Isolation of Thermophilic Bacteria and Investigation of Their Microplastic Degradation Ability Using Polyethylene Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, Pre-Enrichment, and Characterization
2.2. Phylogenetic Analysis and Bioinformatics Processing
2.3. Biodegradation Assay and Analysis
3. Results
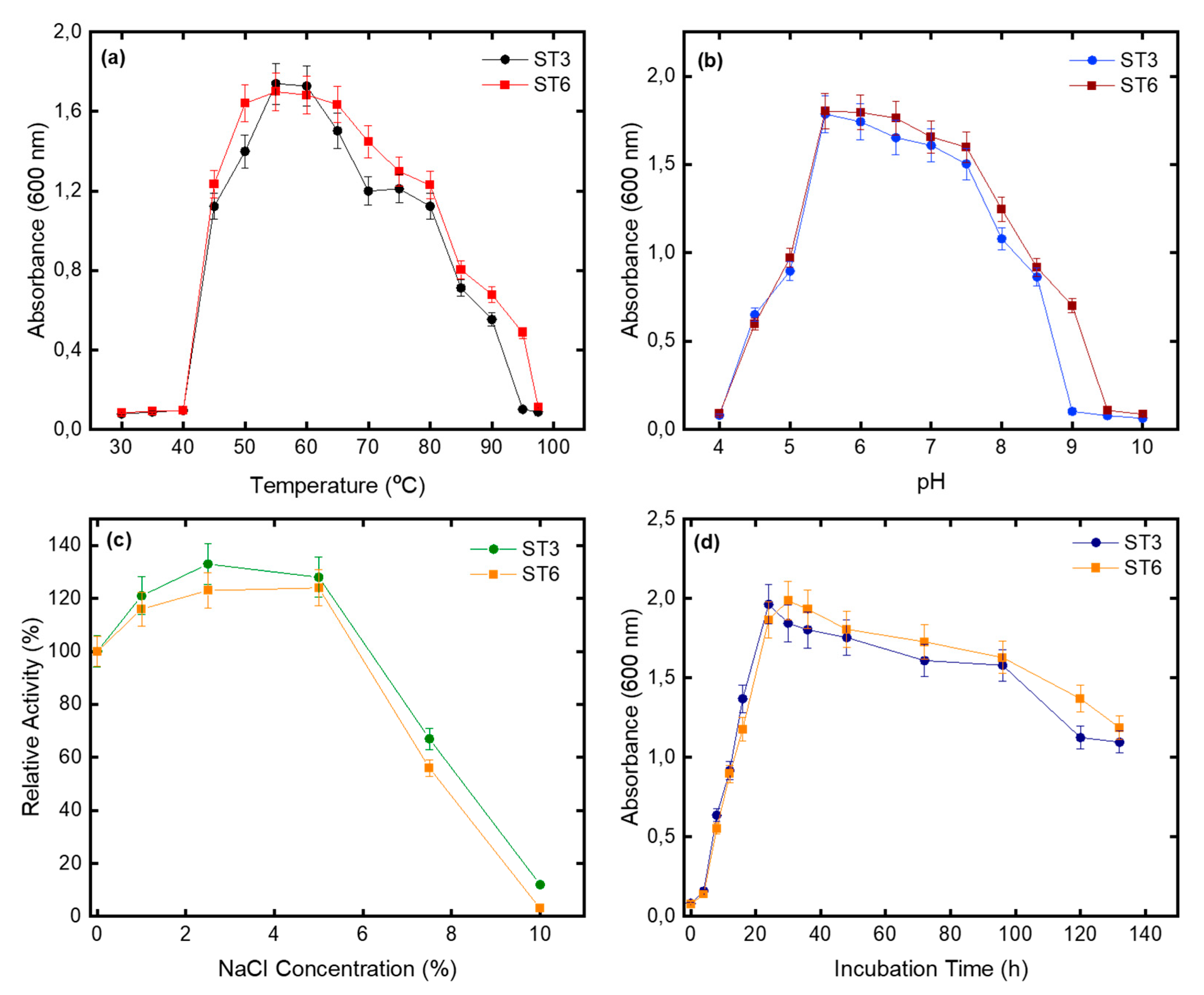
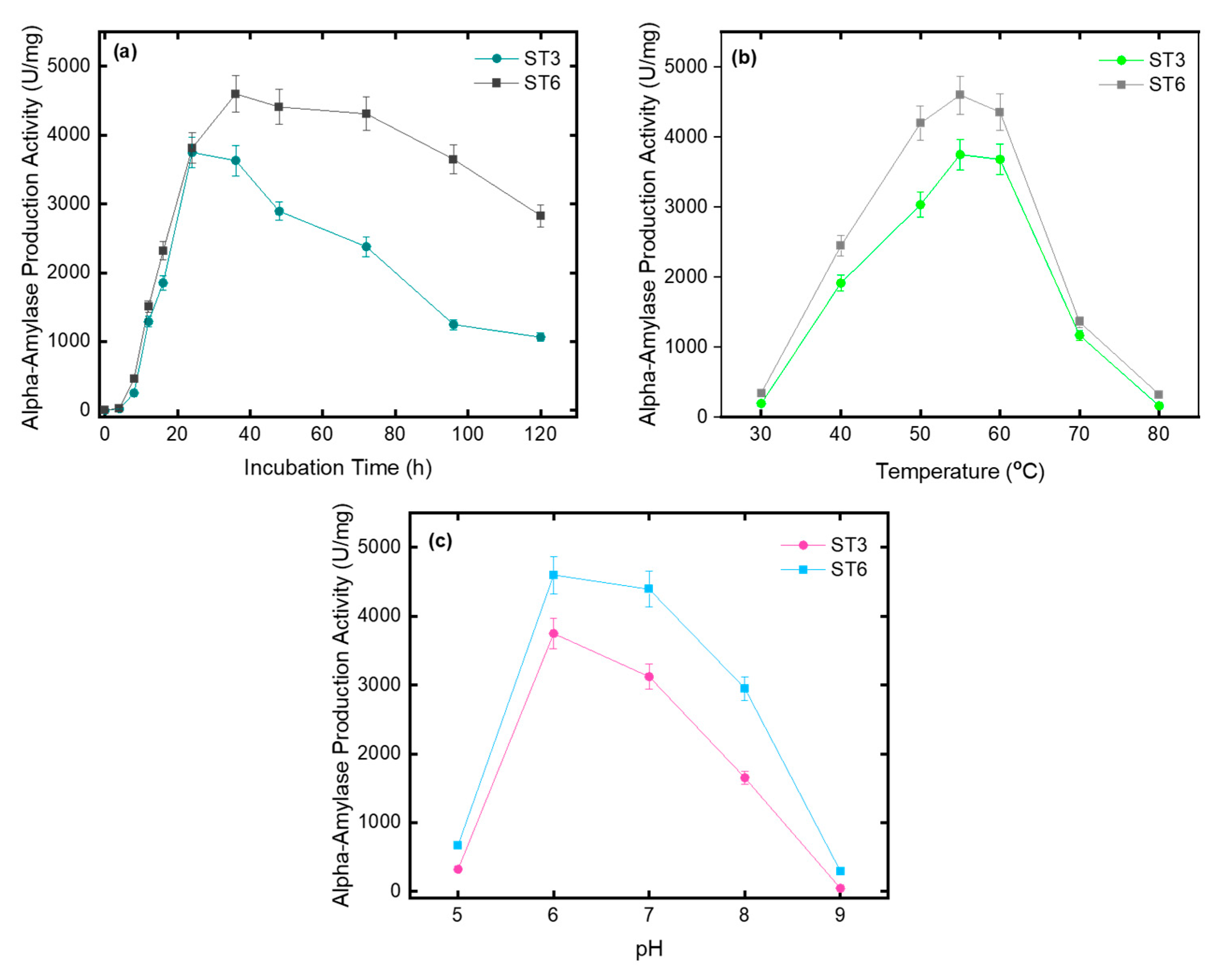
3.1. Characterization of Phenotypic Diversity
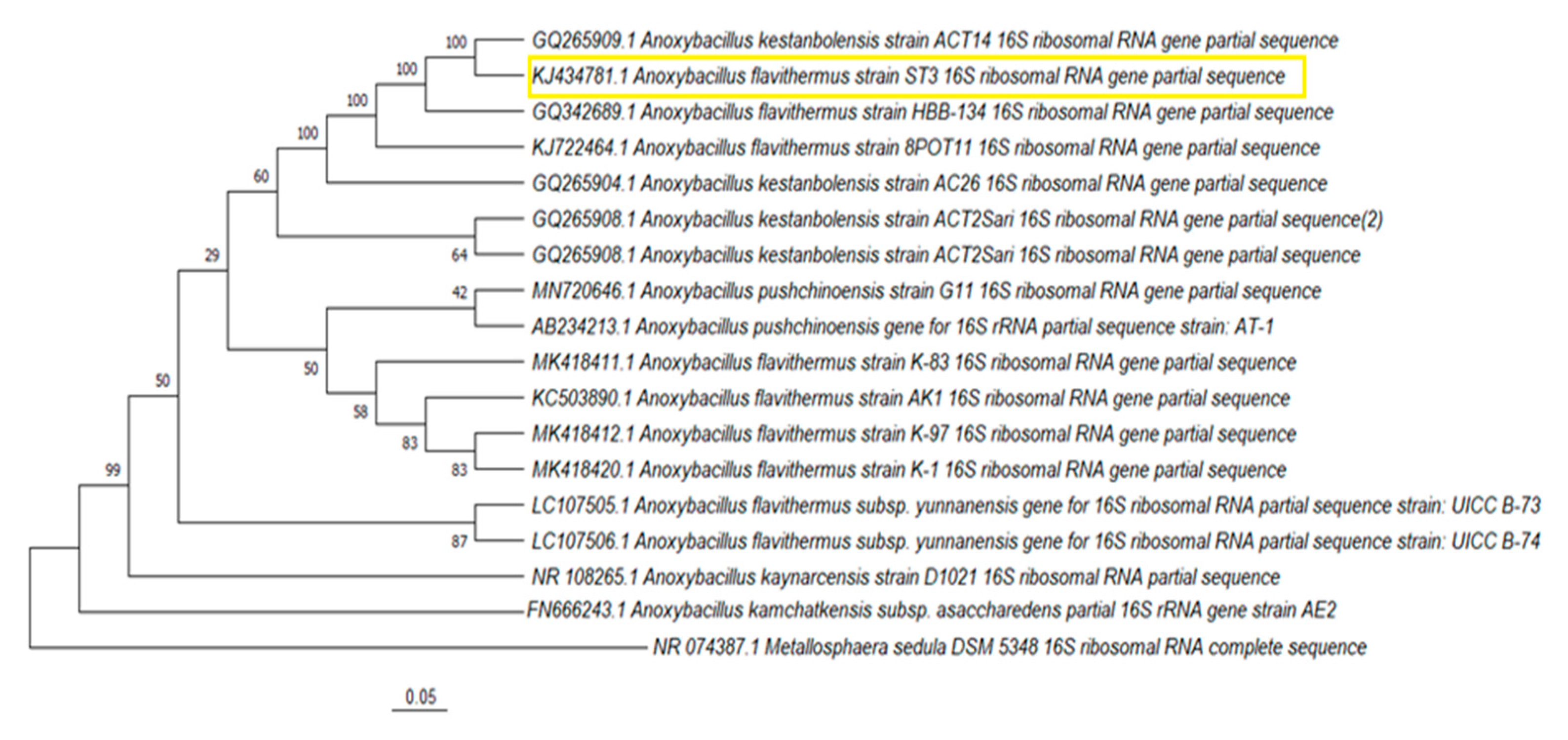
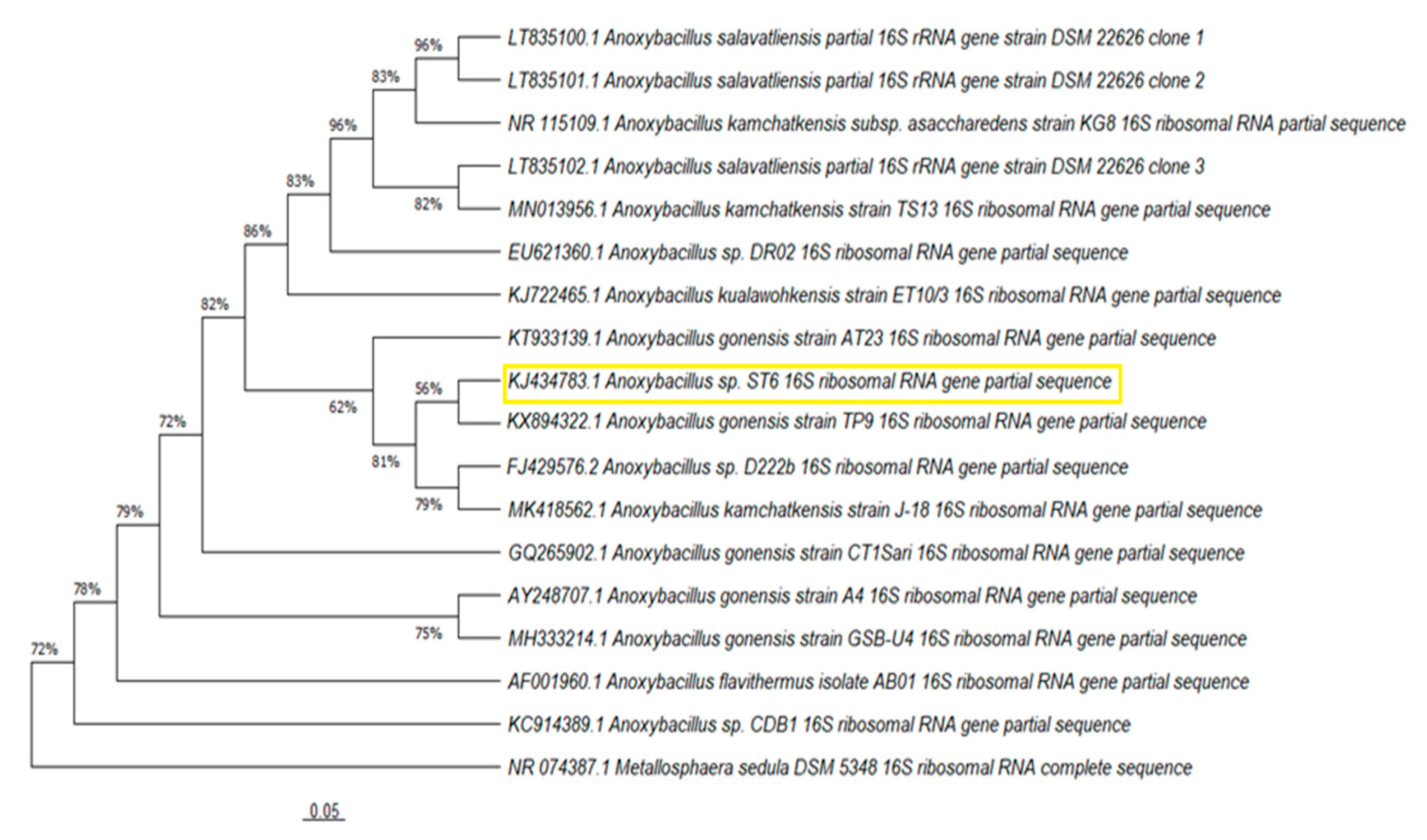
3.2. Phylogenetic Analyses
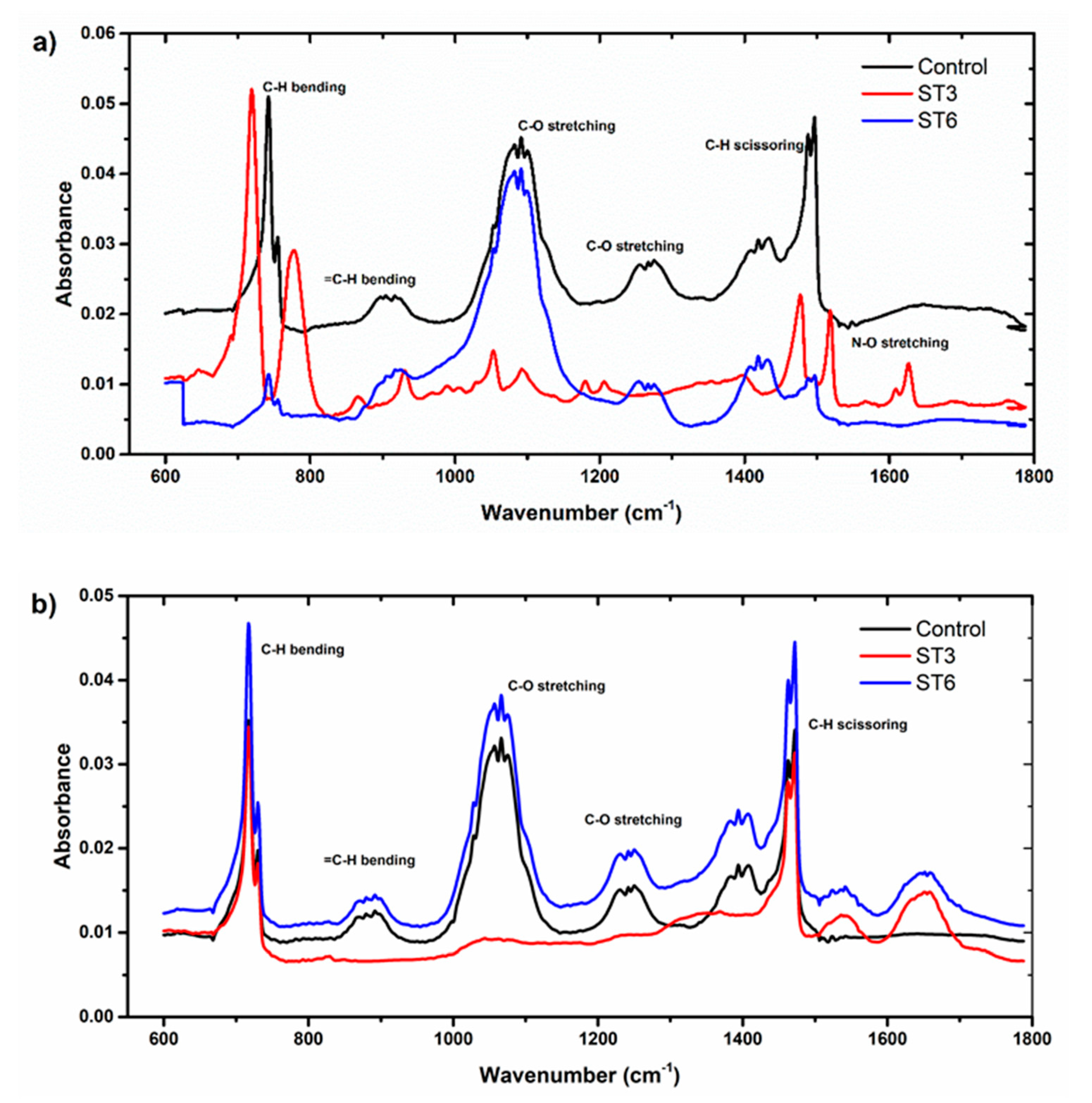
3.3. Determination of Biodegradation by FTIR
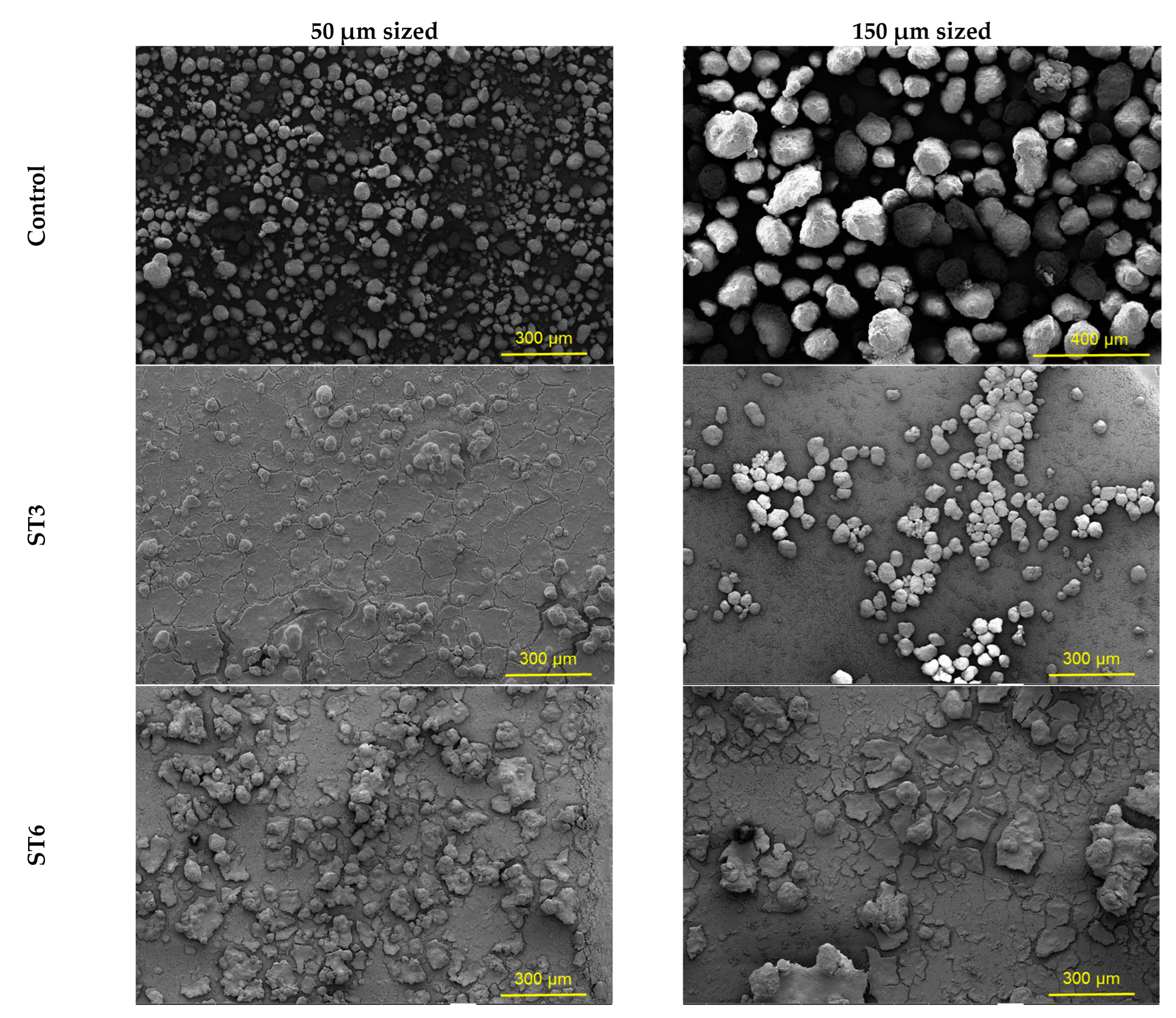
3.4. Morphology of Microplastics
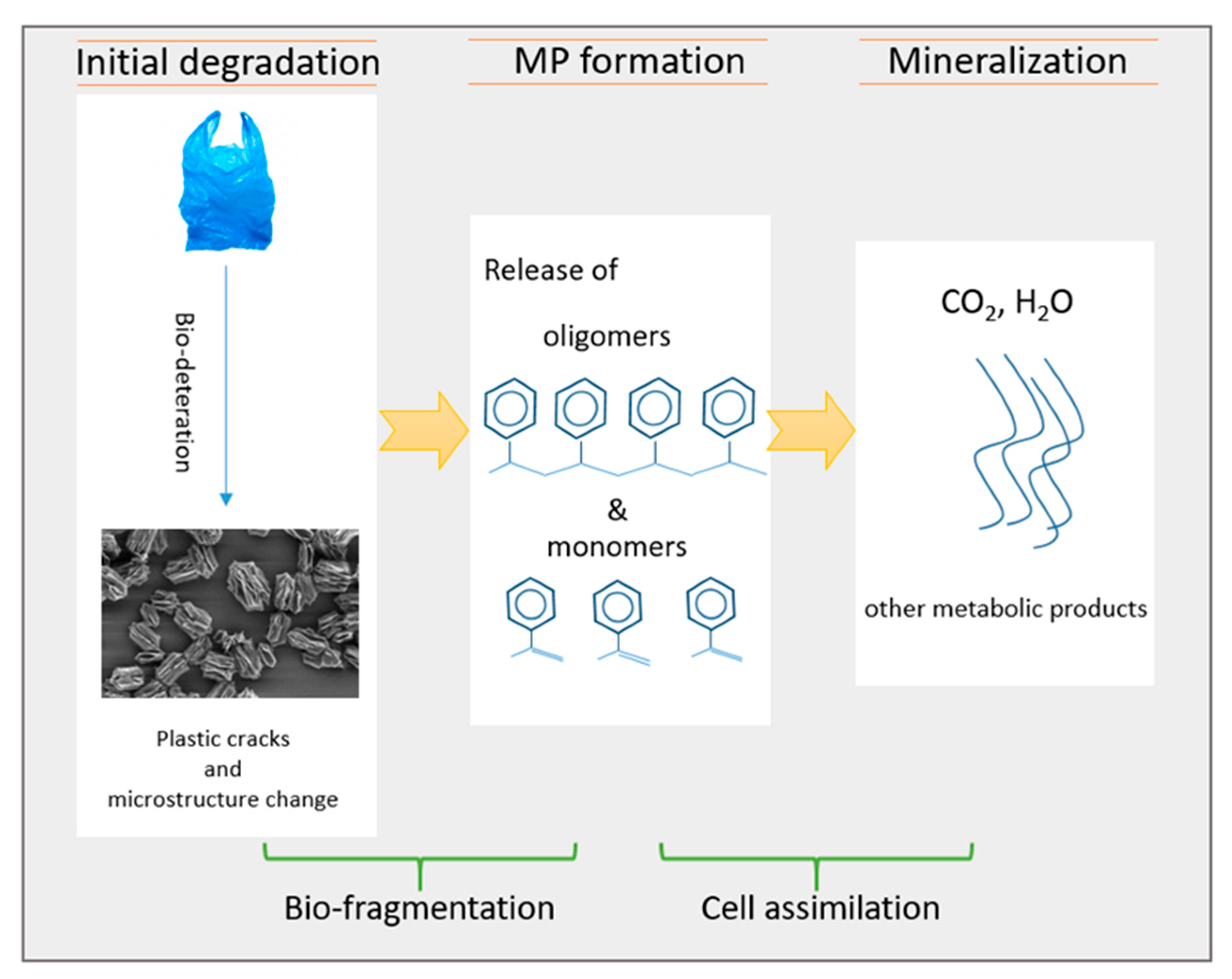
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Pedrouso, M.; Varela, Z.; Franco, D.; Fernandez, J.A.; Aboal, J.R. Can proteomics contribute to biomonitoring of aquatic pollution? A critical review. Environ. Pollut. 2020, 267, 115473. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; İsmail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Dang, T.C.H.; Nguyen, D.T.; Thai, H.; Nguyen, T.C.; Tran, T.T.H.; Le, V.H.; Nguyen, V.H.; Tran, X.B.; Pham, T.P.T.; Nguyen, T.G.; et al. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015014. [Google Scholar] [CrossRef]
- UNEP. United Nations Environment Programme, Beat Plastic Pollution. Available online: https://www.unep.org/ (accessed on 10 October 2022).
- Singh, P.; Sharma, V.P. Integrated Plastic Waste Management: Environmental and Improved Health Approaches. Procedia Environ. Sci. 2016, 35, 692–700. [Google Scholar] [CrossRef]
- Veiga, J.M.; Fleet, D.; Kinsey, S.; Nilsson, P.; Vlachogianni, T.; Werner, S.; Galgani, F.; Thompson, R.C.; Dagevos, J.; Gago, J.; et al. Identifying of Sources Marine Litter, MSFD GES TG Marine Litter Thematic Report; JRC Technical Report; Publications Office of the European Union: Luxemburg, 2016. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2018, 108, 131–139. [Google Scholar] [CrossRef]
- Lechner, A.; Ramler, D. The discharge of certain amounts of industrial microplastic from a production plant into the River Danube is permitted by the Austrian legislation. Environ. Pollut. 2015, 200, 159–160. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, C.; Kumbur, H.; Gökdağ, K.; Kıdeyş, A.E.; Sanchez-Vidal, A. Microplastics composition and load from three wastewater treatment plants discharging into Mersin Bay, north eastern Mediterranean Sea. Mar. Pollut. Bull. 2020, 150, 110776. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Rees, A.; Rowe, R.; Stevens, J.; Wright, P. Microplastics in the Solent estuarine complex, UK: An initial assessment. Mar. Pollut. Bull. 2016, 102, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Habib, R.Z.; Thiemann, T.; Al-Kendi, R. Microplastics and wastewater treatment plants—A review. J. Water Res. Prot. 2020, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Miloloža, M.; Cvetnić, M.; Kučić Grgić, D.; Bulatovic, V.O.; Ukic, S.; Rogosic, M.; Dionysiou, D.D.; Kusic, H.; Bolanca, T. Biotreatment strategies for the removal of microplastics from freshwater systems. A review. Environ. Chem. Lett. 2022, 20, 1377–1402. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Microplastics in Wastewater and Drinking Water Treatment Plants: Occurrence and Removal of Microfibres. Appl. Sci. 2021, 11, 10109. [Google Scholar] [CrossRef]
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Hartmann, N.B.; Jensen, P.R.; Nielsen, T.G.; Brinch, A. Microplastics: Occurrence, Effects and Sources of Releases to the Environment in Denmark; Danish Environmental Protection Agency: Copenhagen, Denmark, 2015; ISBN 978-87-93352-80-3. [Google Scholar]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Blair Espinoza, R.M. Microplastics in Wastewater Treatment Systems and Receiving Waters. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2019. [Google Scholar]
- Atanasova, N.; Stoitsova, S.; Paunova-Krasteva, T.; Kambourova, M. Plastic Degradation by Extremophilic Bacteria. Int. J. Mol. Sci. 2021, 22, 5610. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ. Sci. Pollut. Res. Int. 2013, 20, 4146–4153. [Google Scholar] [CrossRef]
- Gong, J.; Kong, T.; Li, Y.; Li, Q.; Li, Z.; Zhang, J. Biodegradation of Microplastic Derived from Poly(ethylene terephthalate) with Bacterial Whole-Cell Biocatalysts. Polymers 2018, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Enespa Singh, D.P. Microplastic degradation by bacteria in aquatic ecosystem. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 431–467. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Thakur, P. Screening of Plastic Degrading Bacteria from Dumped Soil Area. Master’s Thesis, Department of Life Science National Institute of Technology, Rourkela, India, 2012. [Google Scholar]
- Mohan, A.J.; Sekhar, V.C.; Bhaskar, T.; Nampoothiri, K.M. Microbial assisted High Impact Polystyrene (HIPS) degradation. Bioresour. Technol. 2016, 213, 204–207. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Shah, Z.; Krumholz, L.; Aktas, D.F.; Hasan, F.; Khattak, M.; Shah, A.A. Degradation of polyester polyurethane by a newly isolated soil bacterium, Bacillus subtilis strain MZA-75. Biodegradation 2013, 24, 865–877. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, C.; Moon, J.; Heo, J.; Jung, S.P.; Kim, J.R. Polymer film-based screening and isolation of polylactic acid (PLA)-degrading microorganisms. J. Microbiol. Biotechnol. 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Arévalo, F.R.; Díaz, L.E.; Cobo, M.I.; Valero, M.F. Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J. Biomater. Sci. Polym. Ed. 2016, 27, 1860–1879. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef]
- Syranidou, E.; Karkanorachak, K.; Amorotti, F.; Repouskou, E.; Kroll, K.; Kolvenbach, B.; Corvini, P.F.-X.; Fava, F.; Kalogerakis, N. Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLoS ONE 2017, 12, e0183984. [Google Scholar] [CrossRef] [Green Version]
- Tareen, A.; Saeed, S.; Iqbal, A.; Batool, R.; Jamil, N. Biodeterioration of Microplastics: A Promising Step towards Plastics Waste Management. Polymers 2022, 14, 2275. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, K.S. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini—Review. Clean. Eng. Technol. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 213–225. [Google Scholar] [CrossRef]
- Stepczynska, M.; Rytlewski, P. Enzymatic degradation of flax-fibers reinforced polylactide. Int. Biodeterior. Biodegrad. 2018, 126, 160–166. [Google Scholar] [CrossRef]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Acer, Ö.; Güven, K.; Poli, A.; Di Donato, P.; Leone, L.; Buono, L.; Finore, I. Acinetobacter mesopotamicus sp. nov., petroleum-degrading bacterium, isolated from petroleum-contaminated soil in Diyarbakir, in the southeast of Turkey. Curr. Microbiol. 2020, 77, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Dussault, H.P. An improved technique for staining for halophilic bacteria. J. Bacteriol. 1955, 70, 484–485. [Google Scholar] [CrossRef] [Green Version]
- Lányi, B. Classical and rapid identifcation methods for medically important bacteria. Method Microbiol. 1988, 19, 1–67. [Google Scholar]
- Akarsu, C.; Özdemir, S.; Ozay, Y.; Acer, Ö.; Dizge, N. Investigation of two different size microplastic egradation ability of thermophilic bacteria using polyethylene polymers. Environ. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Ozdemir, S.; Acer, O.; Kılınç, E. Bioaccumulation, tolerance, and removal of U (VI) and Th (IV) by a novel thermophilic Bacillus cereus ST14 isolated from hot spring mud samples in Afyonkarahisar, Turkey. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Trash to treasure: Microwave-assisted conversion of polyethylene to functional chemicals. Ind. Eng. Chem. Res. 2017, 56, 14814–14821. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Aragão Börner, R.; Börner, T.; Muñoz, R. Biodegradation of bioplastics under aerobic and anaerobic aqueous conditions: Kinetics, carbon fate and particle size effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Akarsu, C.; Sönmez, V.Z.; Altay, M.C.; Pehlivan, T.; Sivri, N. The spatial and temporal changes of beach litter on Istanbul (Turkey) beaches as measured by the clean-coast index. Mar. Pollut. Bull. 2022, 176, 113407. [Google Scholar] [CrossRef]
- Wang, X.; Shubella, R.; Ammidown, E.; Chenette, H.C.S. Characteerizing microplastic degradation in a simulated marine environment. J. Undergrad. Chem. Res. 2021, 20, 49. [Google Scholar]
- Idris, S.A.; Mkhatresh, O.A.; Heatley, F. Assignment of 1H NMR spectrum and investigation of oxidative degradation of poly(ethylenimine) using 1H and 13C 1-D and 2-D NMR. Polym. Int. 2006, 55, 1040–1048. [Google Scholar] [CrossRef]
- Akarsu, C.; Madenli, Ö.; Deveci, E.Ü. Characterization of littered face masks in the southeastern part of Turkey. Environ. Sci. Pollut. Res. 2021, 28, 47517–47527. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Berlemont, R.; Gerday, C. Extremophiles. In Comprehensive Biotechnology, 2nd ed.; Pergamon: Oxford, UK, 2011; Volume 1, pp. 229–242. [Google Scholar]
- Koul, B.; Chaudhary, R.; Taak, P. Extremophilic microbes and their application in bioremediation of environmental contaminants. In Microbe Mediated Remediation of Environmental Contaminants; Woodheadd Publishing: Cambridge, UK, 2021; pp. 115–128. [Google Scholar]
- Agustien, A.; Jannah, M.; Djamaan, A. Screening Polyethylene Synthetic Plastic Degrading-Bacteria from Soil. Pharm. Lett. 2016, 8, 183–187. [Google Scholar]
- Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef]
- Skariyachan, S.; Taskeen, N.; Kishore, A.P.; Krishna, B.V.; Naidu, G. Novel consortia of Enterobacter and Pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J. Environ. Manag. 2021, 284, 112030. [Google Scholar] [CrossRef]
- Maroof, L.; Khan, I.; Yoo, H.S.; Kim, S.; Park, H.; Ahmad, B.; Azam, S. Identification and characterization of low density polyethylene-degrading bacteria isolated from soils of waste disposal sites. Environ. Eng. Res. 2021, 26, 200167. [Google Scholar] [CrossRef]
- Andrady, A.L.; Pegram, J.E.; Tropsha, Y. Changes in Carbonyl Index and Average Molecular Weight on Embrittlement of Enhanced-Photodegradable Polyethylenes. J. Environ. Polym. Degrad. 1993, 1, 3. [Google Scholar] [CrossRef]
- Dey, A.S.; Bose, H.; Mohapatra, B.; Sar, P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., Isolated from Waste Dumpsite and Drilling Fluid. Front. Microbiol. 2020, 11, 603210. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, H.V.; Ramalingappa, B.; Nayanashree, G.; Thippeswamy, B.; Krishnappa, M. Polyethylene degradation by fungal consortium. Int. J. Environ. Res. 2015, 9, 823–830. [Google Scholar]
- Gajendiran, A.; Krishnamoorthy, S.; Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheik, S.; Chandrashekar, K.R.; Swaroop, K.; Somashekarappa, H.M. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]

| Genus-Strain | Source | MP Type 1 | Duration Time (Day) | Biodegradation Analysis Method | References | |||
|---|---|---|---|---|---|---|---|---|
| Weighting | FTIR | SEM | Other 2 | |||||
| Bacillus 27 | Mangrove sediment | PP | 40 | + | + | + | + | [37] |
| Rhodococcus 36 | Mangrove sediment | PP | 40 | [37] | ||||
| Bacillus gottheilii | Mangrove ecosystems | PE, PET, PP, PS | 40 | + | + | + | - | [33] |
| Enterobacter asburiae YT1 | Plastic-eating waxworms | PE | 28 | + | + | + | + | [38] |
| Bacillus YP1 | Plastic-eating waxworms | PE | 28 | + | + | + | + | [38] |
| Bacillus subtilis MZA-75 | Soil samples | PUR | 28 | - | + | + | + | [39] |
| Pseudomonas | Digester sludge | PLA | 40 | - | - | + | + | [40] |
| Bacillus MYK2 | Digester sludge | PLA | 40 | - | - | + | + | [40] |
| Pirellulacease | Fresh water | PE, PP | 21 | - | - | - | + | [41] |
| Escherichia coli | - | PU | 3 | + | - | + | + | [42] |
| Alcanivorax borkumensis | Floating plastics in oceans | PET, LDPE, PS | 1–6 | + | + | - | - | [43] |
| Lysinibacillus sp. | Plastic samples at surface water | PE | 180 | + | + | + | - | [44] |
| Alcaligenes faecalis | Municipal dumpsites | LDPE, HDPE, PES | 15 | + | + | + | - | [45] |
| Bacillus cereus | Municipal dumpsites | LDPE, HDPE, PES | 15 | + | + | + | - | [45] |
| Characterization | Strain ST3 | Strain ST6 |
|---|---|---|
| Motility | + | + |
| Gram | + | + |
| Spore | + | + |
| NaCl range (%, w/v) | 1–7.5 | 1–7.5 |
| NaCl optimum (%, w/v) | 2.5–5 | 1–5 |
| Temperature growth range | 45–90 | 45–90 |
| Optimum temperature (°C) | 55–60 | 55–60 |
| pH growth range | 4.5–8.5 | 5.5–9.0 |
| Optimum pH | 5.5–6.0 | 6.0 |
| Urease | - | + |
| β-galactosidase | + | + |
| Amylase | ++ | +++ |
| Protease | - | + |
| Catalase | + | ++ |
| Oxidase | + | + |
| Citrate | + | + |
| Phosphate | + | + |
| Acid production from: | ||
| -Glucose | + | + |
| -Lactose | + | + |
| -Sucrose | + | + |
| Polyethylene | Raw | ST3 | ST6 |
|---|---|---|---|
| 50 µm | 0.709 | 1.888 | 1.905 |
| 150 µm | 0.370 | 0.748 | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, S.; Akarsu, C.; Acer, Ö.; Fouillaud, M.; Dufossé, L.; Dizge, N. Isolation of Thermophilic Bacteria and Investigation of Their Microplastic Degradation Ability Using Polyethylene Polymers. Microorganisms 2022, 10, 2441. https://doi.org/10.3390/microorganisms10122441
Özdemir S, Akarsu C, Acer Ö, Fouillaud M, Dufossé L, Dizge N. Isolation of Thermophilic Bacteria and Investigation of Their Microplastic Degradation Ability Using Polyethylene Polymers. Microorganisms. 2022; 10(12):2441. https://doi.org/10.3390/microorganisms10122441
Chicago/Turabian StyleÖzdemir, Sadin, Ceyhun Akarsu, Ömer Acer, Mireille Fouillaud, Laurent Dufossé, and Nadir Dizge. 2022. "Isolation of Thermophilic Bacteria and Investigation of Their Microplastic Degradation Ability Using Polyethylene Polymers" Microorganisms 10, no. 12: 2441. https://doi.org/10.3390/microorganisms10122441







