Short-Term Exposure to Thermophilic Temperatures Facilitates CO Uptake by Thermophiles Maintained under Predominantly Mesophilic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. CO-Oxidizing Isolates
2.2. Temperature Responses
2.3. CO Uptake Assays
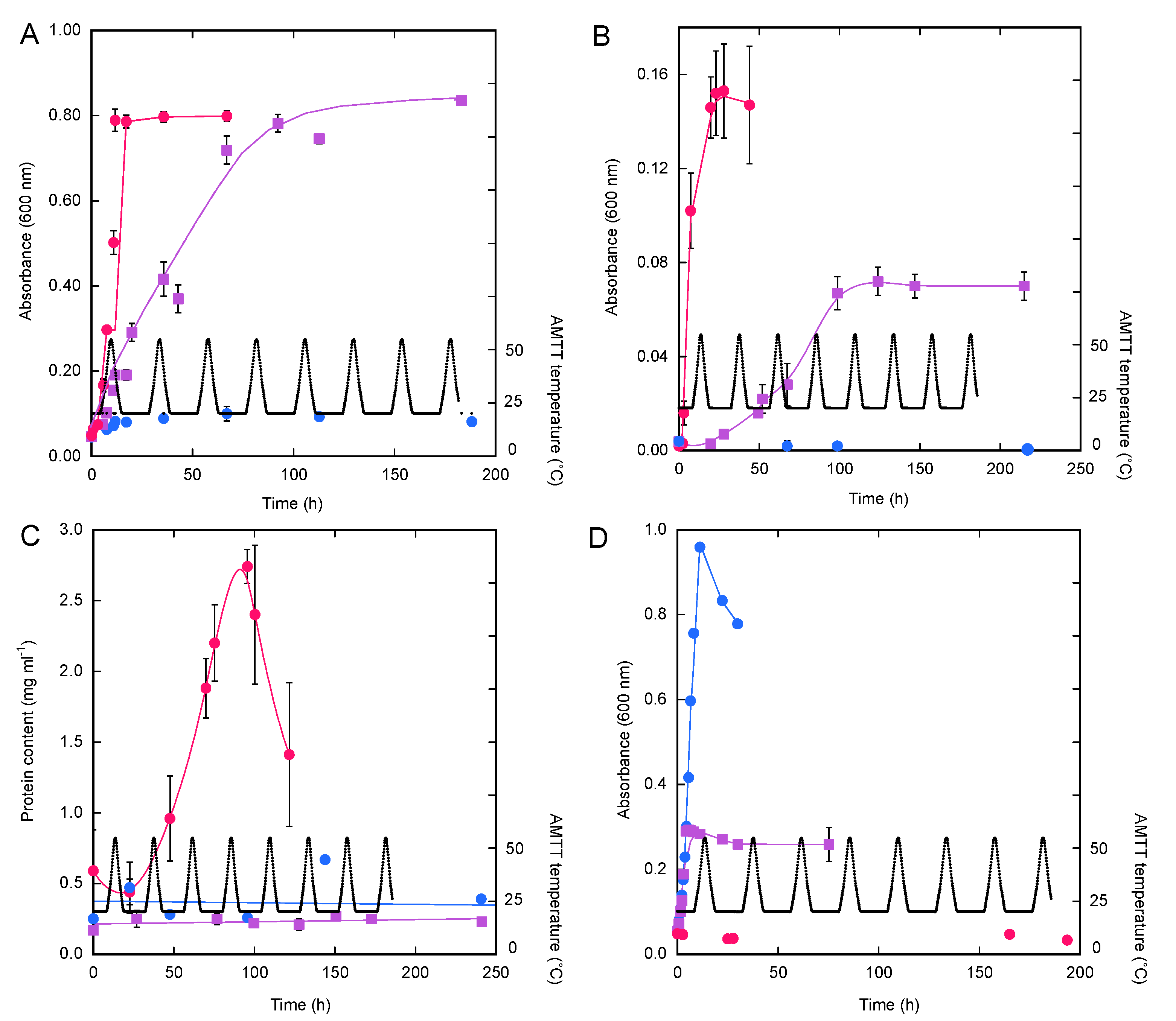
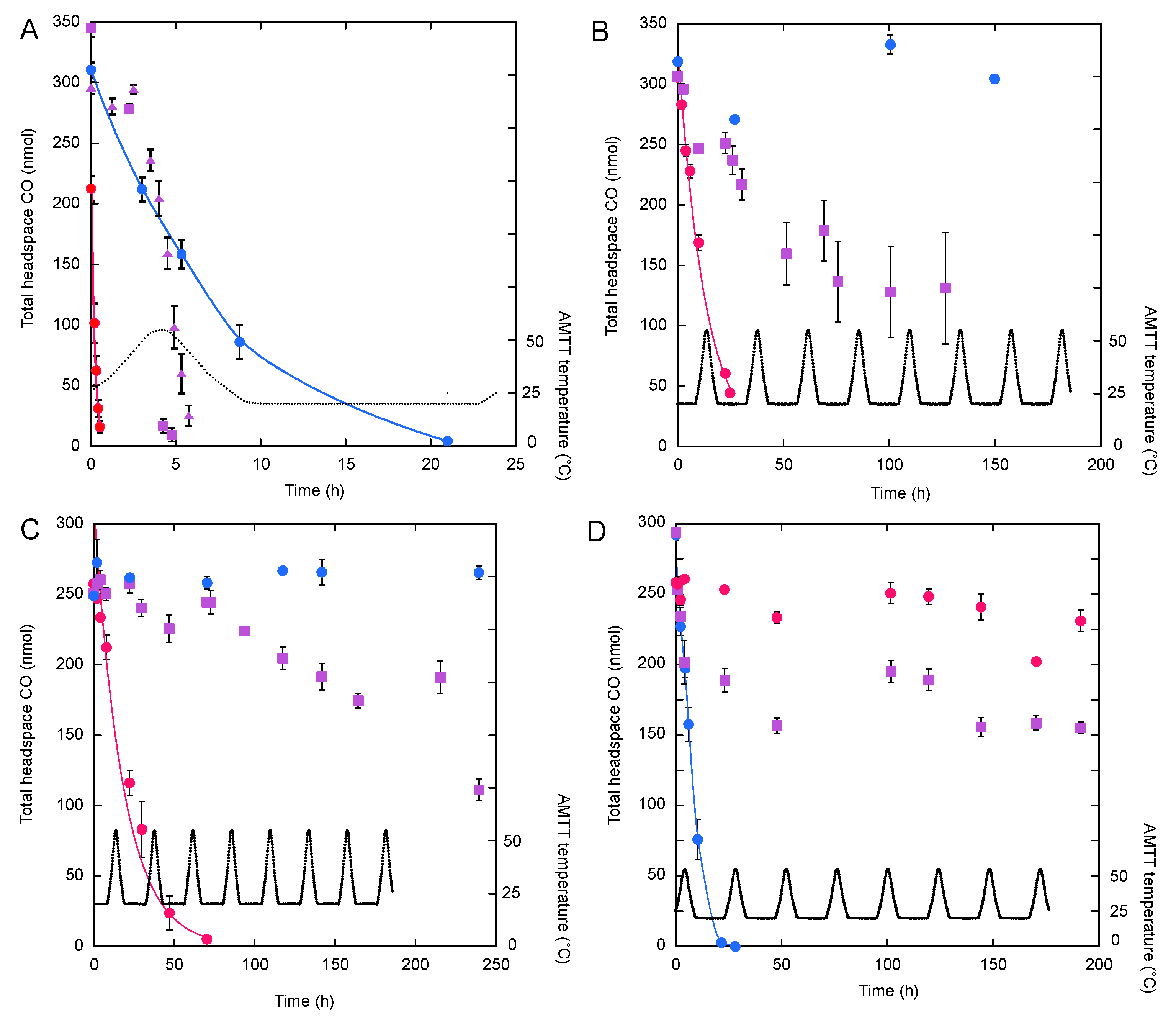
3. Results
3.1. Isolate Growth
3.2. Isolate CO Uptake
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Nussinov, R. How do thermophilic proteins deal with heat? Cell. Mol. Life Sci. 2001, 58, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Fukunaga, N. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. Microbiol. Rev. 1990, 75, 171–182. [Google Scholar] [CrossRef]
- Berezovsky, I.N.; Shakhnovich, E.I. Physics and evolution of thermophilic adaptation. Proc. Natl. Acad. Sci. USA 2005, 102, 12742–12747. [Google Scholar] [CrossRef] [Green Version]
- Puigbò, P.; Pasamontes, A.; Garcia-Vallve, S. Gaining and losing the thermophilic adaptation in prokaryotes. Trends Genet. 2008, 24, 10–14. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Temperature adaptation at homologous sites in proteins from nine thermophile-mesophile species pairs. Genome Biol. Evol. 2010, 2, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.M.; Falconer, C.; Colby, J.; Williams, E. CO metabolism by a thermophilic actinomycete, Streptomyces strain G26. Microbiology 1987, 12, 3445–3456. [Google Scholar] [CrossRef] [Green Version]
- Isaksen, M.F.; Bak, F.; Jørgensen, B.B. Thermophilic sulfate-reducing bacteria in cold marine sediment. FEMS Microbiol. Ecol. 1994, 14, 1–8. [Google Scholar] [CrossRef]
- Kim, S.B.; Goodfellow, M. Streptomyces thermospinisporus sp. nov., a moderately thermophilic carboxydotrophic streptomycete isolated from soil. Int. J. Syst. Evol. Microbiol. 2002, 52, 1225–1228. [Google Scholar]
- Marchant, R.; Banat, I.M.; Rahman, T.J.; Berzano, M. The frequency and characteristics of highly thermophilic bacteria in cool soil environments. Environ. Microbiol. 2002, 4, 595–602. [Google Scholar] [CrossRef]
- Banat, I.M.; Marchant, R.; Rahman, T.J. Geobacillus debilis sp. nov., a novel obligately thermophilic bacterium isolated from a cool soil environment, and reassignment of Bacillus pallidus to Geobacillus pallidus, comb. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 2197–2201. [Google Scholar] [CrossRef] [Green Version]
- Rahman, T.J.; Marchant, R.; Banat, I.M. Distribution and molecular investigation of highly thermophilic bacteria associated with cool soil environments. Biochem. Soc. Trans. 2004, 32, 209–213. [Google Scholar] [PubMed]
- Wu, D.; Raymond, J.; Wu, M.; Chatterji, S.; Ren, Q.; Graham, J.E.; Bryant, D.A.; Robb, F.; Colman, A.; Tallon, L.J.; et al. Complete genome sequence of the aerobic CO-oxidizing thermophile Thermomicrobium roseum. PLoS ONE 2009, 4, e4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorlach-Lira, K.; Coutinho, H.D. Population dynamics and extracellular enzymes activity of mesophilic and thermophilic bacteria isolated from semi-arid soil of northeastern Brazil. Braz. J. Microbiol. 2007, 38, 135–141. [Google Scholar] [CrossRef]
- Hubert, C.; Arnosti, C.; Brüchert, V.; Loy, A.; Vandieken, V.; Jørgensen, B.B. Thermophilic anaerobes in Arctic marine sediments induced to mineralize complex organic matter at high temperature. Environ. Microbiol. 2010, 12, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.A.; Shcherbakova, V.A.; Rivkina, E.M.; Gilichinsky, D.A. Thermophilic bacteria of the genus Geobacillus from permafrost volcanic sedimentary rocks. Microbiology 2013, 82, 389–392. [Google Scholar] [CrossRef]
- DePoy, A.; King, G.M.; Ohta, H. Anaerobic Carbon Monoxide Uptake by Microbial Communities in Volcanic Deposits at Different Stages of Successional Development on O-yama Volcano, Miyake-jima, Japan. Microorganisms 2020, 9, 12. [Google Scholar] [CrossRef]
- DePoy, A.; King, G.M. Putative nickel-dependent anaerobic carbon monoxide uptake occurs commonly in soils and sediments at ambient temperature and may contribute to activity at atmospheric and sub-atmospheric CO levels. Front. Microbiol. 2022. submitted. [Google Scholar]
- Hubert, C.; Loy, A.; Nickel, M.; Arnosti, C.; Baranyi, C.; Brüchert, V.; Ferdelman, T.; Finster, K.; Christensen, F.M.; Rosa de Rezende, J.; et al. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed. Science 2009, 325, 1541–1544. [Google Scholar] [CrossRef] [Green Version]
- Perfumo, A.; Marchant, R. Global transport of thermophilic bacteria in atmospheric dust. Environ. Microbiol. Rep. 2010, 2, 333–339. [Google Scholar] [CrossRef]
- Portillo, M.C.; Santana, M.; Gonzalez, J.M. Presence and potential role of thermophilic bacteria in temperate terrestrial environments. Naturwissenschaften 2008, 99, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Cockell, C.S.; Cousins, C.; Wilkinson, P.T.; Olsson-Francis, K.; Rozitis, B. Are thermophilic microorganisms active in cold environments? Int. J. Astrobiol. 2015, 14, 457–463. [Google Scholar] [CrossRef] [Green Version]
- King, C.E.; King, G.M. Temperature responses of carbon monoxide and hydrogen uptake by vegetated and unvegetated volcanic cinders. ISME J. 2012, 6, 1558–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, C.E.; King, G.M. Description of Thermogemmatispora carboxidivorans sp. nov., a carbon monoxide-oxidizing member of the Ktedonobacteria isolated from a geothermally-heated biofilm, and CO oxidation by members of the Ktedonobacteria. Int. J. Syst. Evol. Microbiol. 2013, 64, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, C.E.; King, G.M. Thermomicrobium carboxidovorans KI3T sp. nov., and Thermorudis peleae KI4T gen. nov., sp. nov., carbon monoxide-oxidizing bacteria from geothermally-heated biofilms. Int. J. Syst. Evol. Microbiol. 2014, 64, 2586–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.F.; King, G.M. Distribution and diversity of carbon monoxide-oxidizing bacteria and bulk bacterial communities across a succession gradient on a Hawaiian volcanic deposit. Environ. Microbiol. 2010, 12, 1855–1867. [Google Scholar] [CrossRef]
- Loginova, L.G.; Egorova, L.A.; Golovacheva, R.S.; Seregina, L.M. Thermus ruber sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1984, 34, 498–499. [Google Scholar] [CrossRef]
- Weber, C.F.; King, G.M. Volcanic soils as sources of novel CO-oxidizing Paraburkholderia and Burkholderia: Paraburkholderia hiiakae sp. nov., Paraburkholderia metrosideri sp. nov., Paraburkholderia paradisi sp. nov., Paraburkholderia peleae sp. nov., and Burkholderia alpina sp. nov. a member of the Burkholderia cepacia complex. Front. Microbiol. 2017, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Darland, G.; Brock, T.D. Bacillus acidocaldarius sp. nov., an acidophilic thermophilic spore-forming bacterium. J. Gen. Microbiol. 1971, 67, 9–15. [Google Scholar] [CrossRef] [Green Version]
- King, G.M. Attributes of atmospheric carbon monoxide oxidation in Maine forest soils. Appl. Environ. Microbiol. 1999, 65, 5257–5264. [Google Scholar] [CrossRef] [Green Version]
- King, G.M.; Weber, C.F. Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat. Rev. Microbiol. 2007, 5, 107–118. [Google Scholar] [CrossRef]
- Cordero, P.R.F.; Bayly, K.; Man Leung, P.; Huang, C.; Islam, Z.F.; Schittenhelm, R.B.; King, G.M.; Greening, C. Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J. 2019, 13, 2868–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, B.; Schübel, U.; Egelseer, C.; Meyer, O. Sequence analysis, characterization and CO-specific transcription of the cox gene cluster on the megaplasmid pHCG3 of Oligotropha carboxidovorans. Gene 1999, 236, 115–124. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.M. Induction of carbon monoxide dehydrogenase during heterotrophic growth of Acinetobacter sp. strain JC1 DSM 3803 in the presence of carbon monoxide. FEMS Microbiol. Lett. 1999, 59, 207–210. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Benlloch, S.; Bonfa, M.; Rodriguez-Valera, F.; Mira, A. Intragenomic 16S rDNA divergence in Haloarcula marismortui is an adaptation to different temperatures. J. Mol. Evol. 2007, 65, 687–696. [Google Scholar] [CrossRef]
- Song, W.; Joo, M.; Yeom, J.-H.; Shin, E.; Lee, M.; Choi, H.-K.; Hwang, J.; Kim, Y.-I.; Seo, R.; Lee, J.E.; et al. Divergent rRNAs as regulators of gene expression at the ribosomal level. Nature Microbiol. 2019, 4, 515–526. [Google Scholar] [CrossRef]
- Weber, C.F.; King, G.M. Water stress impacts on bacterial carbon monoxide oxidation on recent volcanic deposits. ISME J. 2009, 3, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Bérard, A.; Kaisermann, A.; Renult, P. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, W.; Chen, Y.; Liu, S.; Dong, W.; Zhang, H.; Yu, G.; Chen, Z.; He, H.; Guo, W.; et al. Severe summer heatwave and drought strongly reduced carbon uptake in Southern China. Sci. Rep. 2015, 6, 18813. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Cai, M.; Li, D.; Wu, B.; Li, J.; Huang, G.; Duan, H.; Wu, J. Heat waves intensify the effects of drought on bacterial diversity but not community composition in Solanum lycopersicum soil. J. Soils Sediments 2021, 21, 355–363. [Google Scholar] [CrossRef]
- Constant, P.; Chowdhury, S.P.; Pratscher, J.; Conrad, R. Streptomycetes contributing to atmospheric molecular hydrogen soil uptake are widespread and encode a putative high-affinity [NiFe]-hydrogenase. Environ. Microbiol. 2010, 12, 821–829. [Google Scholar] [CrossRef]
- Kim, S.B.; Falconer, C.; Williams, E.; Goodfellow, M. Streptomyces thermocarboxydovorans sp. nov. and Streptomyces thermocarboxydus, two moderately thermophilic carboxydotrophic species from soil. Int. J. Syst. Bacteriol. 1998, 48, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Isolate | 25 °C | AMTT | 55 °C |
|---|---|---|---|
| Meiothermus ruber PS4 | 0.05 (0.001) | 0.11 (0.01) | 0.23 (0.001) |
| Alicyclobacillus macrosporangiidus CPP55 | 0.0 | 0.36 (0.001) | 0.70 (0.10) |
| Thermogemmatispora carboxidovorans PM5 | 0.0 | 0.0 | 0.41 (0.06) |
| Paraburkholderia paradisi WA | 0.33 (0.001) | 0.0 | 0.0 |
| Strain | Medium | 25 °C | AMTT | 55 °C |
|---|---|---|---|---|
| Meiothermus ruber PS4 | unwashed | 2.4 (0.1) | 6.5 (0.1) | 34.0 (1.0) |
| washed | 3.1 (0.1) | 9.5 (0.2) | 53.0 (3.0) | |
| Alicyclobacillus macrosporangiidus CPP55 | unwashed | 0.0 | 1.5 (0.2) | 10.1 (0.5) |
| washed | 0.0 | 2.5 (0.6) | 15.0 (4.0) | |
| Thermogemmatispora carboxidovorans PM5 | unwashed | 0.0 | 0.3 (0.01) | 3.3 (0.8) |
| washed | 0.0 | 0.5 (0.04) | 4.3 (0.7) | |
| Paraburkholderia paradisi WA | unwashed | 2.6 (0.1) | 3.0 (1.3) * | 0.0 |
| washed | 7.6 (0.5) | 3.1 (0.5) * | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, C.K.; King, G.M. Short-Term Exposure to Thermophilic Temperatures Facilitates CO Uptake by Thermophiles Maintained under Predominantly Mesophilic Conditions. Microorganisms 2022, 10, 656. https://doi.org/10.3390/microorganisms10030656
Wilson CK, King GM. Short-Term Exposure to Thermophilic Temperatures Facilitates CO Uptake by Thermophiles Maintained under Predominantly Mesophilic Conditions. Microorganisms. 2022; 10(3):656. https://doi.org/10.3390/microorganisms10030656
Chicago/Turabian StyleWilson, Caitlin K., and Gary M. King. 2022. "Short-Term Exposure to Thermophilic Temperatures Facilitates CO Uptake by Thermophiles Maintained under Predominantly Mesophilic Conditions" Microorganisms 10, no. 3: 656. https://doi.org/10.3390/microorganisms10030656





