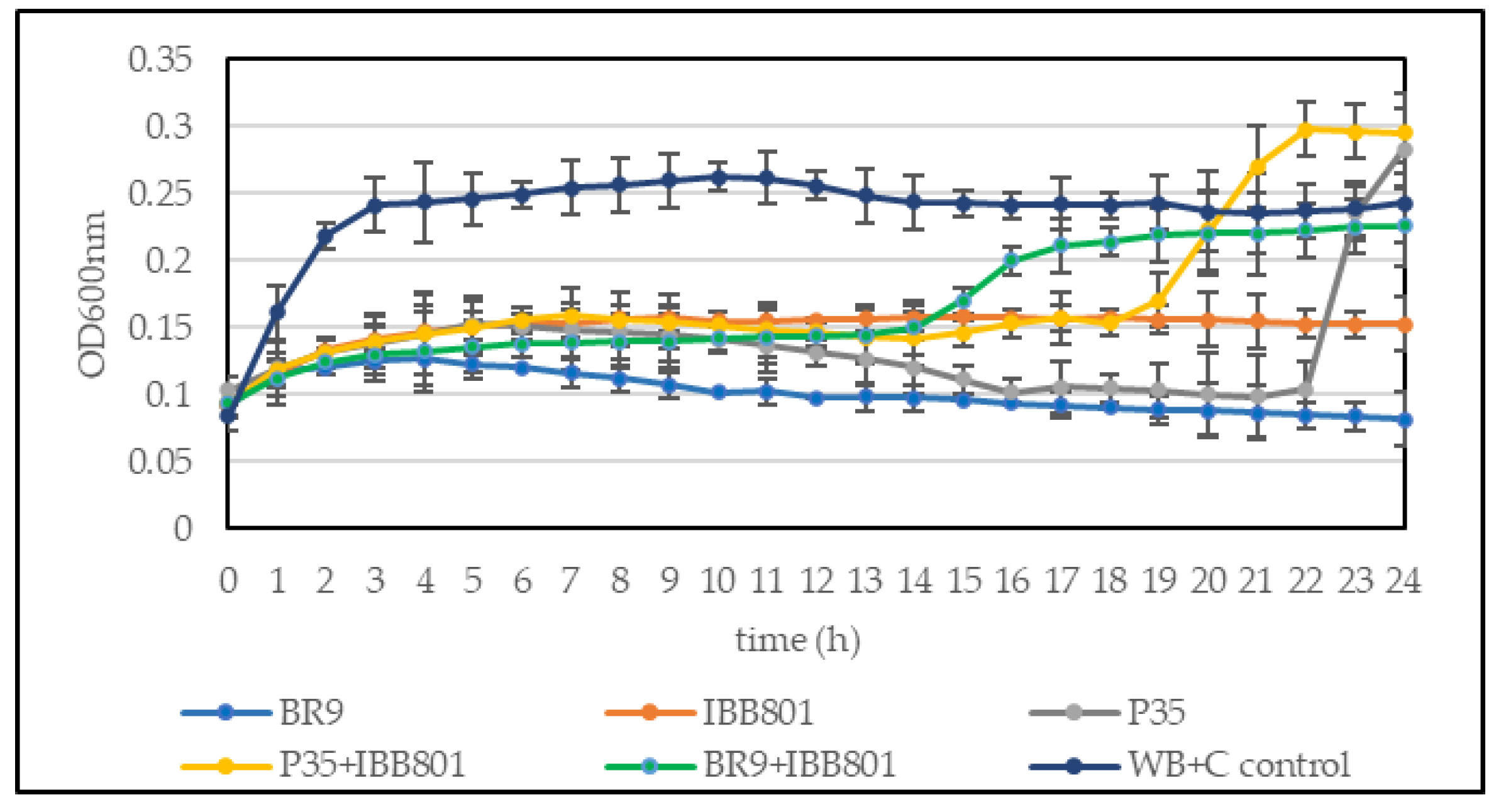
3.3.1. Microbiological Analysis
3.3.2. Physical, Chemical, and Nutritional Characteristics
PH Value
Lactic Acid Production
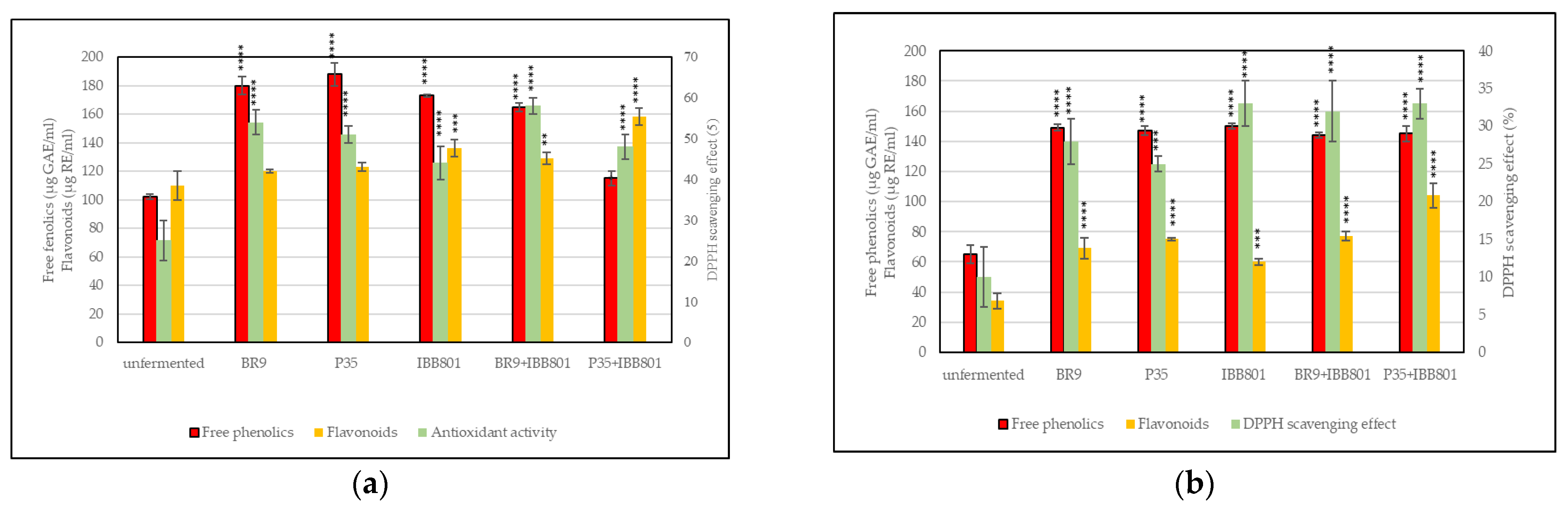
Free Phenolics
Flavonoids Content
Antioxidant Activity
3.3.3. Functional Properties
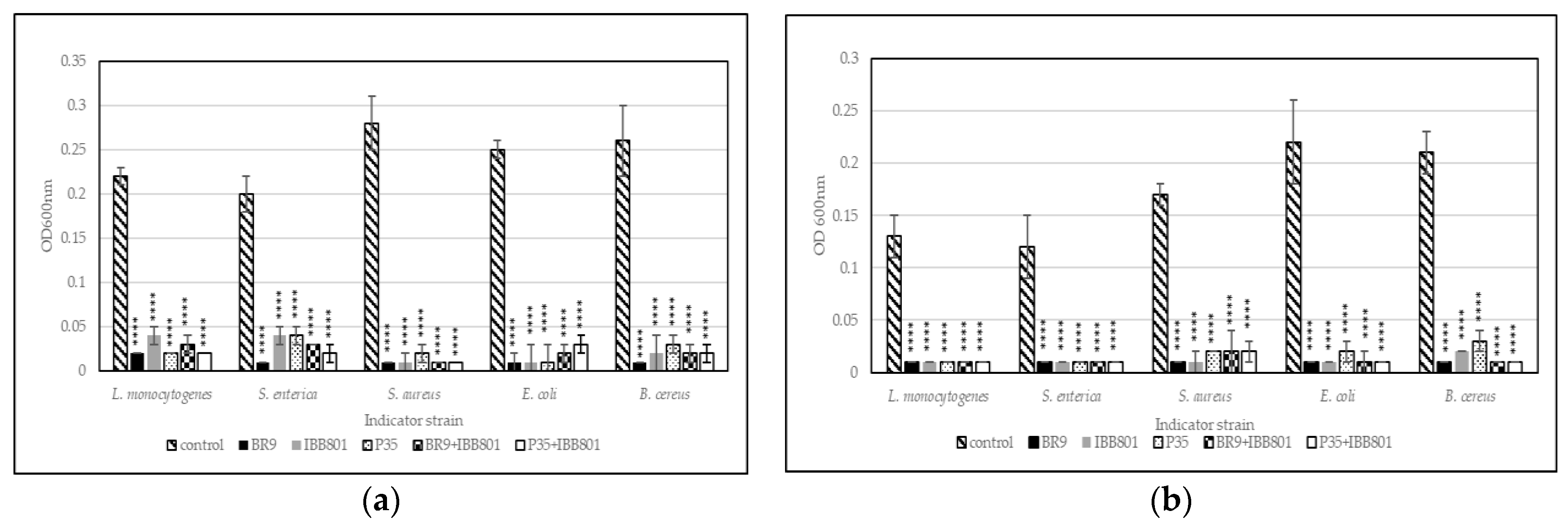
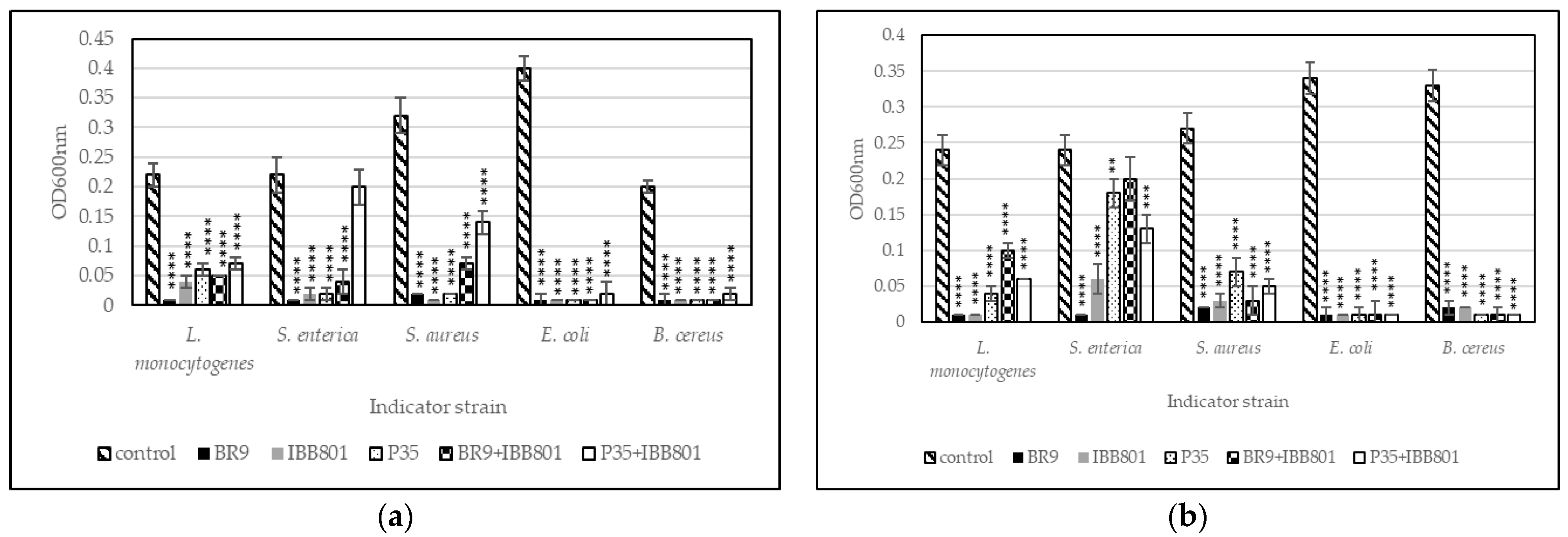
Antibacterial Activity
Prevention of Pathogens Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Dehouegnon, J.A.; Yann, M.; Oyeniran, B.A.; Malthus, D.A.; Ifagbémi, B.C.; Yénoukounmè, E.K.; Paulin, A.; Ilkay, K. Traditional fermented foods and beverages: Indigenous practices of food processing in Benin Republic. Int. J. Gastron. Food Sci. 2022, 27, 100450. [Google Scholar] [CrossRef]
- Cordeiro, M.A.; Souza, E.L.S.; Arantes, R.M.E.; Balthazar, C.F.; Guimaraes, J.T.; Scudino, H.; Silva, H.; Rocha, R.; Freitas, M.; Esmerino, E.; et al. Fermented whey dairy beverage offers protection against Salmonella enterica ssp. enterica serovar typhimurium infection in mice. J. Dairy Sci. 2019, 102, 6756–6765. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Izzo, L.; Graziani, G.; Gaspari, A.; Ritieni, A.; Manes, J.; Meca, G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct. 2018, 9, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Lauridsen, C.; Jensen, B.B. Gastrointestinal ecosystem and immunological responses in E. coli challenged pigs after weaning fed liquid diets containing whey permeate fermented with different lactic acid bacteria. Anim. Feed Sci. Technol. 2015, 207, 278–282. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Pan, D.D.; Wu, Z.; Sun, Y.Y.; Guo, Y.X.; Zeng, X.Q. Antialcoholic liver activity of whey fermented by Lactobacillus casei isolated from koumiss. J. Dairy Sci. 2014, 97, 4062–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, G.; Agosto, M.E.; Cavaglieri, L.; Dogi, C. Effect of fermented whey with a probiotic bacterium on gut immune system. J. Dairy Res. 2020, 87, 134–137. [Google Scholar] [CrossRef]
- Ramos, C.L.; Schwan, R.F. Technological and nutritional aspects of indigenous Latin America fermented foods. Curr. Opin. Food Sci. 2017, 13, 97–102. [Google Scholar] [CrossRef]
- Serafini, M.; Stanzione, A.; Foddai, S. Functional foods: Traditional use and European legislation. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 7–9. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and functional food as future food: A review. Der. Pharmacia. Lett. 2010, 2, 106–116. [Google Scholar]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Pázmándi, M.; Kovács, Z.; Maráz, A. Potential of Lactobacillus strains for the production of fermented functional beverages enriched in galacto-oligosaccharides. LWT Food Sci. Technol. 2021, 143, 111097. [Google Scholar] [CrossRef]
- Iqbal, J.; Yu, D.; Zubair, M.; Rasheed, M.I.; Khizar, H.M.U.; Imran, M. Health Consciousness, Food Safety Concern, and Consumer Purchase Intentions Toward Organic Food: The Role of Consumer Involvement and Ecological Motives. SAGE Open 2021, 11, 21582440211015727. [Google Scholar] [CrossRef]
- Kausar, H.; Saeed, S.; Mushtaq Ahmad, M.; Salam, A. Studies on the development and storage stability of cucumber-melon functional drink. J. Agric. Res. 2012, 50, 239–248. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Hui, Y.H.; Evranuz, E.Ö. Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Saarela, M. Probiotics as ingredients in functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 55–70. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Stefan, I.R.; Stancu, M.M.; Cornea, C.P.; De Vuyst, L.; Zamfir, M. Microbial and nutritional characteristics of fermented wheat bran in traditional Romanian borş production. Rom. Biotechnol. Lett. 2019, 24, 440–447. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Ciz, M.; Cizova, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Singh, A.; Garg, V.K.; Sharma, P.K.; Gupta, S. Wound healing activity of ethanolic extract of Beta vulgaris. Pharmacologyonline 2011, 1, 1031–1038. [Google Scholar] [CrossRef]
- Jain, S.; Garg, V.K.; Sharma, P.K. Anti-inflammatory activity of aqueous extract of Beta vulgaris L. J. Basic Clin. Pharm. 2011, 2, 83–86. [Google Scholar] [PubMed]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Zamfir, M.; Callewaert, R.; Cornea, C.P.; Savu, L.; Vatafu, I.; De Vuyst, L. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB801. J. Appl. Microbiol. 1999, 87, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Angelescu, I.R.; Zamfir, M.; Stancu, M.M.; Grosu-Tudor, S.S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann. Microbiol. 2019, 69, 1557–1565. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Zamfir, M.; Van der Meulen, R.; De Vuyst, L. Isolation of novel homopolysaccharide-producing lactic acid bacteria from Romanian raw milk and fermented dairy products. Eur. Food Res. Technol. 2013, 237, 609–615. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Zamfir, M. Functional properties of lactic acid bacteria isolated from Romanian fermented vegetables. Food Biotechnol. 2013, 27, 235–248. [Google Scholar] [CrossRef]
- Cornea, C.P.; Israel Roming, F.; Sicuia, O.A.; Voaideș, C.; Zamfir, M.; Grosu-Tudor, S.S. Biosurfactant production by Lactobacillus spp. strains isolated from Romanian traditional fermented food products. Rom. Biotechnol. Lett. 2016, 21, 11312–11320. [Google Scholar]
- Pieniz, S.; de Moura, T.M.; Cassenego, A.P.V.; Andreazza, R.; Frazzon, A.P.G.; de Oliveira Camargo, F.A.; Brandelli, A. Evaluation of resistance genes and virulence factors in a food isolated Enterococcus durans with potential probiotic effect. Food Control 2015, 2, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Turpin, W.; Humblot, C.; Guyot, J.P. Genetic Screening of Functional Properties of Lactic Acid Bacteria in a Fermented Pearl Millet Slurry and in the Metagenome of Fermented Starchy Foods. Appl. Environ. Microbiol. 2011, 77, 8722–8734. [Google Scholar] [CrossRef] [Green Version]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Moon, J.H.; Terao, J. Antioxidant effect of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Kumari, S.; Deori, M.; Elancheran, R.; Kotoky, J.; Devi, R. In vitro and In vivo Antioxidant, Anti-hyperlipidemic Properties and Chemical Characterization of Centella asiatica (L.) Extract. Front. Pharmacol. 2016, 7, 400. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2016.00400 (accessed on 8 October 2022). [CrossRef] [Green Version]
- De Vuyst, L.; Callewaert, R.; Pot, B. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst. Appl. Microbiol. 1996, 19, 9–20. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.L.; Liu, C.L.; Yin, T.T.; Yan, X.T.; Wu, C.; He, H.; Yi, C.P. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Klewicka, E.; Motyl, I.; Libudzisz, Z. Fermentation of beet juice by bacteria of genus Lactobacillus sp. Eur. Food Res. Technol. 2004, 218, 178–183. [Google Scholar] [CrossRef]
- Hattingh, M.; Alexander, A.; Meijering, I.; Van Reenen, C.A.; Dicks, L.M.T. Amylolytic strains of Lactobacillus plantarum isolated from barley. Afr. J. Biotechnol. 2015, 14, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Krungleviciute, V.; Zelvyte, R.; Monkeviciene, I.; Kantautaite, J.; Stankevicius, R.; Ruzauskas, M.; Bartkiene, E. Applicability of Pediococcus strains for fermentation of cereal bran and its influence on the milk yield of dairy cattle. Zemdirb. -Agric. 2017, 104, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Avila, C.L.S.; Carvalho, B.F. Silage fermentation-Updates focusing on the performance of microorganisms. J. Appl. Microbiol. 2019, 128, 966–984. [Google Scholar] [CrossRef] [Green Version]
- Keddari, S.; Aldib, I.; Riazi, A. In vivo stimulatory effects of wheat bran on intestinal microbial ecosystem of mice. South Asian J. Exp. Biol. 2014, 4, 24–32. [Google Scholar] [CrossRef]
- Shumoy, H.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and bound phenolic contents and antioxidant capacity of tef injera as affected by traditional fermentation. J. Food Compos. Anal. 2017, 58, 52–59. [Google Scholar] [CrossRef]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen Guido, R.M.M. Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects ex Vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, W.; Zou, J.; Zhou, H.; Liu, C.; Yang, H. Flavor and antioxidant activity improvement of carrot juice by fermentation with Lactobacillus plantarum WZ-01. Food Meas. 2019, 13, 3366–3375. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Loscos, J.; Dietz, K.J.; Aparicio-Tejo, P.M.; Becana, M. Function of antioxidant enzymes and metabolites during maturation of pea fruits. J. Exp. Bot. 2010, 61, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosanic, M.; Rankovic, B.; Vukojevic, J. Antioxidant properties of some lichen species. J. Food Sci. Technol. 2011, 48, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
| Strain | Isolation Source | Properties | References |
|---|---|---|---|
| Lactobacillus acidophilus IBB801 | yogurt | antibacterial activity, bacteriocin production | [32] |
| Lactiplantibacillus plantarum BR9 | braga | antibacterial activity, probiotic potential, antifungal activity 1 | [33] |
| Lactiplantibacillus plantarum CR1 | water kefir | antibacterial activity, probiotic potential, antifungal activity 1 | [33] |
| Leuconostoc mesenteroides 21.2 | milk | exopolysaccharide production | [34] |
| Leuconostoc citreum 52 | fermented vegetables | exopolysaccharide production | [35] |
| Lactiplantibacillus plantarum P35 | bors | antifungal activity, surfactant production, antibacterial activity 1 | [36] |
| Lactiplantibacillus plantarum P26 | bors | antifungal activity, surfactant production | [36] |
| General Function | Gene | Predicted Function | Nucleotide Sequence | Melting Temperature (°C) | Expected Amplicon Size (bp) | References |
|---|---|---|---|---|---|---|
| Survival at low pH | LBA1272 | Cyclopropane FA synthase | f: GGCCGGTGTTCCACTAGTCC r: ACGTTGGGTCGATTTGACGA | 60 | 203 pb | [38] |
| dltD | D-alanine transfer protein | f: TTCGCCTGTTCAAGCCACAT r: ACGTGCCCTTCTTTGGTTCC | 283 pb | |||
| Folate synthesis | folP | Dihydropteroate synthase/dihydropteroate pyrophosphorylase | f: CCASGRCSGCTTGCATGAC r: TKACGCCGGACTCCTTTTWY | 61 | 261 pb | |
| folK | 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase | f: CCATTTCCAGGTGGGGAATC r: GGGGTGGTCCAAGCAAACTT | 214 pb | |||
| Starch metabolism | agl | α -Glucosidase | f: GCSAAAATGCTAGCGACYMT r: CCACTGCATYGGYGTACGY | 62 | 236 pb | |
| α-amy | α-amilase | f: AGATCAGGCGCAAGTTCAGT r: TTTTATGGGCACACCACTCA | 220 pb | |||
| malL | Oligo-1,6-glucosidase | f: TTGCCTAACAACTGGGGTTC r: ATCAACGCCTTTGTTCAACC | 177 pb | |||
| Riboflavin synthesis | ribA | 3,4-dihydroxy-2-butanone 4-phosphate synthase/GTP cyclohydrolase II | f: TTTACGGGCGATGTTTTAGG r: CGACCCTCTTGCCGTAAATA | 62 | 121 pb | |
| Virulence | ace | adhesion collagen protein | f: AAAGTAGAATTAGATCACAC r: TCTATCACATTCGGTTGCG | 45 | 320 pb | [37] |
| agg | aggregation | f: AAGAAAAAGTAGACCAAC r: AACGGCAAGACAAGTAAATA | 44 | 1553 pb | ||
| asa | aggregation | f: GATACAAAGCCAATGTGGTTCCT r: TAAAGAGTCGCCACGTTTCACA | 56 | 101 pb |
| Substrate Code | Substrate Composition 1 |
|---|---|
| 1 | 5% WB + 5% BR |
| 2 | 5% WB + 5% C |
| 3 | 5% WB + 10% BR |
| 4 | 5% WB + 10% C |
| 5 | 10% WB + 5% BR |
| 6 | 10% WB + 5% C |
| 7 | 10% WB + 10% BR |
| 8 | 10% WB + 10% C |
| 9 | 5% WB + 5% BR + 5% C |
| 10 | 10% WB + 5% BR + 10% C |
| Strain | Gene | |||||||
|---|---|---|---|---|---|---|---|---|
| LBA1272 | dltD | folP | folK | agl | α-amy | malL | ribA | |
| Lb. plantarum P26 | + | − | + | − | + | + | − | + |
| Lb. plantarum P35 | + | − | + | − | + | + | − | + |
| Lb. plantarum BR9 | + | − | + | − | + | + | + | + |
| Lb. plantarum CR1 | + | − | + | − | + | + | + | + |
| Leuc. citreum 52 | + | − | + | − | + | + | + | + |
| Lb. acidophilus IBB801 | − | − | − | − | + | − | − | − |
| Leuc. mesenteroides 21.2 | + | − | + | − | + | + | − | − |
| Inoculum | Substrate | Sampling Time (h) | pH | Lactic Acid (mg/mL) | log CFU/ml | Total Free Phenolics | Flavonoids (µg RE/mL) | DPPH Scavenging Effect (%) | Inhibitory Activity (AU/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| MRS | VRBG | (µg GAE/mL) | ||||||||
| L. plantarum BR9 | 5% WB + 10% BR | 0 | 6.3 ± 0.1 | nd | 6.0 ± 0 | nd | 121 ± 10 | 110 ± 22 | 33 ± 2 | nd |
| 3 | 6.0 ± 0.1 | nd | 6.8 ± 0.2 | nd | 132 ± 2 | 110 ± 7 | 40 ± 3 | nd | ||
| 6 | 5.0 ± 0.3 | 1.2 ± 0.3 | 7.8 ± 0.2 | 1.2 ± 0.6 | 148 ± 8 | 109 ± 12 | 50 ± 2 | nd | ||
| 9 | 3.8 ± 0.1 | 4.5 ± 0.1 | 8.3 ± 0.2 | nd | 158 ± 4 | 102 ± 9 | 51 ± 4 | nd | ||
| 12 | 3.5 ± 0.1 | 7.0 ± 0.3 | 8.7 ± 0.3 | nd | 161 ± 1 | 101 ± 6 | 50 ± 1 | nd | ||
| 24 | 3.3 ± 0 | 11.0 ± 0.2 | 9.1 ± 0.2 | nd | 180 ± 6 | 120 ± 1 | 54 ± 2 | nd | ||
| 1week | - | - | 8.6 ± 0.3 | nd | - | - | - | - | ||
| 5% WB + 10% C | 0 | 6.3 ± 0.2 | nd | 5.9 ± 0.1 | 0.6 ± 0.1 | 81 ± 1 | 44 ± 2 | 35 ± 5 | nd | |
| 3 | 6.0 ± 0 | nd | 6.6 ± 0.1 | 1.3 ± 0.1 | 92 ± 3 | 52 ± 6 | 29 ± 3 | nd | ||
| 6 | 5.1 ± 0.3 | nd | 7.4 ± 0.4 | 1.8 ± 0.2 | 100 ± 3 | 49 ± 5 | 27 ± 4 | nd | ||
| 9 | 3.8 ± 0.2 | 4.4 ± 0.2 | 8.4 ± 0.3 | 0.8 ± 0.1 | 114 ± 2 | 47 ± 1 | 22 ± 2 | nd | ||
| 12 | 3.6 ± 0.1 | 6.3 ± 0.1 | 8.8 ± 0.4 | nd | 119 ± 0 | 59 ± 5 | 23 ± 2 | nd | ||
| 24 | 3.3 ± 0 | 11.1 ± 0.1 | 8.9 ± 0.4 | nd | 149 ± 2 | 69 ± 7 | 28 ± 3 | nd | ||
| 1 week | - | - | 7.5 ± 0.2 | nd | - | - | - | - | ||
| L. plantarum P35 | 5% WB + 10% BR | 0 | 6.1 ± 0 | nd | 7.4 ± 0.2 | nd | 128 ± 7 | 116 ± 8 | 41 ± 1 | nd |
| 3 | 4.9 ± 0.1 | nd | 7.7 ± 0.1 | nd | 148 ± 10 | 123 ± 8 | 50 ± 5 | 100 | ||
| 6 | 3.9 ± 0 | 0.8 ± 0.1 | 8.4 ± 0.5 | nd | 152 ± 10 | 110 ± 9 | 48 ± 3 | 100 | ||
| 9 | 3.5 ± 0.1 | 3.8 ± 0.4 | 8.6 ± 0.3 | nd | 155 ± 10 | 117 ± 2 | 48 ± 6 | 100 | ||
| 12 | 3.4 ± 0 | 5.5 ± 0.5 | 8.8 ± 0.1 | nd | 156 ± 9 | 114 ± 3 | 49 ± 2 | 100 | ||
| 24 | 3.2 ± 0.1 | 12.1 ± 0.1 | 8.7 ± 0.2 | nd | 188 ± 8 | 123 ± 3 | 51 ± 1 | 100 | ||
| 1 week | - | - | 8.8 ± 0.1 | nd | - | - | - | - | ||
| 5% WB + 10% C | 0 | 6.0 ± 0 | nd | 8.2 ± 0.2 | nd | 84 ± 6 | 35 ± 1 | 20 ± 4 | nd | |
| 3 | 4.7 ± 0.1 | nd | 8.6 ± 0.1 | nd | 95 ± 1 | 36 ± 1 | 18 ± 1 | nd | ||
| 6 | 3.8 ± 0 | 3.7 ± 0.5 | 8.9 ± 0.5 | nd | 107 ± 2 | 46 ± 2 | 19 ± 3 | 100 | ||
| 9 | 3.5 ± 0.1 | 6.7 ± 0.5 | 9.6 ± 0.9 | nd | 114 ± 3 | 53 ± 7 | 23 ± 2 | 100 | ||
| 12 | 3.3 ± 0.1 | 8.8 ± 0.5 | 9.8 ± 1.0 | nd | 123 ± 5 | 58 ± 7 | 22 ± 3 | 100 | ||
| 24 | 3.2 ± 0 | 15.0 ± 1.8 | 9.8 ± 1.1 | nd | 147 ± 3 | 75 ± 1 | 25 ± 1 | 100 | ||
| 1 week | - | - | 8.9 ± 0.4 | nd | - | - | - | - | ||
| L. acidophilus IBB801 | 5% WB + 10% BR | 0 | 6.2 ± 0.1 | nd | 6.9 ± 0.3 | nd | 112 ± 2 | 83 ± 3 | 33 ± 3 | nd |
| 3 | 5.6 ± 0.1 | nd | 7.0 ± 0.1 | nd | 131 ± 1 | 92 ± 4 | 39 ± 6 | nd | ||
| 6 | 4.9 ± 0 | 1.5 ± 0.2 | 7.7 ± 0.1 | 2.4 ± 0.2 | 138 ± 4 | 83 ± 1 | 40 ± 2 | nd | ||
| 9 | 4.1 ± 0.1 | 3.0 ± 0.4 | 7.9 ± 0.2 | 2.5 ± 0.3 | 146 ± 5 | 95 ± 5 | 43 ± 4 | nd | ||
| 12 | 3.9 ± 0.1 | 4.2 ± 0.2 | 8.0 ± 0.1 | nd | 160 ± 7 | 103 ± 2 | 42 ± 1 | 100 | ||
| 24 | 3.6 ± 0.1 | 7.4 ± 1.1 | 8.1 ± 0.2 | nd | 173 ± 1 | 136 ± 6 | 44 ± 3 | 100 | ||
| 1 week | - | - | 8.2 ± 0.3 | nd | - | - | - | - | ||
| 5% WB + 10% C | 0 | 6.1 ± 0 | nd | 6.9 ± 0.1 | nd | 68 ± 1 | 40 ± 3 | 32 ± 3 | nd | |
| 3 | 5.5 ± 0.1 | nd | 7.0 ± 0.2 | nd | 88 ± 3 | 41 ± 1 | 25 ± 1 | nd | ||
| 6 | 4.7 ± 0.2 | 1.9 ± 0.1 | 7.6 ± 0.1 | 2.0 ± 0.1 | 92 ± 4 | 39 ± 2 | 18 ± 2 | nd | ||
| 9 | 4.1 ± 0.1 | 3.3 ± 0.3 | 7.9 ± 0.3 | 2.3 ± 0.2 | 105 ± 6 | 47 ± 1 | 18 ± 3 | 100 | ||
| 12 | 3.8 ± 0 | 4.2 ± 0.4 | 8.0 ± 0.1 | 2.8 ± 0.2 | 123 ± 2 | 44 ± 3 | 20 ± 1 | 200 | ||
| 24 | 3.4 ± 0 | 8.0 ± 0.9 | 8.1 ± 0.1 | nd | 150 ± 2 | 60 ± 2 | 33 ± 1 | 100 | ||
| 1 week | - | - | 8.4 ± 0.4 | nd | - | - | - | - | ||
| L. plantarum BR9 + L. acidophilus IBB801 | 5% WB + 10% BR | 0 | 6.2 ± 0.1 | nd | 7.0 ± 0.1 | nd | 114 ± 4 | 95 ± 2 | 46 ± 5 | nd |
| 3 | 5.9 ± 0 | nd | 7.0 ± 0.1 | nd | 128 ± 3 | 111 ± 1 | 52 ± 2 | nd | ||
| 6 | 5.2 ± 0.2 | 1.5 ± 0.2 | 7.6 ± 0.3 | 1.3 ± 0.1 | 134 ± 4 | 96 ± 4 | 51 ± 2 | nd | ||
| 9 | 4.3 ± 0.1 | 3.3 ± 0.4 | 7.9 ± 0.3 | nd | 141 ± 1 | 122 ± 2 | 54 ± 6 | nd | ||
| 12 | 3.9 ± 0.1 | 5.0 ± 0.5 | 8.2 ± 0.2 | nd | 148 ± 6 | 98 ± 5 | 53 ± 2 | 100 | ||
| 24 | 3.4 ± 0 | 11.2 ± 1.2 | 8.3 ± 0.1 | nd | 165 ± 2 | 129 ± 4 | 58 ± 4 | 100 | ||
| 1 week | - | - | 8.3 ± 0.2 | nd | - | - | - | - | ||
| 5% WB + 10% C | 0 | 6.1 ± 0 | nd | 6.7 ± 0.2 | nd | 81 ± 1 | 53 ± 2 | 34 ± 5 | nd | |
| 3 | 5.6 ± 0.1 | nd | 6.8 ± 0.1 | nd | 89 ± 2 | 49 ± 2 | 32 ± 2 | nd | ||
| 6 | 4.8 ± 0 | 1.9 ± 0.1 | 7.5 ± 0.3 | nd | 96 ± 6 | 40 ± 1 | 26 ± 3 | 100 | ||
| 9 | 3.8 ± 0.2 | 4.8 ± 0.5 | 8.0 ± 0.1 | nd | 115 ± 5 | 62 ± 5 | 32 ± 4 | 200 | ||
| 12 | 3.6 ± 0.1 | 6.8 ± 0.8 | 8.2 ± 0.1 | nd | 117 ± 3 | 58 ± 4 | 33 ± 3 | 200 | ||
| 24 | 3.2 ± 0.2 | 11.5 ± 1.5 | 8.6 ± 0.4 | nd | 144 ± 4 | 77 ± 3 | 32 ± 2 | 100 | ||
| 1 week | - | - | 8.2 ± 0.5 | nd | - | - | - | - | ||
| L. plantarum P35 + L. acidophilus IBB801 | 5% WB + 10% BR | 0 | 6.1 ± 0.2 | nd | 7.3 ± 0.3 | nd | 119 ± 2 | 92 ± 2 | 53 ± 5 | nd |
| 3 | 5.7 ± 0.1 | nd | 7.3 ± 0.2 | nd | 130 ± 4 | 94 ± 3 | 49 ± 5 | nd | ||
| 6 | 4.8 ± 0 | 1.8 ± 0.1 | 7.8 ± 0.2 | nd | 135 ± 5 | 100 ± 8 | 53 ± 2 | nd | ||
| 9 | 3.8 ± 0 | 4.5 ± 0.4 | 8.4 ± 0.1 | nd | 135 ± 3 | 126 ± 6 | 49 ± 6 | 100 | ||
| 12 | 3.6 ± 0.1 | 7.2 ± 0.5 | 8.6 ± 0.1 | nd | 137± 3 | 105 ± 4 | 51 ± 2 | 100 | ||
| 24 | 3.2 ± 0.1 | 12.3 ± 0.7 | 8.7 ± 0.3 | nd | 158 ± 2 | 115 ± 5 | 48 ± 4 | 100 | ||
| 1 week | - | - | 8.9 ± 0.6 | nd | - | - | - | - | ||
| 5% WB + 10% C | 0 | 6.1 ± 0.1 | nd | 7.3 ± 0.1 | nd | 83 ± 3 | 42 ± 3 | 32 ± 2 | nd | |
| 3 | 5.1 ± 0.2 | nd | 7.4 ± 0.1 | nd | 91 ± 1 | 56 ± 5 | 27 ± 3 | 100 | ||
| 6 | 3.9 ± 0.1 | 3.6 ± 0.3 | 8.4 ± 0.4 | nd | 103 ± 5 | 46 ± 4 | 29 ± 3 | 200 | ||
| 9 | 3.5 ± 0.1 | 7.3 ± 0.5 | 8.7 ± 0.1 | nd | 108 ± 8 | 60 ± 8 | 31 ± 5 | 200 | ||
| 12 | 3.4 ± 0.1 | 9.5 ± 0.2 | 8.6 ± 0.2 | nd | 117 ± 2 | 65 ± 5 | 33 ± 3 | 100 | ||
| 24 | 3.1 ± 0.1 | 13.6 ± 0.8 | 8.8 ± 0.2 | nd | 145 ± 3 | 104 ± 2 | 33 ± 4 | 100 | ||
| 1 week | - | - | 8.9 ± 0.3 | nd | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamfir, M.; Angelescu, I.-R.; Voaides, C.; Cornea, C.-P.; Boiu-Sicuia, O.; Grosu-Tudor, S.-S. Non-Dairy Fermented Beverages Produced with Functional Lactic Acid Bacteria. Microorganisms 2022, 10, 2314. https://doi.org/10.3390/microorganisms10122314
Zamfir M, Angelescu I-R, Voaides C, Cornea C-P, Boiu-Sicuia O, Grosu-Tudor S-S. Non-Dairy Fermented Beverages Produced with Functional Lactic Acid Bacteria. Microorganisms. 2022; 10(12):2314. https://doi.org/10.3390/microorganisms10122314
Chicago/Turabian StyleZamfir, Medana, Iulia-Roxana Angelescu, Catalina Voaides, Calina-Petruta Cornea, Oana Boiu-Sicuia, and Silvia-Simona Grosu-Tudor. 2022. "Non-Dairy Fermented Beverages Produced with Functional Lactic Acid Bacteria" Microorganisms 10, no. 12: 2314. https://doi.org/10.3390/microorganisms10122314





