Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry
Abstract
:1. Introduction
2. Intestinal Microbiota in Poultry

3. Intestinal Barrier and Tight Junctions
4. Biomarkers Related to Intestinal Health of Animals
5. Probiotics
6. Prebiotics
7. Synbiotics
7.1. Role of Synbiotics in Poultry Production
7.2. The Role of Short Chain Fatty Acids (SCFAs) on Digestive Physiology
7.2.1. SCFAs and Muscular Activity
7.2.2. SCFAs and Enterocyte Proliferation
7.2.3. SCFAs and Mucin Production
8. Phytogenic Feed Additives
8.1. Berberine
8.2. Boswellia
8.3. Capsaicin
8.4. Triterpenoids of Marigold
8.5. Phytocannabinoids
8.6. Eugenol
8.7. Isoflavones (ISF)
8.8. Isoquinoline Alkaloids
8.9. Phenolic Derivatives
8.10. Quercetin
8.11. Thymol/Carvacrol

8.12. Resveratrol
8.13. Curcumin
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BisBischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Zoetendal, E.G.; Rajilic-Stojanovic, M.; de Vos, W.M. High-Throughput Diversity and Functionality Analysis of the Gastrointestinal Tract Microbiota. Gut 2008, 57, 1605–1615. [Google Scholar] [CrossRef]
- Ackermann, W.; Coenen, M.; Schrödl, W.; Shehata, A.A.; Krüger, M. The Influence of Glyphosate on the Microbiota and Production of Botulinum Neurotoxin During Ruminal Fermentation. Curr. Microbiol. 2015, 70, 374–382. [Google Scholar] [CrossRef]
- Schrödl, W.; Krüger, S.; Konstantinova-Müller, T.; Shehata, A.A.; Rulff, R.; Krüger, M. Possible Effects of Glyphosate on Mucorales Abundance in the Rumen of Dairy Cows in Germany. Curr. Microbiol. 2014, 69, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Neuhaus, J.; Herrenthey, A.G.; Gökce, M.M.; Schrödl, W.; Shehata, A.A. Chronic Botulism in a Saxony Dairy Farm: Sources, Predisposing Factors, Development of the Disease and Treatment Possibilities. Anaerobe 2014, 28, 220–225. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.S.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; et al. Inflammatory Biomarkers Are Associated with Ketosis in Periparturient Holstein Cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef]
- Hafez, H.M.; Shehata, A.A. Turkey Production and Health: Current Challenges. Ger. J. Vet. Res. 2021, 1, 3–14. [Google Scholar] [CrossRef]
- Gernat, A.A.; Santos, F.B.O.; Grimes, J.L. Alternative Approaches to Antimicrobial Use in the Turkey Industry: Challenges and Perspectives. Ger. J. Vet. Res. 2021, 1, 37–47. [Google Scholar] [CrossRef]
- López-García, P.; Eme, L.; Moreira, D. Symbiosis in Eukaryotic Evolution. J. Theor. Biol. 2017, 434, 20–33. [Google Scholar] [CrossRef]
- Wren, B.W. Microbial Genome Analysis: Insights into Virulence, Host Adaptation and Evolution. Nat. Rev. Genet. 2000, 1, 30–39. [Google Scholar] [CrossRef] [PubMed]
- McFall-Ngai, M.J. Identifying “Prime Suspects”: Symbioses and the Evolution of Multicellularity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 711–723. [Google Scholar] [CrossRef]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P.; et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; La, T.-M.; Lee, H.-J.; Choi, I.-S.; Song, C.-S.; Park, S.-Y.; Lee, J.-B.; Lee, S.-W. Characterization of Microbial Communities in the Chicken Oviduct and the Origin of Chicken Embryo Gut Microbiota. Sci. Rep. 2019, 9, 6838. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.-Y.; Kamagata, Y.; Fukatsu, T. Host-Symbiont Co-Speciation and Reductive Genome Evolution in Gut Symbiotic Bacteria of Acanthosomatid Stinkbugs. BMC Biol. 2009, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Tellez, G. Prokaryotes Versus Eukaryotes: Who Is Hosting Whom? Front. Vet. Sci. 2014, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, M.J. Who Are We? Indigenous Microbes and the Ecology of Human Diseases. EMBO Rep. 2006, 7, 956–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Hadrich, D. Microbiome Research Is Becoming the Key to Better Understanding Health and Nutrition. Front. Genet. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Wielen, P.W.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; van Knapen, F. Role of Volatile Fatty Acids in Development of the Cecal Microflora in Broiler Chickens during Growth. Appl. Environ. MicroBiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef] [Green Version]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC MicroBiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the Landscape of Gut Microbial Community Composition. Nat. MicroBiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Knight, R.; Gordon, J.I. The Human Microbiome: Eliminating the Biomedical/Environmental Dichotomy in Microbial Ecology. Environ. MicroBiol. 2007, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, M.T.R. Nutrigenetics and Nutraceuticals: The next Wave Riding on Personalized Medicine. Transl. Res. 2007, 149, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.M.; Paswan, V.K.; Attia, Y.A.; Abdel-Moneim, A.-M.E.; Abougabal, M.S.; Sharaf, M.; Elmazoudy, R.; Alghafari, W.T.; Osman, M.A.; Farag, M.R.; et al. Managing Gut Microbiota through in Ovo Nutrition Influences Early-Life Programming in Broiler Chickens. Animals 2021, 11, 3491. [Google Scholar] [CrossRef]
- Sugiharto, S. Role of Nutraceuticals in Gut Health and Growth Performance of Poultry. J. Saudi Soc. Agric. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Hailu, G.; Boecker, A.; Henson, S.; Cranfield, J. Consumer Valuation of Functional Foods and Nutraceuticals in Canada. A Conjoint Study Using Probiotics. Appetite 2009, 52, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Sprague, L.D.; Neubauer, H.; Pletz, M.W. Klebsiella Pneumoniae in Germany: An Overview on Spatiotemporal Distribution and Resistance Development in Humans. Ger. J. Microbiol. 2021, 1, 16–25. [Google Scholar] [CrossRef]
- Kaonga, N.; Hang’ombe, B.M.; Lupindu, A.M.; Hoza, A.S. Detection of CTX-M-Type Extended-Spectrum Beta-Lactamase Producing Salmonella typhimurium in Commercial Poultry Farms in Copperbelt Province, Zambia. Ger. J. Vet. Res. 2021, 1, 27–34. [Google Scholar] [CrossRef]
- Njeru, J. Emerging Carbapenem Resistance in ESKAPE Organisms in Sub-Saharan Africa and the Way Forward. Ger. J. Microbiol. 2021, 1, 3–6. [Google Scholar] [CrossRef]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals. Vet. Clin. North Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The Prolongation of Life. Optimistic Studies; Chalmers, P.M., Ed.; Heinemann: London, UK, 1907. [Google Scholar]
- Dhama, K.; Tiwari, R.; Khan, R.U.; Chakrabort, S.; Gopi, M.; Karthik, K.; Saminathan, M.; Desingu, P.A.; Sunkara, L.T. Growth Promoters and Novel Feed Additives Improving Poultry Production and Health, Bioactive Principles and Beneficial Applications: The Trends and Advances-A Review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar] [CrossRef] [Green Version]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The Role of Gut Microbiota (Commensal Bacteria) and the Mucosal Barrier in the Pathogenesis of Inflammatory and Autoimmune Diseases and Cancer: Contribution of Germ-Free and Gnotobiotic Animal Models of Human Diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Zhao, X. Prebiotics and Gut Microbiota in Chickens. FEMS MicroBiol. Lett. 2015, 362, fnv122. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, S.T.; Hunter, J.H.; Taylor, A.E. Regulation of Tight-Junction Permeability During Nutrient Absorption Across the Intestinal Epithelium. Annu. Rev. Nutr. 1995, 15, 35–55. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of Tight Junction Components with Signaling Pathways. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhaj, N.S.; Antonetti, D.A. Regulation of Tight Junctions and Loss of Barrier Function in Pathophysiology. Int. J. Biochem. Cell. Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef] [PubMed]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In Vitro Permeability Enhancement of Curcumin across Caco-2 Cells Monolayers Using Electrospun Xanthan-Chitosan Nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin Improves Intestinal Barrier Function: Modulation of Intracellular Signaling, and Organization of Tight Junctions. Am. J. Physiol. Cell. Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. BioSci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat Stress and Reduced Plane of Nutrition Decreases Intestinal Integrity and Function in Pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef]
- Gilani, S.; Chrystal, P.V.; Barekatain, R. Current Experimental Models, Assessment and Dietary Modulations of Intestinal Permeability in Broiler Chickens. Anim. Nutr. 2021, 7, 801–811. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Dickson, M.J.; Abuajamieh, M.; Snider, D.B.; Fernandez, M.V.S.; Johnson, J.S.; Keating, A.F.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; et al. Intentionally Induced Intestinal Barrier Dysfunction Causes Inflammation, Affects Metabolism, and Reduces Productivity in Lactating Holstein Cows. J. Dairy Sci. 2017, 100, 4113–4127. [Google Scholar] [CrossRef] [Green Version]
- Gilani, S.; Howarth, G.S.; Kitessa, S.M.; Tran, C.D.; Forder, R.E.A.; Hughes, R.J. New Biomarkers for Increased Intestinal Permeability Induced by Dextran Sodium Sulphate and Fasting in Chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, e237–e245. [Google Scholar] [CrossRef]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for Monitoring Intestinal Health in Poultry: Present Status and Future Perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef] [Green Version]
- Tellez, G.; Latorre, J.D.; Kuttappan, V.A.; Kogut, M.H.; Wolfenden, A.; Hernandez-Velasco, X.; Hargis, B.M.; Bottje, W.G.; Bielke, L.R.; Faulkner, O.B. Utilization of Rye as Energy Source Affects Bacterial Translocation, Intestinal Viscosity, Microbiota Composition, and Bone Mineralization in Broiler Chickens. Front. Genet. 2014, 5, 339. [Google Scholar] [CrossRef]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Ruff, J.; Tellez, G.; Forga, A.J.; Señas-Cuesta, R.; Vuong, C.N.; Greene, E.S.; Hernandez-Velasco, X.; Uribe, Á.J.; Martínez, B.C.; Angel-Isaza, J.A.; et al. Evaluation of Three Formulations of Essential Oils in Broiler Chickens under Cyclic Heat Stress. Animals 2021, 11, 1084. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen Duijghuijsen, L.M.; Grefte, S.; de Boer, V.C.J.; Zeper, L.; van Dartel, D.A.M.; van der Stelt, I.; Bekkenkamp-Grovenstein, M.; van Norren, K.; Wichers, H.J.; Keijer, J. Mitochondrial ATP Depletion Disrupts Caco-2 Monolayer Integrity and Internalizes Claudin 7. Front. Physiol. 2017, 8, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.-X.; Cao, C.-Y.; Sun, Y.-C.; Wang, L.-L.; Li, N.; Xu, S.-W.; Li, J.-L. Effects on Liver Hydrogen Peroxide Metabolism Induced by Dietary Selenium Deficiency or Excess in Chickens. Biol. Trace Elem. Res. 2014, 159, 174–182. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Latorre, J.D.; Dridi, S.; Merino-Guzman, R.; Hernandez-Velasco, X.; Hargis, B.M.; Tellez-Isaias, G. Identification of Serum Biomarkers for Intestinal Integrity in a Broiler Chicken Malabsorption Model. Front. Vet. Sci. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windmueller, H.G.; Spaeth, A.E. Source and Fate of Circulating Citrulline. Am. J. Physiol. Endocrinol. Metab. 1981, 241, E473–E480. [Google Scholar] [CrossRef] [PubMed]
- Berkeveld, M.; Langendijk, P.; Verheijden, J.H.M.; Taverne, M.A.M.; van Nes, A.; van Haard, P.; Koets, A.P. Citrulline and Intestinal Fatty Acid-Binding Protein: Longitudinal Markers of Postweaning Small Intestinal Function in Pigs? J. Anim. Sci. 2008, 86, 3440–3449. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, M. Wound Healing of Intestinal Epithelial Cells. WJG 2011, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking Stress and Immunity: Immunoglobulin A as a Non-Invasive Physiological Biomarker in Animal Welfare Studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Utech, M.; Ivanov, A.I.; Samarin, S.N.; Bruewer, M.; Turner, J.R.; Mrsny, R.J.; Parkos, C.A.; Nusrat, A. Mechanism of IFN-γ-Induced Endocytosis of Tight Junction Proteins: Myosin II-Dependent Vacuolarization of the Apical Plasma Membrane. MBoC 2005, 16, 5040–5052. [Google Scholar] [CrossRef] [Green Version]
- Nava, P.; Koch, S.; Laukoetter, M.G.; Lee, W.Y.; Kolegraff, K.; Capaldo, C.T.; Beeman, N.; Addis, C.; Gerner-Smidt, K.; Neumaier, I.; et al. Interferon-γ Regulates Intestinal Epithelial Homeostasis through Converging β-Catenin Signaling Pathways. Immunity 2010, 32, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, W.A.; Ghareeb, K.; Böhm, J. Evaluation of the Chicory Inulin Efficacy on Ameliorating the Intestinal Morphology and Modulating the Intestinal Electrophysiological Properties in Broiler Chickens: Evaluation of the Chicory Inulin Efficacy in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2011, 95, 65–72. [Google Scholar] [CrossRef]
- Wideman, R.F.; Hamal, K.R.; Stark, J.M.; Blankenship, J.; Lester, H.; Mitchell, K.N.; Lorenzoni, G.; Pevzner, I. A Wire-Flooring Model for Inducing Lameness in Broilers: Evaluation of Probiotics as a Prophylactic Treatment. Poult. Sci. 2012, 91, 870–883. [Google Scholar] [CrossRef]
- Wideman, R.F.; Al-Rubaye, A.; Kwon, Y.M.; Blankenship, J.; Lester, H.; Mitchell, K.N.; Pevzner, I.Y.; Lohrmann, T.; Schleifer, J. Prophylactic Administration of a Combined Prebiotic and Probiotic, or Therapeutic Administration of Enrofloxacin, to Reduce the Incidence of Bacterial Chondronecrosis with Osteomyelitis in Broilers. Poult. Sci. 2015, 94, 25–36. [Google Scholar] [CrossRef]
- Saleh, M.; Elson, C.O. Experimental Inflammatory Bowel Disease: Insights into the Host-Microbiota Dialog. Immunity 2011, 34, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Latorre, J.D.; Hernandez-Velasco, X.; Bielke, L.R.; Vicente, J.L.; Wolfenden, R.; Menconi, A.; Hargis, B.M.; Tellez, G. Evaluation of a Bacillus Direct-Fed Microbial Candidate on Digesta Viscosity, Bacterial Translocation, Microbiota Composition and Bone Mineralisation in Broiler Chickens Fed on a Rye-Based Diet. Br. Poult. Sci. 2015, 56, 723–732. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Merino-Guzman, R.; Latorre, J.D.; Mahaffey, B.D.; Yang, Y.; Teague, K.D.; Graham, L.E.; Wolfenden, A.D.; Hernandez-Velasco, X.; Bielke, L.R.; et al. Optimizing Fluorescein Isothiocyanate Dextran Measurement as a Biomarker in a 24-h Feed Restriction Model to Induce Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2017, 4, 56. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty Acid-Binding Proteins: Role in Metabolic Diseases and Potential as Drug Targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albala, C.; Santos, J.L.; Cifuentes, M.; Villarroel, A.C.; Lera, L.; Liberman, C.; Angel, B.; Pérez-Bravo, F. Intestinal FABP2 A54T Polymorphism: Association with Insulin Resistance and Obesity in Women. Obes. Res. 2004, 12, 340–345. [Google Scholar] [CrossRef]
- Dal Pont, G.C.; Belote, B.L.; Lee, A.; Bortoluzzi, C.; Eyng, C.; Sevastiyanova, M.; Khadem, A.; Santin, E.; Farnell, Y.Z.; Gougoulias, C.; et al. Novel Models for Chronic Intestinal Inflammation in Chickens: Intestinal Inflammation Pattern and Biomarkers. Front. Immunol. 2021, 12, 676628. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, Prebiotics, and Synbiotics-Approaching a Definition. Am J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [CrossRef] [Green Version]
- Yurong, Y.; Ruiping, S.; Shimin, Z.; Yibao, J. Effect of Probiotics on Intestinal Mucosal Immunity and Ultrastructure of Cecal Tonsils of Chickens. Arch. Anim. Nutr. 2005, 59, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of Probiotic Action: Implications for Therapeutic Applications in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef]
- Prado-Rebolledo, O.F.; de Jesus Delgado-Machuca, J.; Macedo-Barragan, R.J.; Garcia-Márquez, L.J.; Morales-Barrera, J.E.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G. Evaluation of a Selected Lactic Acid Bacteria-Based Probiotic on Salmonella enterica Serovar Enteritidis Colonization and Intestinal Permeability in Broiler Chickens. Avian Pathol. 2017, 46, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinaro, F.; Paschetta, E.; Cassader, M.; Gambino, R.; Musso, G. Probiotics, Prebiotics, Energy Balance, and Obesity: Mechanistic Insights and Therapeutic Implications. Gastroenterol. Clin. North Am. 2012, 41, 843–854. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Vicente, J.L.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Temporal Effects of Lactic Acid Bacteria Probiotic Culture on Salmonella in Neonatal Broilers. Poult. Sci. 2007, 86, 1662–1666. [Google Scholar] [CrossRef]
- Vicente, J.; Wolfenden, A.; Torres-Rodriguez, A.; Higgins, S.; Tellez, G.; Hargis, B. Effect of a Lactobacillus Species-Based Probiotic and Dietary Lactose Prebiotic on Turkey Poult Performance with or Without Salmonella enteritidis Challenge. J. Appl. Poult. Res. 2007, 16, 361–364. [Google Scholar] [CrossRef]
- Menconi, A.; Wolfenden, A.D.; Shivaramaiah, S.; Terraes, J.C.; Urbano, T.; Kuttel, J.; Kremer, C.; Hargis, B.M.; Tellez, G. Effect of Lactic Acid Bacteria Probiotic Culture for the Treatment of Salmonella Enterica Serovar Heidelberg in Neonatal Broiler Chickens and Turkey Poults. Poult. Sci. 2011, 90, 561–565. [Google Scholar] [CrossRef]
- Higgins, S.E.; Torres-Rodriguez, A.; Vicente, J.L.; Sartor, C.D.; Pixley, C.M.; Nava, G.M.; Tellez, G.; Barton, J.T.; Hargis, B.M. Evaluation of Intervention Strategies for Idiopathic Diarrhea in Commercial Turkey Brooding Houses. J. Appl. Poult. Res. 2005, 14, 345–348. [Google Scholar] [CrossRef]
- Torres-Rodriguez, A.; Higgins, S.E.; Vicente, J.L.S.; Wolfenden, A.D.; Gaona-Ramirez, G.; Barton, J.T.; Tellez, G.; Donoghue, A.M.; Hargis, B.M. Effect of Lactose as a Prebiotic on Turkey Body Weight Under Commercial Conditions. J. Appl. Poult. Res. 2007, 16, 635–641. [Google Scholar] [CrossRef]
- Higgins, S.E.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M.; Porter, T.E. Transcriptional Profiling of Cecal Gene Expression in Probiotic- and Salmonella-Challenged Neonatal Chicks. Poult Sci. 2011, 90, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Isaias, G.; Vuong, C.N.; Graham, B.D.; Selby, C.M.; Graham, L.E.; Señas-Cuesta, R.; Barros, T.L.; Beer, L.C.; Coles, M.E.; Forga, A.J.; et al. Developing Probiotics, Prebiotics, and Organic Acids to Control Salmonella Spp. in Commercial Turkeys at the University of Arkansas, USA. Ger. J. Vet. Res. 2021, 1, 7–12. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Rosenzweig, W.D.; Powers, D.W. Isolation of a 250 Million-Year-Old Halotolerant Bacterium from a Primary Salt Crystal. Nature 2000, 407, 897–900. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The Use of Bacterial Spore Formers as Probiotics. FEMS MicroBiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef] [Green Version]
- Shivaramaiah, S.; Pumford, N.R.; Morgan, M.J.; Wolfenden, R.E.; Wolfenden, A.D.; Torres-Rodríguez, A.; Hargis, B.M.; Téllez, G. Evaluation of Bacillus Species as Potential Candidates for Direct-Fed Microbials in Commercial Poultry. Poult. Sci. 2011, 90, 1574–1580. [Google Scholar] [CrossRef]
- Wolfenden, R.E.; Pumford, N.R.; Morgan, M.J.; Shivaramaiah, S.; Wolfenden, A.D.; Pixley, C.M.; Green, J.; Tellez, G.; Hargis, B.M. Evaluation of Selected Direct-Fed Microbial Candidates on Live Performance and Salmonella Reduction in Commercial Turkey Brooding Houses. Poult. Sci. 2011, 90, 2627–2631. [Google Scholar] [CrossRef]
- Wolfenden, R.E.; Pumford, N.R.; Morgan, M.J.; Shivaramai, S.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Evaluation of a Screening and Selection Method for Bacillus Isolates for Use as Effective Direct-Fed Microbials in Commercial Poultry. Int. J. Poult. Sci. 2010, 9, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Ingale, S.L.; Kim, Y.W.; Kim, J.S.; Kim, K.H.; Lohakare, J.D.; Kim, E.K.; Kim, H.S.; Ryu, M.H.; Kwon, I.K.; et al. Effect of Supplementation of Bacillus Subtilis LS 1-2 to Broiler Diets on Growth Performance, Nutrient Retention, Caecal Microbiology and Small Intestinal Morphology. Res. Vet. Sci. 2012, 93, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N.R.; Morgan, M.J.; Layton, S.L.; Bielke, L.R.; Hargis, B.M.; Téllez, G. Evaluation of Germination, Distribution, and Persistence of Bacillus subtilis Spores through the Gastrointestinal Tract of Chickens. Poult. Sci. 2014, 93, 1793–1800. [Google Scholar] [CrossRef]
- Bedford, M.R.; Schulze, H. Exogenous Enzymes for Pigs and Poultry. Nutr. Res. Rev. 1998, 11, 91–114. [Google Scholar] [CrossRef]
- Esteve-Garcia, E.; Brufau, J.; Pérez-Vendrell, A.; Miquel, A.; Duven, K. Bioefficacy of Enzyme Preparations Containing Beta-Glucanase and Xylanase Activities in Broiler Diets Based on Barley or Wheat, in Combination with Flavomycin. Poult. Sci. 1997, 76, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Kogut, M.H.; Vicente, J.L.; Wolfenden, R.; Wolfenden, A.; Hargis, B.M.; Kuttappan, V.A.; Tellez, G. Role of a Bacillus Subtilis Direct-Fed Microbial on Digesta Viscosity, Bacterial Translocation, and Bone Mineralization in Turkey Poults Fed with a Rye-Based Diet. Front. Vet. Sci. 2014, 1, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.; Hernandez-Patlan, D.; Solis-Cruz, B.; Kwon, Y.M.; Arreguin, M.A.; Latorre, J.D.; Hernandez-Velasco, X.; Hargis, B.M.; Tellez-Isaias, G. Evaluation of the Antimicrobial and Anti-Inflammatory Properties of Bacillus-DFM (NorumTM) in Broiler Chickens Infected with Salmonella Enteritidis. Front. Vet. Sci. 2019, 6, 282. [Google Scholar] [CrossRef] [Green Version]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.M.; Pontin, K.P.; Latorre, J.D.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Arreguin, M.A.; Hargis, B.M.; et al. Evaluation of a Bacillus -Based Direct-Fed Microbial on Aflatoxin B1 Toxic Effects, Performance, Immunologic Status, and Serum Biochemical Parameters in Broiler Chickens. Avian Dis. 2019, 63, 659–669. [Google Scholar] [CrossRef]
- Black, S.; Fahrenholz, A.; Grimes, J.L. The effect of a direct-fed microbial and dietary fat inclusion on performance and energy metabolism in broiler chicks and turkey poults. Ger. J. Vet. Res. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Probiotics and Prebiotics in the Elderly. Postgrad. Med. J. 2004, 80, 447–451. [Google Scholar] [CrossRef]
- Hedin, C.; Whelan, K.; Lindsay, J.O. Evidence for the Use of Probiotics and Prebiotics in Inflammatory Bowel Disease: A Review of Clinical Trials. Proc. Nutr. Soc. 2007, 66, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A Review on Prebiotics and Probiotics for the Control of Dysbiosis: Present Status and Future Perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M. Toward a Better Understanding of Mechanisms of Probiotics and Prebiotics Action in Poultry Species. J. Appl. Poult. Res. 2016, 25, 277–283. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, Prebiotics, and Synbiotics: Approaches for Modulating the Microbial Ecology of the Gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics- a Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Hinz, S.W.A.; Beldman, G.; Vincken, J.-P.; Voragen, A.G.J. Bifidobacterium Carbohydrases-Their Role in Breakdown and Synthesis of (Potential) Prebiotics. Mol. Nutr. Food Res. 2008, 52, 146–163. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Tomar, S.; Chauhan, R. Beneficial Effects of Probiotics and Prebiotics in Livestock and Poultry: The Current Perspectives. Intas. Polivet. 2008, 9, 1–12. [Google Scholar]
- Janssens, G.P.J.; Millet, S.; Van Immerseel, F.; De Buck, J.; Hesta, M. The Impact of Prebiotics and Salmonellosis on Apparent Nutrient Digestibility and Salmonella typhimurium Var. Copenhagen Excretion in Adult Pigeons (Columba Livia Domestica). Poult. Sci. 2004, 83, 1884–1890. [Google Scholar] [CrossRef]
- Parracho, H.; McCartney, A.L.; Gibson, G.R. Probiotics and Prebiotics in Infant Nutrition. Proc. Nutr. Soc. 2007, 66, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, J.E.; Walker, W.A. Nutritional Impact of Pre- and Probiotics as Protective Gastrointestinal Organisms. Annu. Rev. Nutr. 2002, 22, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Ilyas, O.; Handan, E. Effects of dietary yeast cell wall on performance, egg quality and humoral immune response in laying hens. Ank. Üniv. Vet. Fakültesi Derg. 2014, 61, 289–294. [Google Scholar] [CrossRef]
- Harms, R.H.; Miles, R.D. Influence of Fermacto on the Performance of Laying Hens When Fed Diets with Different Levels of Methionine. Poult. Sci. 1988, 67, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodriguez, A.; Sartor, C.; Higgins, S.E.; Wolfenden, A.D.; Bielke, L.R.; Pixley, C.M.; Sutton, L.; Tellez, G.; Hargis, B.M. Effect of Aspergillus Meal Prebiotic (Fermacto) on Performance of Broiler Chickens in the Starter Phase and Fed Low Protein Diets. J. Appl. Poult. Res. 2005, 14, 665–669. [Google Scholar] [CrossRef]
- Uchima, C.A.; Tokuda, G.; Watanabe, H.; Kitamoto, K.; Arioka, M. Heterologous Expression and Characterization of a Glucose-Stimulated β-Glucosidase from the Termite Neotermes Koshunensis in Aspergillus Oryzae. Appl. MicroBiol. Biotechnol. 2011, 89, 1761–1771. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Hargis, B.M.; Tellez, G. Chitoneous Materials for Control of Foodborne Pathogens and Mycotoxins in Poultry. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; Dongre, R.S., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-406-0. [Google Scholar]
- Jonker, D.; Kuper, C.F.; Maquet, V.; Nollevaux, G.; Gautier, S. Subchronic (13-Week) Oral Toxicity Study in Rats with Fungal Chitin-Glucan from Aspergillus Niger. Food Chem. Toxicol. 2010, 48, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Hooge, D.M.; Sims, M.D.; Sefton, A.E.; Spring, P.; Connolly, A. Effect of Dietary Mannan Oligosaccharide, With or Without Bacitracin or Virginiamycin, on Live Performance of Broiler Chickens at Relatively High Stocking Density on New Litter. J. Appl. Poult. Res. 2003, 12, 461–467. [Google Scholar] [CrossRef]
- Kim, W.K.; Donalson, L.M.; Mitchell, A.D.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Effects of Alfalfa and Fructooligosaccharide on Molting Parameters and Bone Qualities Using Dual Energy X-ray Absorptiometry and Conventional Bone Assays. Poult. Sci. 2006, 85, 15–20. [Google Scholar] [CrossRef]
- Tellez, G.; Nava, G.M.; Vicente, J.L.; De Frances, M.; Morales, E.J.; Prado, O.; Terraes, J.C.; Hargis, B.M. Evaluation of Dietary Aspergillus Meal on Intestinal Morphometry in Turkey Poults. Int. J. Poult. Sci. 2010, 9, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Amirdahri, S.; Janmohammadi, H.; Taghizadeh, A.; Rafat, S.A. Effect of Dietary Aspergillus Meal Prebiotic on Growth Performance, Carcass Characteristics, Nutrient Digestibility, and Serum Lipid Profile in Broiler Chick Low-Protein Diets. Turk. J. Vet. Anim. Sci. 2012, 36. [Google Scholar] [CrossRef]
- Reginatto, A.R.; Menconi, A.; Londero, A.; Lovato, M.; Rosa, A.P.; Shivaramai, S.; Wolfenden, A.D.; Huff, W.E.; Huff, G.R.; Rath, N.C.; et al. Effects of Dietary Aspergillus Meal Prebiotic on Turkey Poults Production Parameters and Bone Qualities. Int. J. Poult. Sci. 2011, 10, 496–499. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Açil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; De Buck, J.; De Smet, I.; Mast, J.; Haesebrouck, F.; Ducatelle, R. Dynamics of Immune Cell Infiltration in the Caecal Lamina Propria of Chickens after Neonatal Infection with a Salmonella enteritidis Strain. Dev. Comp. Immunol. 2002, 26, 355–364. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of Stressors on Normal Intestinal Microbiota, Intestinal Morphology, and Susceptibility to Salmonella enteritidis Colonization in Broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Filipkowska, U.; Jóźwiak, T.; Szymczyk, P. Application of cross-linked chitosan for phosphate removal from aqueous solutions. Prog. Chem. Appl. Chitin Its Deriv. Volume XIX 2014, 19, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Londero, A.; Menconi, A.; Reginatto, A.R.; Bacocina, I.; Wolfenden, A.; Shivaramai, S.; Hargis, B.M.; Tellez, G. Effect of an Aspergillus Meal Prebiotic on Salmonella Infection in Turkeys and Broiler Chickens. Int. J. Poult. Sci. 2011, 10, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Yalçin, S.; Yalçin, S.; Eser, H.; Şahin, A.; Yalçin, S.S.; Güçer, Ş. Effects of Dietary Yeast Cell Wall Supplementation on Performance, Carcass Characteristics, Antibody Production and Histopathological Changes in Broilers. Kafkas Univ. Vet. Fak. Derg. 2014, 20. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of Dietary Inclusion of Probiotic and Synbiotic on Growth Performance, Organ Weights, and Intestinal Histomorphology of Broiler Chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef]
- Maiorano, G.; Sobolewska, A.; Cianciullo, D.; Walasik, K.; Elminowska-Wenda, G.; Slawinska, A.; Tavaniello, S.; Zylinska, J.; Bardowski, J.; Bednarczyk, M. Influence of in Ovo Prebiotic and Synbiotic Administration on Meat Quality of Broiler Chickens. Poult. Sci. 2012, 91, 2963–2969. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, W.L.; Feng, Y.; Yao, J.H. Effects of Immune Stress on Growth Performance, Immunity, and Cecal Microflora in Chickens. Poult. Sci. 2011, 90, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.V. The Human Gutome: Nutrigenomics of the Host-Microbiome Interactions. OMICS 2011, 15, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.; Higgins, S.E.; Donoghue, A.M.; Hargis, B.M. Digestive Physiology and the Role of Microorganisms. J. Appl. Poult. Res. 2006, 15, 136–144. [Google Scholar] [CrossRef]
- Weiss, A.S.; Burrichter, A.G.; Durai Raj, A.C.; von Strempel, A.; Meng, C.; Kleigrewe, K.; Münch, P.C.; Rössler, L.; Huber, C.; Eisenreich, W.; et al. In Vitro Interaction Network of a Synthetic Gut Bacterial Community. ISME J. 2021, 1–15. [Google Scholar] [CrossRef]
- Plöger, S.; Stumpff, F.; Penner, G.B.; Schulzke, J.-D.; Gäbel, G.; Martens, H.; Shen, Z.; Günzel, D.; Aschenbach, J.R. Microbial Butyrate and Its Role for Barrier Function in the Gastrointestinal Tract. Ann. N. Y. Acad. Sci. 2012, 1258, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Guan, N.L.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Obin, M.S.; Zhao, L. The Gut Microbiota, Obesity and Insulin Resistance. Mol. Aspects Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The Interaction between Bacteria and Bile. FEMS MicroBiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.F. Bile Acids: The Good, the Bad, and the Ugly. News Physiol. Sci. 1999, 14, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Calik, A.; Ceylan, A.; Ekim, B.; Adabi, S.G.; Dilber, F.; Bayraktaroglu, A.G.; Tekinay, T.; Özen, D.; Sacakli, P. The Effect of Intra-Amniotic and Posthatch Dietary Synbiotic Administration on the Performance, Intestinal Histomorphology, Cecal Microbial Population, and Short-Chain Fatty Acid Composition of Broiler Chickens. Poult. Sci. 2017, 96, 169–183. [Google Scholar] [CrossRef]
- Montagne, L.; Piel, C.; Lallès, J.P. Effect of Diet on Mucin Kinetics and Composition: Nutrition and Health Implications. Nutr. Rev. 2004, 62, 105–114. [Google Scholar] [CrossRef]
- Schippa, S.; Conte, M. Dysbiotic Events in Gut Microbiota: Impact on Human Health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef] [PubMed]
- EC (European Commission). Regulation 1831 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Off. J. Eur. Union L. 2003, 268, 29–43. [Google Scholar]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of Dietary Supplementation with Oregano Essential Oil on Performance of Broilers after Experimental Infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Isabel, B.; Santos, Y. Effects of Dietary Organic Acids and Essential Oils on Growth Performance and Carcass Characteristics of Broiler Chickens. J. Appl. Poult. Res. 2009, 18, 472–476. [Google Scholar] [CrossRef]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of Active Substances of Plant Origin in Chicken Diets Based on Maize and Locally Grown Cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar] [CrossRef]
- McReynolds, J.; Waneck, C.; Byrd, J.; Genovese, K.; Duke, S.; Nisbet, D. Efficacy of Multistrain Direct-Fed Microbial and Phytogenetic Products in Reducing Necrotic Enteritis in Commercial Broilers. Poult. Sci. 2009, 88, 2075–2080. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Yener, Y.; Yalçin, S.; Çolpan, İ. Effects of Dietary Supplementation of Red Ginseng Root Powder on Performance, Immune System, Cecal Microbial Population and Some Blood Parameters in Broilers. Ank. Üniv. Vet. Fakültesi Derg. 2020, 68, 137–145. [Google Scholar] [CrossRef]
- Mathe, A. Essential Oils: Basic and Applied Research. Allured Publishing Corporation, Carol Stream. In Essential Oils as Phytogenic Feed Additives; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 315–325. [Google Scholar]
- Salaheen, S.; Kim, S.-W.; Haley, B.J.; Van Kessel, J.A.S.; Biswas, D. Alternative Growth Promoters Modulate Broiler Gut Microbiome and Enhance Body Weight Gain. Front. Microbiol. 2017, 8, 2088. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal Microbiome-Metabolome Responses to Essential Oils in Piglets. Front. Microbiol. 2018, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Díaz Carrasco, J.M.; Redondo, E.A.; Pin Viso, N.D.; Redondo, L.M.; Farber, M.D.; Fernández Miyakawa, M.E. Tannins and Bacitracin Differentially Modulate Gut Microbiota of Broiler Chickens. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Berrocoso, J.D. Review: Dietary Fiber Utilization and Its Effects on Physiological Functions and Gut Health of Swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of Microbiota in One-Day Old Broiler Chickens with Antibiotic for 24 Hours Negatively Affects Intestinal Immune Development. BMC Genom. 2017, 18, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathlouthi, N.; Mallet, S.; Saulnier, L.; Quemener, B.; Larbier, M. Effects of Xylanase and?—Glucanase Additionon Performance, Nutrient Digestibility and Physico-Chemical Conditions in the Small Intestine Contents and Caecal Microflora of Broiler Chickens Feda Wheat and Barley-Based Diet. Anim. Res. 2002, 51, 395–406. [Google Scholar] [CrossRef]
- Cherbut, C. Motor Effects of Short-Chain Fatty Acids and Lactate in the Gastrointestinal Tract. Proc. Nutr. Soc. 2003, 62, 95–99. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic Effect of Berberine on Metabolic Diseases: Both Pharmacological Data and Clinical Evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.-Q.; Fan, D.-J.; Yang, S.-S.; Lin, X.; Meng, L.-K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tong, Q.; Shou, J.-W.; Zhao, Z.-X.; Li, X.-Y.; Zhang, X.-F.; Ma, S.-R.; He, C.-Y.; Lin, Y.; Wen, B.-Y.; et al. Gut Microbiota-Mediated Personalized Treatment of Hyperlipidemia Using Berberine. Theranostics 2017, 7, 2443–2451. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.-D.; Zhao, W.; Wang, Z.-Z.; Wang, S.-K.; Zhou, Z.-X.; Song, D.-Q.; Wang, Y.-M.; et al. Berberine Lowers Blood Glucose in Type 2 Diabetes Mellitus Patients through Increasing Insulin Receptor Expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S.; et al. Berberine Improved Experimental Chronic Colitis by Regulating Interferon-γ- and IL-17A-Producing Lamina Propria CD4+ T Cells through AMPK Activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Deng, H.; Li, Y.; Fan, T.; Liu, Y.; Tang, S.; Wei, W.; Liu, X.; Guo, X.; Jiang, J.; et al. Berberine Directly Targets the NEK7 Protein to Block the NEK7–NLRP3 Interaction and Exert Anti-Inflammatory Activity. J. Med. Chem. 2021, 64, 768–781. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The Effect of Berberine on Weight Loss in Order to Prevent Obesity: A Systematic Review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Ke, X.; Huang, Y.; Li, L.; Xin, F.; Xu, L.; Zhang, Y.; Zeng, Z.; Lin, F.; Song, Y. Berberine Attenuates Arterial Plaque Formation in Atherosclerotic Rats with Damp-Heat Syndrome via Regulating Autophagy. DDDT 2020, 14, 2449–2460. [Google Scholar] [CrossRef]
- Tan, W.; Wang, Y.; Wang, K.; Wang, S.; Liu, J.; Qin, X.; Dai, Y.; Wang, X.; Gao, X. Improvement of Endothelial Dysfunction of Berberine in Atherosclerotic Mice and Mechanism Exploring through TMT-Based Proteomics. Oxid. Med. Cell. Longev. 2020, 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, M.; Hu, Y.; Zhao, Y.; Teng, F.; Lv, X.; Li, J.; Zhang, Y.; Hatch, G.M.; Chen, L. Increased Bioavailable Berberine Protects Against Myocardial Ischemia Reperfusion Injury Through Attenuation of NFκB and JNK Signaling Pathways. Int. Heart J. 2018, 59, 1378–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Ameliorates Intestinal Epithelial Tight-Junction Damage and down-Regulates Myosin Light Chain Kinase Pathways in a Mouse Model of Endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Hu, M.; Huang, Z.; Fang, K.; Wang, D.; Chen, Q.; Li, J.; Yang, D.; Zou, X.; Xu, L.; et al. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front. Pharmacol. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Li, N.; Li, Q.; Zhang, Q.; Wang, C.; Zhu, W.; Li, J. The Effect of Berberine in Vitro on Tight Junctions in Human Caco-2 Intestinal Epithelial Cells. Fitoterapia 2009, 80, 241–248. [Google Scholar] [CrossRef]
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Attenuates Pro-Inflammatory Cytokine-Induced Tight Junction Disruption in an in Vitro Model of Intestinal Epithelial Cells. Eur. J. Pharm. Sci. 2010, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.P.; Afrin, F.; Flores, R.A.; Kim, W.H.; Jeong, J.; Kim, S.; Chang, H.H.; Lillehoj, H.S.; Min, W. Downregulation of Inflammatory Cytokines by Berberine Attenuates Riemerella anatipestifer Infection in Ducks. Dev. Comp. Immunol. 2017, 77, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine Pharmacology and the Gut Microbiota: A Hidden Therapeutic Link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Lin, L.; Xie, Y.; Li, D.; Xiao, M.; Zhang, Y.; Cheung, S.C.K.; Shaw, P.C.; Yang, X.; Chan, P.K.S.; et al. Blood-Glucose-Lowering Effect of Coptidis Rhizoma Extracts from Different Origins via Gut Microbiota Modulation in Db/Db Mice. Front. Pharmacol. 2021, 12, 684358. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, K.; Bai, Y.; Feng, X.; Gong, L.; Wei, C.; Huang, H.; Zhang, H. Dietary Supplementation with Berberine Improves Growth Performance and Modulates the Composition and Function of Cecal Microbiota in Yellow-Feathered Broilers. Poult. Sci. 2021, 100, 1034–1048. [Google Scholar] [CrossRef]
- Xiang Yu, D.; He, Z.; Pouton, C.; Hoerr, F.J.; Xiao, Z.C. Target Animal Safety and Residual Study for Berberine and Other Phytogenic Compounds in Broiler Chickens. Arch. Clin. MicroBiol. 2017, 8, 69. [Google Scholar] [CrossRef]
- Malik, T.A.; Kamili, A.N.; Chishti, M.Z.; Tanveer, S.; Ahad, S.; Johri, R.K. Synergistic Approach for Treatment of Chicken Coccidiosis Using Berberine—A Plant Natural Product. Microb. Pathog. 2016, 93, 56–62. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Liang, X.; Wang, M.; Zhu, X.; Luo, Y.; Shang, Y.; Yang, J.; Zhou, P.; Gu, X. Effects of Berberine on the Growth Performance, Antioxidative Capacity and Immune Response to Lipopolysaccharide Challenge in Broilers. Anim. Sci. J. 2019, 90, 1229–1238. [Google Scholar] [CrossRef]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P. The Effects of Berberine on the Magnitude of the Acute Inflammatory Response Induced by Escherichia Coli Lipopolysaccharide in Broiler Chickens. Poult. Sci. 2010, 89, 13–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Huang, J.; Sun, Y.; He, F.; Zloh, M.; Wang, L. Inhibitory Effect of Berberine on Broiler P-Glycoprotein Expression and Function: In Situ and In Vitro Studies. IJMS 2019, 20, 1966. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.B.; Yang, Q.; Osban, J.; Azzarello, J.T.; Saban, M.R.; Saban, R.; Ashley, R.A.; Welter, J.C.; Fung, K.-M.; Lin, H.-K. Frankincense Oil Derived from Boswellia Carteri Induces Tumor Cell Specific Cytotoxicity. BMC Complement. Altern. Med. 2009, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Poeckel, D.; Werz, O. Boswellic Acids: Biological Actions and Molecular Targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef]
- Anthoni, C.; Laukoetter, M.G.; Rijcken, E.; Vowinkel, T.; Mennigen, R.; Müller, S.; Senninger, N.; Russell, J.; Jauch, J.; Bergmann, J.; et al. Mechanisms Underlying the Anti-Inflammatory Actions of Boswellic Acid Derivatives in Experimental Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1131–G1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammon, H.P.T. Modulation of the Immune System by Boswellia Serrata Extracts and Boswellic Acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An Overall Assessment of in Vitro, Preclinical, Pharmacokinetic and Clinical Data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia Serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.E.; Abdelnour, S.A.; Shehata, S.A.; Abd El-Hack, M.E.; El-Edel, M.A.; Taha, A.E.; Schiavitto, M.; Tufarelli, V. Effect of Dietary Boswellia Serrata Resin on Growth Performance, Blood Biochemistry, and Cecal Microbiota of Growing Rabbits. Front. Vet. Sci. 2019, 6, 471. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.H.; Attia, A.I.; Reda, F.M.; Abd El-Hack, M.E.; Ismail, I.E. Impacts of Dietary Supplementation of Boswellia Serrata on Growth, Nutrients Digestibility, Immunity, Antioxidant Status, Carcase Traits and Caecum Microbiota of Broilers. Ital. J. Anim. Sci. 2021, 20, 205–214. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.M.; Kiczorowska, B.; Samolińska, W.; Kowalczuk-Vasilev, E.; Kowalczyk-Pecka, D. The Effect of Boswellia Serrata Resin Diet Supplementation on Production, Hematological, Biochemical and Immunological Parameters in Broiler Chickens. Animal 2017, 11, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Santos, E.A.D.; Alvarez-Leite, J.I. Capsaicin: A Potential Therapy Adjuvant for Intestinal Bowel Disease. JDDD 2019, 2, 8–16. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, J.R.; Maruniak, J.A. Behavioral and Physiological Effects of Capsaicin in Red-Winged Blackbirds. Pharmacol. Biochem. Behav. 1983, 19, 857–862. [Google Scholar] [CrossRef]
- Geisthövel, E.; Ludwig, O.; Simon, E. Capsaicin Fails to Produce Disturbances of Autonomic Heat and Cold Defence in an Avian Species (Anas platyrhynchos). Pflugers. Arch. 1986, 406, 343–350. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Jancsó-Gábor, A. Sensory Effects of Capsaicin Congeners I. Relationship between Chemical Structure and Pain-Producing Potency of Pungent Agents. Arzneimittelforschung 1975, 25, 1877–1881. [Google Scholar]
- Muley, B.; Khadabadi, S.; Banarase, N. Phytochemical Constituents and Pharmacological Activities of Calendula Officinalis Linn (Asteraceae): A Review. Trop. J. Pharm. Res. 2009, 8. [Google Scholar] [CrossRef] [Green Version]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-Inflammatory, Anti-Tumor-Promoting, and Cytotoxic Activities of Constituents of Marigold (Calendula officinalis) Flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Kageura, T.; Matsuda, H. Medicinal Flowers. III. Marigold. (1): Hypoglycemic, Gastric Emptying Inhibitory, and Gastroprotective Principles and New Oleanane-Type Triterpene Oligoglycosides, Calendasaponins A, B, C, and D, from Egyptian Calendula Officinalis. Chem. Pharm. Bull. 2001, 49, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-Oedematous Activities of the Main Triterpendiol Esters of Marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV Activity of Extracts from Calendula officinalis Flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Catanzaro, D.; Cocetta, V.; Igl, N.; Ragazzi, E.; Giron, M.C.; Cecconello, L.; Montopoli, M. Protective Effects of ψ Taraxasterol 3-O-Myristate and Arnidiol 3-O-Myristate Isolated from Calendula officinalis on Epithelial Intestinal Barrier. Fitoterapia 2016, 109, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroutankhah, M.; Toghyani, M.; Landy, N. Evaluation of Calendula officinalis L. (Marigold) Flower as a Natural Growth Promoter in Comparison with an Antibiotic Growth Promoter on Growth Performance, Carcass Traits and Humoral Immune Responses of Broilers. Anim. Nutr. 2019, 5, 314–318. [Google Scholar] [CrossRef]
- Rajput, N.; Naeem, M.; Ali, S.; Rui, Y.; Tian, W. Effect of Dietary Supplementation of Marigold Pigment on Immunity, Skin and Meat Color, and Growth Performance of Broiler Chickens. Braz. J. Poult. Sci. 2012, 4, 233–304. [Google Scholar] [CrossRef]
- Page, J.E.; Nagel, J. Chapter Eight Biosynthesis of Terpenophenolic Metabolites in Hop and Cannabis. In Recent Advances in Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Klein, T.W.; Cabral, G.A. Cannabinoid-Induced Immune Suppression and Modulation of Antigen-Presenting Cells. J. Neuroimmune Pharmacol. 2006, 1, 50–64. [Google Scholar] [CrossRef]
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the Gut: New Developments and Emerging Concepts. Pharmacol. Ther. 2010, 126, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Szkopek, D.; Kinsner, M.; Fotschki, B.; Juśkiewicz, J.; Banach, J. Cannabis-Derived Cannabidiol and Nanoselenium Improve Gut Barrier Function and Affect Bacterial Enzyme Activity in Chickens Subjected to C. Perfringens Challenge. Vet. Res. 2020, 51, 141. [Google Scholar] [CrossRef]
- Alhamoruni, A.; Lee, A.C.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Pharmacological Effects of Cannabinoids on the Caco-2 Cell Culture Model of Intestinal Permeability. J. Pharmacol. Exp. Ther. 2010, 335, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Barceloux, D. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants and Venomous Animals; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Han, X.; Parker, T.L. Anti-Inflammatory Activity of Clove (Eugenia caryophyllata) Essential Oil in Human Dermal Fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Pirgozliev, V.; Rose, S.; Catherine, I.; Blanchard, A. Phytogenic Feed Additives Can Alleviate the Negative Impact of Necrotic Enteritis in Broilers. In Proceedings of the 6th International Conference on Poultry Intestinal Health, Rome, Italy, 3–5 April 2019. [Google Scholar]
- Kumar, A.; Kheravii, S.K.; Ionescu, C.; Blanchard, A.; Barekatain, R.; Bajagai, Y.S.; Wu, S.-B. A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers. Microorganisms 2021, 9, 1451. [Google Scholar] [CrossRef]
- Ajdžanović, V.Z.; Medigović, I.M.; Pantelić, J.B.; Milošević, V.Lj. Soy Isoflavones and Cellular Mechanics. J. Bioenerg. Biomembr. 2014, 46, 99–107. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Jiang, S.Q.; Lin, Y.C.; Xi, P.B.; Yu, D.Q.; Wu, T.X. Effects of Soybean Isoflavone on Growth Performance, Meat Quality, and Antioxidation in Male Broilers. Poult. Sci. 2007, 86, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Shi, D.-X.; Zhou, B.-C.; Zeng, C.-K.; Pang, S.-J. Study on the Structure of C-Phycocyanin in Spirulina Platensis with Scanning Tunneling Microscope. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao 1997, 29, 521–525. [Google Scholar] [PubMed]
- Messina, M.; Ho, S.; Alekel, D.L. Skeletal Benefits of Soy Isoflavones: A Review of the Clinical Trial and Epidemiologic Data. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D.R. Factors Affecting the Bioavailability of Soy Isoflavones in Humans after Ingestion of Physiologically Relevant Levels from Different Soy Foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Azzam, M.M.; Jiang, S.; Chen, J.; Lin, X.; Gou, Z.; Fan, Q.; Wang, Y.; Li, L.; Jiang, Z. Effect of Soybean Isoflavones on Growth Performance, Immune Function, and Viral Protein 5 MRNA Expression in Broiler Chickens Challenged with Infectious Bursal Disease Virus. Animals 2019, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A. Potential Risks and Benefits of Phytoestrogen-Rich Diets. Int. J. Vitam. Nutr. Res. 2003, 73, 120–126. [Google Scholar] [CrossRef]
- Yousef, M.I.; Esmail, A.M.; Baghdadi, H.H. Effect of Isoflavones on Reproductive Performance, Testosterone Levels, Lipid Peroxidation, and Seminal Plasma Biochemistry of Male Rabbits. J. Environ. Sci. Health B 2004, 39, 819–833. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, J.-M.; Bak, D.-J.; Jean, W.-M.; Song, J.-C.; Kim, S.-K.; An, B.-K.; Kang, C.-W.; Jung, W.-S.; Kim, J.-M. Effects of Germinated and Fermented Unmarketable Soybean on Laying Performance and Egg Quality in Laying Hens. Korean J. Food Sci. Anim. Resour. 2008, 28, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.R.; Gu, H.; Chang, L.L.; Wang, Z.Y.; Tong, H.B.; Zou, J.M. Safety Evaluation of Daidzein in Laying Hens: Part I. Effects on Laying Performance, Clinical Blood Parameters, and Organs Development. Food Chem. Toxicol. 2013, 55, 684–688. [Google Scholar] [CrossRef]
- Zhao, X.; Shao, T.; Wang, Y.Q.; Lu, X.L.; Luo, J.B.; Zhou, W.D. The Phytoestrogen Daidzein May Affect Reproductive Performance of Zhedong White Geese by Regulating Gene MRNA Levels in the HPG Axis. Br. Poult. Sci. 2013, 54, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Q.; Zhou, Y.C.; Ni, Y.D.; Lu, L.Z.; Tao, Z.R.; Chen, W.H.; Chen, J. Effect of Daidzein on Egg-Laying Performance in Shaoxing Duck Breeders during Different Stages of the Egg Production Cycle. Br. Poult. Sci. 2005, 46, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.D.; Wu, J.; Tong, H.Y.; Huang, Y.B.; Lu, L.Z.; Grossmann, R.; Zhao, R.Q. Effect of Dietary Daidzein Supplementation on Egg Laying Rate Was Associated with the Change of Hepatic VTG-II MRNA Expression and Higher Antioxidant Activities during the Post-Peak Egg Laying Period of Broiler Breeders. Anim. Feed. Sci. Technol. 2012, 177, 116–123. [Google Scholar] [CrossRef]
- Sahin, N.; Onderci, M.; Balci, T.A.; Cikim, G.; Sahin, K.; Kucuk, O. The Effect of Soy Isoflavones on Egg Quality and Bone Mineralisation during the Late Laying Period of Quail. Br. Poult. Sci. 2007, 48, 363–369. [Google Scholar] [CrossRef]
- Kajiya, H.; Okabe, K.; Okamoto, F.; Tsuzuki, T.; Soeda, H. Protein Tyrosine Kinase Inhibitors Increase Cytosolic Calcium and Inhibit Actin Organization as Resorbing Activity in Rat Osteoclasts. J. Cell Physiol. 2000, 183, 83–90. [Google Scholar] [CrossRef]
- Kosina, P.; Gregorova, J.; Gruz, J.; Vacek, J.; Kolar, M.; Vogel, M.; Roos, W.; Naumann, K.; Simanek, V.; Ulrichova, J. Phytochemical and Antimicrobial Characterization of Macleaya Cordata Herb. Fitoterapia 2010, 81, 1006–1012. [Google Scholar] [CrossRef]
- Le, H.H.; Shakeri, M.; Suleria, H.A.R.; Zhao, W.; McQuade, R.M.; Phillips, D.J.; Vidacs, E.; Furness, J.B.; Dunshea, F.R.; Artuso-Ponte, V.; et al. Betaine and Isoquinoline Alkaloids Protect against Heat Stress and Colonic Permeability in Growing Pigs. Antioxidants 2020, 9, 1024. [Google Scholar] [CrossRef]
- Vrublova, E.; Vostalova, J.; Ehrmann, J.; Palikova, I.; Vrbkova, J.; Vacek, J.; Cibicek, N.; Vecera, R.; Ulrichova, J.; Simanek, V. The Phytogenetic Feed Additive Sangrovit Modulates Dextran Sulfate Sodium-Induced Colitis in Rats. Vet. Med. 2010, 55, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Robbins, R.C.; Artuso-Ponte, V.C.; Moeser, A.J.; Morrow, W.E.M.; Spears, J.W.; Gebreyes, W.A. Effects of Quaternary Benzo(c)Phenanthridine Alkaloids on Growth Performance, Shedding of Organisms, and Gastrointestinal Tract Integrity in Pigs Inoculated with Multidrug-Resistant Salmonella Spp. Am. J. Vet. Res. 2013, 74, 1530–1535. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral Bioavailability of Curcumin: Problems and Advancements. J. Drug Target 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive Tree (Olea europaea) Leaves: Potential Beneficial Effects on Human Health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Talhaoui, N.; Vezza, T.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Gálvez, J.; Segura-Carretero, A. Phenolic Compounds and in Vitro Immunomodulatory Properties of Three Andalusian Olive Leaf Extracts. J. Funct. Foods 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Deiana, M.; Serra, G.; Corona, G. Modulation of Intestinal Epithelium Homeostasis by Extra Virgin Olive Oil Phenolic Compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and Immunologically Beneficial Impact of Extra Virgin Olive and Flaxseed Oils on Composition of Gut Microbiota in Mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef] [Green Version]
- Şenay, S.; Dursen, Ü. The Use of Grape Seed-, Olive Leaf- and Pomegranate Peel-Extracts as Alternative Natural Antimicrobial Feed Additives in Broiler Diets; Verlag Eugen Ulmer: Stuttgart, Germany, 2016. [Google Scholar]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.J.; Mereu, A.; Ipharraguerre, I.R.; Menoyo, D. Effects of a Bioactive Olive Pomace Extract from Olea Europaea on Growth Performance, Gut Function, and Intestinal Microbiota in Broiler Chickens. Poult. Sci. 2020, 99, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodríguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory Properties of Olea Europaea Leaf Extract in Intestinal Inflammation. Mol. Nutr. Food Res. 2017, 61, 1601066. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of Potentially Anticarcinogenic Flavonoids of 28 Vegetables and 9 Fruits Commonly Consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium f Alciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-Alanine Ligase as a New Target for the Flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal MRNA Expression Genes in Broiler Chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin Enhances Intestinal Barrier Function through the Assembly of Zonula [Corrected] Occludens-2, Occludin, and Claudin-1 and the Expression of Claudin-4 in Caco-2 Cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef]
- Amasheh, M.; Grotjohann, I.; Amasheh, S.; Fromm, A.; Söderholm, J.D.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Regulation of Mucosal Structure and Barrier Function in Rat Colon Exposed to Tumor Necrosis Factor Alpha and Interferon Gamma in Vitro: A Novel Model for Studying the Pathomechanisms of Inflammatory Bowel Disease Cytokines. Scand. J. Gastroenterol. 2009, 44, 1226–1235. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols Protect the Epithelial Barrier Function of Caco-2 Cells Exposed to Indomethacin through the Modulation of Occludin and Zonula Occludens-1 Expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W.; et al. Structure Activity Relationship of Antioxidative Property of Flavonoids and Inhibitory Effect on Matrix Metalloproteinase Activity in UVA-Irradiated Human Dermal Fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Agullo, G.; Gamet-Payrastre, L.; Manenti, S.; Viala, C.; Rémésy, C.; Chap, H.; Payrastre, B. Relationship between Flavonoid Structure and Inhibition of Phosphatidylinositol 3-Kinase: A Comparison with Tyrosine Kinase and Protein Kinase C Inhibition. Biochem. Pharmacol. 1997, 53, 1649–1657. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement Alternat. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. IJCM 2015, 06, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. MicroBiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalçin, S.; Eser, H.; Onbaşilar, İ.; Yalçin, S. Effects of Dried Thyme (Thymus vulgaris L.) Leaves on Performance, Some Egg Quality Traits and Immunity in Laying Hens. Ank. Üniv. Vet. Fakültesi Derg. 2020, 67, 303–311. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular Basis of Epithelial Barrier Regulation: From Basic Mechanisms to Clinical Application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [Green Version]
- Placha, I.; Chrastinova, L.; Laukova, A.; Cobanova, K.; Takacova, J.; Strompfova, V.; Chrenkova, M.; Formelova, Z.; Faix, S. Effect of Thyme Oil on Small Intestine Integrity and Antioxidant Status, Phagocytic Activity and Gastrointestinal Microbiota in Rabbits. Acta Vet. Hung. 2013, 61, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Higashi, N.; Koga, K. Antioxidant and Antiinflammatory Activities of Oregano Extract. J. Health Sci. 2006, 52, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Parker, T.L. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of Thymol and Carvacrol Supplementation on Intestinal Integrity and Immune Responses of Broiler Chickens Challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of Thymol and Carvacrol Feed Supplementation on Performance, Antioxidant Enzyme Activities, Fatty Acid Composition, Digestive Enzyme Activities, and Immune Response in Broiler Chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential Oil and Aromatic Plants as Feed Additives in Non-Ruminant Nutrition: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary Oregano Essential Oil Supplementation Improves Intestinal Functions and Alters Gut Microbiota in Late-Phase Laying Hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol Alleviates Heat Stress-Induced Impairment of Intestinal Morphology, Microflora, and Barrier Integrity in Broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-Kappa B, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol Induces Antioxidant and Heat Shock Protein MRNA Expression in Response to Heat Stress in Black-Boned Chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Mayangsari, Y.; Suzuki, T. Resveratrol Enhances Intestinal Barrier Function by Ameliorating Barrier Disruption in Caco-2 Cell Monolayers. J. Funct. Foods 2018, 51, 39–46. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Han, X.; Hu, D.; Hu, X.; Li, Y.; Huang, P.; Yao, W. Resveratrol Suppresses Gut-Derived NLRP3 Inflammasome Partly through Stabilizing Mast Cells in a Rat Model. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Zhao, X.; Chen, X.; Wang, L.; Geng, Z. Effect of Dietary Resveratrol Supplementation on Meat Quality, Muscle Antioxidative Capacity and Mitochondrial Biogenesis of Broilers. J. Sci. Food Agric. 2018, 98, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Chen, X.; Geng, Z.; Hu, H.; Zhang, C. Effects of Resveratrol on Growth Performance, Intestinal Development, and Antioxidant Status of Broilers under Heat Stress. Animals 2021, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Antiobesity Molecular Mechanisms of Action: Resveratrol and Pterostilbene. Biofactors 2018, 44, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.-T.; Ye, X.-L.; Li, R.-R.; Chen, H.; Wang, Y.-Y.; Yong, H.-J.; Pan, M.-L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol Modulates the Gut Microbiota to Prevent Murine Colitis Development through Induction of Tregs and Suppression of Th17 Cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Miyazawa, T. Occurrence of Orally Administered Curcuminoid as Glucuronide and Glucuronide/Sulfate Conjugates in Rat Plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the Cancer Chemopreventive Agent Curcumin in Human and Rat Intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of Curcumin through Reduction and Glucuronidation in Mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in Cancer Chemoprevention: Molecular Targets, Pharmacokinetics, Bioavailability, and Clinical Trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-K. The Effects of Black Pepper on the Intestinal Absorption and Hepatic Metabolism of Drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-Fold When Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.-H. Recent Advances in the Investigation of Curcuminoids. Chin. Med. 2008, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin Alleviates Ethanol-Induced Hepatocytes Oxidative Damage Involving Heme Oxygenase-1 Induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-Alpha-Induced Increase in Intestinal Epithelial Tight Junction Permeability Requires NF-Kappa B Activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [Green Version]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 and Protects Endothelial Cells against Oxidative Stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin Induces Heme Oxygenase 1 through Generation of Reactive Oxygen Species, P38 Activation and Phosphatase Inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef]
- Huang, M.T.; Newmark, H.L.; Frenkel, K. Inhibitory Effects of Curcumin on Tumorigenesis in Mice. J. Cell Biochem. Suppl. 1997, 27, 26–34. [Google Scholar] [CrossRef]
- Carmody, R.N.; Turnbaugh, P.J. Host-Microbial Interactions in the Metabolism of Therapeutic and Diet-Derived Xenobiotics. J. Clin. Invest. 2014, 124, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Curcumin-Attenuated Trinitrobenzene Sulphonic Acid Induces Chronic Colitis by Inhibiting Expression of Cyclooxygenase-2. WJG 2006, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, E2499. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23. [Google Scholar] [CrossRef]
- Li, S.; Fu, C.; Zhao, Y.; He, J. Intervention with α -Ketoglutarate Ameliorates Colitis-Related Colorectal Carcinoma via Modulation of the Gut Microbiome. BioMed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, G.; Hao, J.; Ma, J.; Wang, Y.; Jiang, X.; Jiang, H. Curcumin Ameliorates Hydrogen Peroxide-Induced Epithelial Barrier Disruption by Upregulating Heme Oxygenase-1 Expression in Human Intestinal Epithelial Cells. Dig. Dis. Sci. 2012, 57, 1792–1801. [Google Scholar] [CrossRef]
- Leyva-Diaz, A.A.; Hernandez-Patlan, D.; Solis-Cruz, B.; Adhikari, B.; Kwon, Y.M.; Latorre, J.D.; Hernandez-Velasco, X.; Fuente-Martinez, B.; Hargis, B.M.; Lopez-Arellano, R.; et al. Evaluation of Curcumin and Copper Acetate against Salmonella Typhimurium Infection, Intestinal Permeability, and Cecal Microbiota Composition in Broiler Chickens. J. Anim. Sci. Biotechnol. 2021, 12, 23. [Google Scholar] [CrossRef]



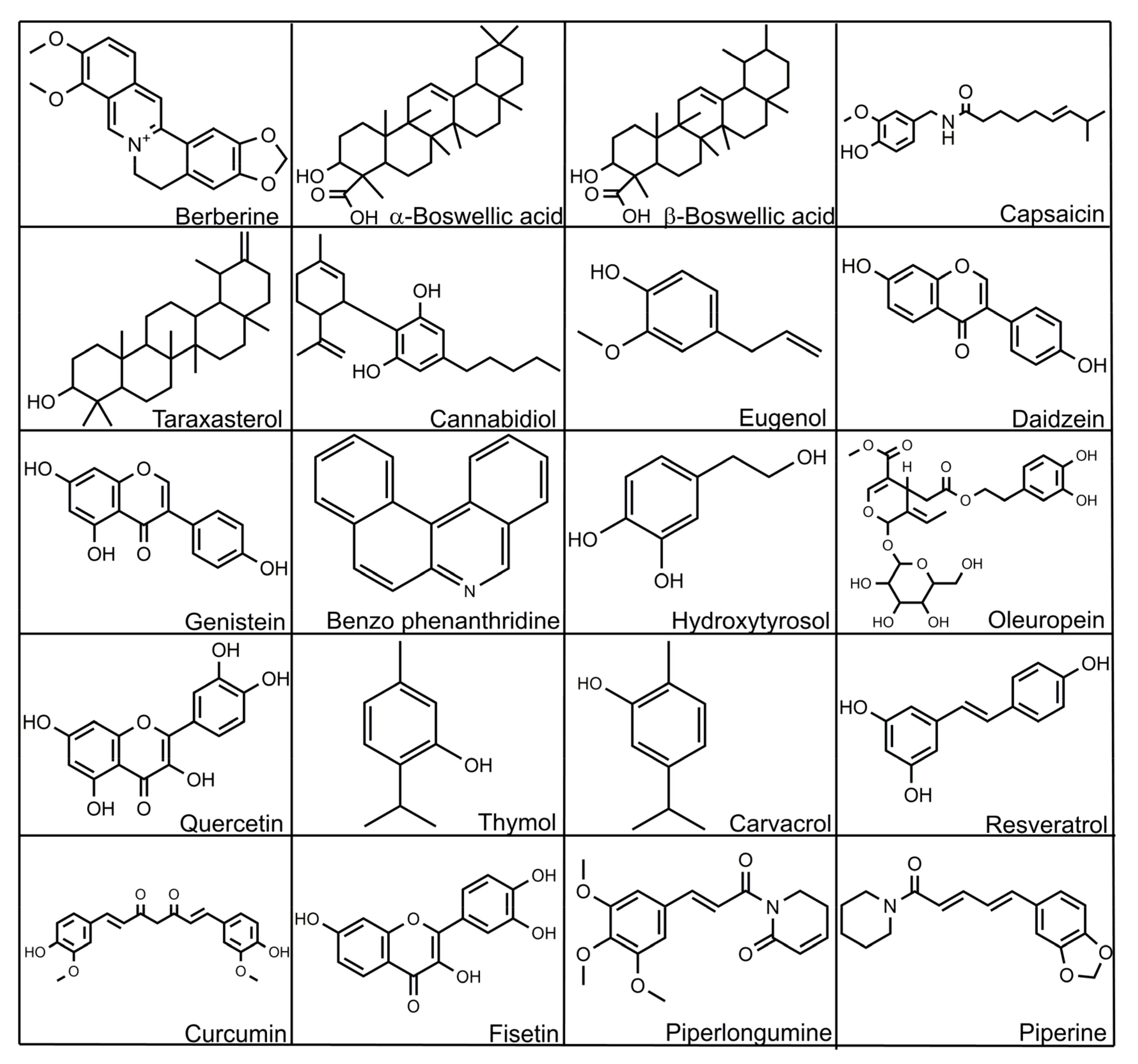
| Measurement/Function | Biomarker Type |
|---|---|
| Antioxidant activity | Superoxide dismutase (SOD), Thiobarbituric acid reactive substances (TBARS), Total antioxidant capacity |
| Gene expression of host protein biomarkers and tight junction | Fatty acid binding protein (FABP), Fibronectin, Occludin, Zonula occludens, Claudins |
| Immune activity | Acute phase proteins, Calprotectin, Lipocalin, Immunoglobulins (IgA), Interferon gamma (INF-γ) |
| Intestinal permeability | Fluorescein isothiocyanate dextran (FITC-d), Trans epithelial electrical resistance (TEER), Bacterial translocation |
| Enterocyte function | Extracellular signal-regulated kinase (ERK), Citrulline |
| Enterotypes | Biological Activities | Favorable Substance/s |
|---|---|---|
| Bacteroides |
| proteins and fats |
| Prevotella |
| high fiber diet |
| Ruminococcus |
| Sugars |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. https://doi.org/10.3390/microorganisms10020395
Shehata AA, Yalçın S, Latorre JD, Basiouni S, Attia YA, Abd El-Wahab A, Visscher C, El-Seedi HR, Huber C, Hafez HM, et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms. 2022; 10(2):395. https://doi.org/10.3390/microorganisms10020395
Chicago/Turabian StyleShehata, Awad A., Sakine Yalçın, Juan D. Latorre, Shereen Basiouni, Youssef A. Attia, Amr Abd El-Wahab, Christian Visscher, Hesham R. El-Seedi, Claudia Huber, Hafez M. Hafez, and et al. 2022. "Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry" Microorganisms 10, no. 2: 395. https://doi.org/10.3390/microorganisms10020395











