Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria
Abstract
1. Introduction
2. Antibiotic Resistance Mechanisms
| Resistance Mechanism | Examples | References |
|---|---|---|
| Reduced drug uptake |
| [32,34,43,44,45] |
| Antibiotic degrading enzymes |
| [25,28,34,46,47] |
| Antibiotic modifying enzymes |
| [25,28,48,49,50] |
| Proteases and Peptidases |
| [51,52,53,54] |
| Efflux pumps |
| [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Reduced affinity of targets to the antibiotics |
| [78,79,80,81,82,83,84,85] |
| Modification of the targets |
| [79,86,87,88,89,90,91,92,93] |
| Target protection |
| [94,95,96,97] |
| Ribosomal protection |
| [98,99,100,101,102,103] |
| Biofilm- embedded bacteria |
| [1,13,14,20,104,105,106] |
2.1. Acquisition of Various Antibiotic-Resistant Genes via Horizontal Gene Transfer
2.2. Decreased Membrane Permeability
2.3. Increased Production of Antibiotic Degrading Enzymes
2.4. Increased Production of Antibiotic Modification Enzymes
2.5. Alterations of the Target That Disable the Binding of Antibiotics
2.6. Overexpression of Efflux Pumps
2.6.1. Inducible Efflux Pumps
2.6.2. Mechanisms Resulting in Constitutive Overexpression of Efflux Pump
2.6.3. Major Efflux Pumps in Pseudomonas aeruginosa
2.6.4. Major Efflux Pumps in Enterobacter spp.
2.6.5. Major Efflux Pumps in Staphylococcus aureus Contributing to the MRSA and MDRSA Phenotypes
2.7. Involvement of rRNA Methyltransferase in Antibiotic Resistance
2.8. Involvement of DNA Methyltransferase in Antibiotic Resistance
2.9. Involvement of Ribosomal Protection in Antibiotic Resistance
2.10. Involvement of Non-Coding RNAs in Antibiotic Resistance
2.11. Involvement of Bacterial Proteases in Antibiotic Resistance
3. Quorum Sensing
3.1. TCSs in Vibrio Strains
3.2. TCSs in Pseudomonas aeruginosa
3.3. TCSs in Staphylococcus aureus
3.4. Involvement of Two-Component Systems in Promoting Antibiotic Resistance
4. Biofilms
4.1. Regulation of Biofilm Formation
4.1.1. Induction of Biofilm Formation by Low Antibiotic Concentrations
4.1.2. Involvement of Two-Component Systems in Biofilm Formation
| Biofilm Formation Regulating Factors | Function | Species | Reference |
|---|---|---|---|
| Agr |
| Staphylococcus aureus | [370] |
| AlgD-A |
| Pseudomonas aeruginosa | [509,543,544] |
| AlsSD |
| Staphylococcus aureus | [545,546] |
| ArgR |
| Staphylococcus aureus | [547,548] |
| ArlRS |
| Staphylococcus aureus | [549,550,551,552] |
| AtlA/AtlE |
| Staphylococcus aureus, Staphylococcus epidermidis | [492,493,553] |
| BasSR |
| Escherichia coli | [554] |
| BfiRS |
| Pseudomonas aeruginosa | [555,556,557] |
| BfmRS (RtsAB) |
| Acinetobacter baumannii, Pseudomonas aeruginosa | [518,558,559,560,561] |
| cAMP-CRP |
| Escherichia coli, Klebsiella pneumoniae | [562,563,564,565] |
| CidABC |
| Staphylococcus aureus | [495,546,566] |
| c-di-GMP |
| Pseudomonas aeruginosa | [508,517,567,568,569,570,571] |
| CodY |
| Staphylococcus aureus | [572,573,574] |
| CqsA |
| Vibrio harveyi | [336] |
| CpxRA |
| Escherichia coli, Salmonellaenteritidis | [575] |
| CreBC (BlrAB) |
| Pseudomonas aeruginosa | [426,576] |
| CsgD |
| Escherichia coli, Salmonella enterica | [577,578,579,580] |
| DltA |
| Staphylococcus aureus | [581] |
| FsrBDC |
| Enterococcus faecalis | [582,583,584] |
| GacSA |
| Pseudomonas aeruginosa, Acinetobacter baumannii | [507,559,585,586,587,588] |
| GraRS |
| Staphylococcus aureus | [441,589] |
| HapR |
| Vibrio cholerae | [335,504] |
| LadS |
| Pseudomonas aeruginosa | [507,585,590] |
| LasR/LasI |
| Pseudomonas aeruginosa | [296,505,509,591] |
| LecA |
| Pseudomonas aeruginosa | [592] |
| LrgAB |
| Staphylococcus aureus | [289,593] |
| LuxS |
| Salmonella species, Vibrio species, Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae | [525,530,538,541,594,595,596] |
| LytA |
| Streptococcus pneumoniae | [597] |
| LytSR |
| Staphylococcus aureus | [598,599] |
| MgrA |
| Staphylococcus aureus | [374,398,549,550,552,600] |
| MifRS |
| Pseudomonas aeruginosa | [557,601] |
| QseBC |
| Escherichia coli, Salmonella Typhimurium | [249,541,602,603,604] |
| PA1161 |
| Pseudomonas aeruginosa | [605] |
| PilSR |
| Pseudomonas aeruginosa | [606,607] |
| (p)ppGpp |
| Escherichia coli, Acinetobacter baumannii, Staphylococcus aureus | [570,608,609,610,611,612,613] |
| PprAB |
| Pseudomonas aeruginosa | [614,615] |
| Rbf |
| Staphylococcus aureus, Staphylococcus epidermidis | [616,617,618] |
| RcsCDB |
| Escherichia coli, Salmonella enterica serovar Typhimurium, Pseudomonas aeruginosa | [484,619,620,621,622,623,624] |
| RetS |
| Pseudomonas aeruginosa | [524,625] |
| RhlR/RhlI |
| Pseudomonas aeruginosa | [509,626,627] |
| RocS1A1R |
| Pseudomonas aeruginosa | [628,629] |
| Rot |
| Staphylococcus aureus | [630] |
| RpoS |
| Escherichia coli, Pseudomonas aeruginosa | [578,631,632,633,634,635,636,637] |
| SadARS |
| Pseudomonas aeruginosa | [638] |
| SaeRS |
| Staphylococcus aureus | [639,640] |
| SagS |
| Pseudomonas aeruginosa | [230,555,568,641,642,643,644] |
| SarA |
| Staphylococcus aureus | [645,646,647,648] |
| SarX |
| Staphylococcus aureus, Staphylococcus epidermidis | [616,649,650,651] |
| SdiA |
| Escherichia coli, Salmonella spp. | [652,653,654] |
| SigB |
| Staphylococcus aureus | [655] |
| SrrAB |
| Staphylococcus aureus | [549] |
| TcaR/IcaR |
| Staphylococcus aureus | [656,657,658] |
| VraSR |
| Staphylococcus epidermidis | [467] |
4.1.3. Role of Cyclic di-GMP (c-di-GMP) in Biofilm Formation
4.1.4. Role of Non-Coding RNAs (ncRNAs) or Small Regulatory RNA (sRNA) in Regulating Biofilm Formation
4.2. Biofilm Formation by Vibrio cholerae
4.3. Biofilm Formation by Escherichia coli
4.4. Biofilm Formation by Pseudomonas aeruginosa
4.5. Biofilm Formation by Staphylococcus Species
4.6. Biofilm Formation by Klebsiella pneumoniae
4.7. Antibiotic Resistance of Biofilm-Embedded Bacteria
4.7.1. Prevention of Antibiotic Penetration through the Biofilm
4.7.2. Antibiotic Tolerance Due to Low Metabolic State of Biofilm-Associated Bacteria
4.7.3. Antibiotic Tolerance Due to Altered Chemical Microenvironment within the Biofilm
4.7.4. Activation of Protective Stress Responses
4.7.5. Altered Expression of Antibiotic-Resistant Genes in Biofilm-Embedded Bacteria
4.7.6. Increased Efflux Pump Expression in Biofilm-Embedded Bacteria
4.8. The Relationship between Biofilm Formation and Efflux Pumps
5. Targeting Quorum Sensing and Biofilms as a Strategy to Overcome Antibiotic Resistance
5.1. Antibiotic Adjuvants
Repurposing Clinically Approved Drugs as Antibiotic Adjuvants
5.2. Quorum Sensing Inhibitors and Quenchers
5.3. Inhibition of Biofilm Formation
5.4. Inhibition of Efflux Pumps
5.5. Targeting Cell Wall Teichoic Acid Synthesis
| Compound | Effects on Bacteria | References |
|---|---|---|
| Clomiphene |
| [1066] |
| HSGN-94 and HSGN-189 |
| [1067] |
| Targocil |
| [1065] |
| Tarocin A and Tarocin B |
| [1068] |
| Ticlopidine |
| [798] |
| Tunicamycin |
| [1043,1058,1069] |
5.6. Inactivation of PBP2a as an Approach to Sensitize MRSA to β-Lactams
5.7. Targeting Cell Division Proteins to Sensitize MRSA to β-Lactams
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum sensing: A prospective therapeutic target for bacterial diseases. BioMed. Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert Rev. Anti-Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Tonkin, M.; Khan, S.; Wani, M.Y.; Ahmad, A. Quorum sensing—A stratagem for conquering multi-drug resistant pathogens. Curr. Pharm. Des. 2020, 27, 2835–2847. [Google Scholar] [CrossRef]
- Xiang, Y.; Ding, Y.; Cao, J.; Sun, Y.; Wang, F.; Ju, S.; Yu, J. Non-antibiotic methods against Pseudomonas aeruginosa include QS inhibitors: A narrative review. Ann. Palliat. Med. 2021, 10, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; He, S. Quorum Sensing inhibition or quenching in Acinetobacter baumannii: The novel therapeutic strategies for new drug development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Shin, H.-J.; Yang, S.; Lim, Y. Antibiotic susceptibility of Staphylococcus aureus with different degrees of biofilm formation. J. Anal. Sci. Techol. 2021, 12, 41. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. 1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Vale de Macedo, G.H.R.; Costa, G.D.E.; Oliveira, E.R.; Damasceno, G.V.; Mendonça, J.S.P.; Silva, L.D.S.; Chagas, V.L.; Bazán, J.M.N.; Aliança, A.; Miranda, R.C.M.; et al. Interplay between ESKAPE Pathogens and immunity in skin infections: An overview of the major determinants of virulence and antibiotic resistance. Pathogens 2021, 10, 148. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Dar, D.; Sorek, R. Regulation of antibiotic-resistance by non-coding RNAs in bacteria. Curr. Opin. Microbiol. 2017, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; He, L.L.; Pironti, A.; Laibinis, H.H.; Ernst, C.M.; Manson, A.L.; Bhattacharyya, R.P.; Earl, A.M.; Livny, J.; Hung, D.T. Genetic determinants facilitating the evolution of resistance to carbapenem antibiotics. Elife 2021, 10, e67310. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Larrouy-Maumus, G.J.; Meiqi Tan, M.G.C.; Wareham, D.W. Antibiotic resistance mechanisms and their transmission in Acinetobacter baumannii. Adv. Exp. Med. Biol. 2021, 1313, 135–153. [Google Scholar] [CrossRef]
- Mediati, D.G.; Wu, S.; Wu, W.; Tree, J.J. Networks of resistance: Small RNA control of antibiotic resistance. Trends Genet. 2021, 37, 35–45. [Google Scholar] [CrossRef]
- Goldberger, O.; Livny, J.; Bhattacharyya, R.; Amster-Choder, O. Wisdom of the crowds: A suggested polygenic plan for small-RNA-mediated regulation in bacteria. iScience 2021, 24, 103096. [Google Scholar] [CrossRef]
- Haaber, J.; Penadés, J.R.; Ingmer, H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, VMBF-0016-2015. [Google Scholar] [CrossRef]
- Chetri, S.; Singha, K.; Bhowmik, D.; Chanda, D.D.; Chakravarty, A.; Bhattacharjee, A. Sub-inhibitory concentration of ertapenem induces overexpression of regulator of antibiotic resistance A in Escherichia coli. Indian J. Med. Microbiol. 2018, 36, 569–571. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Limansky, A.S.; Mussi, M.A.; Viale, A.M. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J. Clin. Microbiol. 2002, 40, 4776–4778. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B. Imipenem resistance among Acinetobacter baumannii: Association with reduced expression of a 33–36 kDa outer membrane protein. J. Antimicrob. Chemother. 1996, 38, 245–251. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, X.; Wu, Q.; Wei, L.; Li, J.; Ying, C. Efflux pump overexpression in conjunction with alternation of outer membrane protein may induce Acinetobacter baumannii resistant to imipenem. Chemotherapy 2011, 57, 77–84. [Google Scholar] [CrossRef]
- Zieliński, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and functional insights into esterase-mediated macrolide resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Golkar, T.; Zieliński, M.; Berghuis, A.M. Look and outlook on enzyme-mediated macrolide resistance. Front. Microbiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Fong, D.H.; Burk, D.L.; Blanchet, J.; Yan, A.Y.; Berghuis, A.M. Structural basis for kinase-mediated macrolide antibiotic resistance. Structure 2017, 25, 750–761.e755. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Cui, C.Y.; Zhang, Y.; Liu, X.; Cui, Z.H.; Ma, X.Y.; Feng, Y.; Fang, L.X.; Lian, X.L.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Rigby, K.; Wang, R.; Queck, S.Y.; Braughton, K.R.; Whitney, A.R.; Teintze, M.; DeLeo, F.R.; Otto, M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010, 6, e1001133. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Lee, S.; Jacoby, K.; Manoil, C. Membrane proteases and aminoglycoside antibiotic resistance. J. Bacteriol. 2011, 193, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhong, Z.; Hou, P.; Zhang, W.P.; Qian, P.Y. Resistance to nonribosomal peptide antibiotics mediated by D-stereospecific peptidases. Nat. Chem. Biol. 2018, 14, 381–387. [Google Scholar] [CrossRef]
- Meziane-Cherif, D.; Stogios, P.J.; Evdokimova, E.; Savchenko, A.; Courvalin, P. Structural basis for the evolution of vancomycin resistance D,D-peptidases. Proc. Natl. Acad. Sci. USA 2014, 111, 5872–5877. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, K.D.; Nisbet, R.; Stephens, D.S. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob. Agents Chemother. 2005, 49, 4203–4209. [Google Scholar] [CrossRef] [PubMed]
- Anes, J.; McCusker, M.P.; Fanning, S.; Martins, M. The ins and outs of RND efflux pumps in Escherichia coli. Front. Microbiol. 2015, 6, 587. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Lv, F.; Cai, J.; He, Q.; Wang, W.; Luo, Y.; Wang, X.; Mi, N.; Zhao, Z.; Li, G.; Luo, W. Overexpression of efflux pumps mediate pan resistance of Klebsiella pneumoniae sequence type 11. Microb. Drug Resist. 2021, 27, 1405–1411. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Chancey, S.T.; Zhou, X.; Zähner, D.; Stephens, D.S. Induction of efflux-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.B.; Singh, B.B.; Priyadarshi, N.; Chauhan, N.K.; Rajamohan, G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae. PLoS ONE 2014, 9, e96288. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Pages, J.M.; Ferrand, A. Clinical status of efflux resistance mechanisms in gram-negative bacteria. Antibiotics 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Warren, M.S.; Lee, A.; Mistry, A.; Lomovskaya, O. MexXY-OprM efflux pump is required for antagonism of aminoglycosides by divalent cations in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001, 45, 2001–2007. [Google Scholar] [CrossRef]
- Muller, C.; Plésiat, P.; Jeannot, K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and β-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1211–1221. [Google Scholar] [CrossRef]
- Coyne, S.; Rosenfeld, N.; Lambert, T.; Courvalin, P.; Périchon, B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4389–4393. [Google Scholar] [CrossRef]
- Damier-Piolle, L.; Magnet, S.; Brémont, S.; Lambert, T.; Courvalin, P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 557–562. [Google Scholar] [CrossRef]
- Schaffner, S.H.; Lee, A.V.; Pham, M.T.N.; Kassaye, B.B.; Li, H.; Tallada, S.; Lis, C.; Lang, M.; Liu, Y.; Ahmed, N.; et al. Extreme acid modulates fitness trade-offs of multidrug efflux pumps MdtEF-TolC and AcrAB-TolC in Escherichia coli K-12. Appl. Environ. Microbiol. 2021, 87, e0072421. [Google Scholar] [CrossRef]
- Yousefian, N.; Ornik-Cha, A.; Poussard, S.; Decossas, M.; Berbon, M.; Daury, L.; Taveau, J.C.; Dupuy, J.W.; Đorđević-Marquardt, S.; Lambert, O.; et al. Structural characterization of the EmrAB-TolC efflux complex from E. coli. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183488. [Google Scholar] [CrossRef]
- Yoshikai, H.; Kizaki, H.; Saito, Y.; Omae, Y.; Sekimizu, K.; Kaito, C. Multidrug-resistance transporter AbcA secretes Staphylococcus aureus cytolytic toxins. J. Infect. Dis. 2016, 213, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.S.; Joo, H.S.; Duong, A.C.; Dieringer, T.D.; Tan, V.Y.; Song, Y.; Fischer, E.R.; Cheung, G.Y.; Li, M.; Otto, M. Essential Staphylococcus aureus toxin export system. Nat. Med. 2013, 19, 364–367. [Google Scholar] [CrossRef]
- Short, F.L.; Liu, Q.; Shah, B.; Clift, H.E.; Naidu, V.; Li, L.; Prity, F.T.; Mabbutt, B.C.; Hassan, K.A.; Paulsen, I.T. The Acinetobacter baumannii disinfectant resistance protein, AmvA, is a spermidine and spermine efflux pump. Commun. Biol. 2021, 4, 1114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Lin, Z.W.; Sun, X.; Lin, W.H.; Chen, Z.; Wu, Y.; Qi, G.B.; Deng, Q.W.; Qu, D.; Yu, Z.J. Overexpression of OqxAB and MacAB efflux pumps contributes to eravacycline resistance and heteroresistance in clinical isolates of Klebsiella pneumoniae. Emerg. Microbes Infect. 2018, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.; Courvalin, P.; Périchon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Eswarappa, S.M.; Panguluri, K.K.; Hensel, M.; Chakravortty, D. The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology 2008, 154, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.; Peters, B.M.; Li, L.; Li, B.; Xu, Z.; Shirliff, M.E. Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2016, 101, 56–67. [Google Scholar] [CrossRef]
- Roberts, M.C. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef]
- Canu, A.; Malbruny, B.; Coquemont, M.; Davies, T.A.; Appelbaum, P.C.; Leclercq, R. Diversity of ribosomal mutations conferring resistance to macrolides, clindamycin, streptogramin, and telithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2002, 46, 125–131. [Google Scholar] [CrossRef]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.C.; Seol, S.Y.; Gurung, M.; Jin, J.S.; Choi, C.H.; Kim, J.; Lee, Y.C.; Cho, D.T.; Lee, J.C. Emergence of a new mutation and its accumulation in the topoisomerase IV gene confers high levels of resistance to fluoroquinolones in Escherichia coli isolates. Int. J. Antimicrob. Agents 2010, 35, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.; Feudi, C.; Fortini, D.; García-Fernández, A.; Carattoli, A. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob. Agents Chemother. 2014, 58, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Tanaka, K.; Yamamichi, F.; Shirakawa, T.; Miyake, H.; Fujisawa, M. Does mutation in gyrA and/or parC or efflux pump expression play the main role in fluoroquinolone resistance in Escherichia coli urinary tract infections?: A statistical analysis study. Int. J. Antimicrob. Agents 2012, 40, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, Y.S.; Park, Y.K.; Kim, B.S. Mutations in the gyrA and parC genes in ciprofloxacin-resistant clinical isolates of Acinetobacter baumannii in Korea. Microbiol. Immunol. 2005, 49, 647–653. [Google Scholar] [CrossRef]
- Dalmolin, T.V.; de Lima-Morales, D.; Barth, A.L. Plasmid-mediated Colistin resistance: What do we know? J. Infect. 2018, 1, 16–22. [Google Scholar] [CrossRef]
- Bugg, T.D.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef]
- Hong, H.J.; Hutchings, M.I.; Buttner, M.J. Vancomycin resistance VanS/VanR two-component systems. Adv. Exp. Med. Biol. 2008, 631, 200–213. [Google Scholar] [CrossRef]
- Gupta, P.; Sothiselvam, S.; Vázquez-Laslop, N.; Mankin, A.S. Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible. Nat. Commun. 2013, 4, 1984. [Google Scholar] [CrossRef]
- Fernández, L.; Alvarez-Ortega, C.; Wiegand, I.; Olivares, J.; Kocíncová, D.; Lam, J.S.; Martínez, J.L.; Hancock, R.E. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 110–119. [Google Scholar] [CrossRef]
- Thaipisuttikul, I.; Hittle, L.E.; Chandra, R.; Zangari, D.; Dixon, C.L.; Garrett, T.A.; Rasko, D.A.; Dasgupta, N.; Moskowitz, S.M.; Malmström, L.; et al. A divergent Pseudomonas aeruginosa palmitoyltransferase essential for cystic fibrosis-specific lipid A. Mol. Microbiol. 2014, 91, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Vester, B. The cfr and cfr-like multiple resistance genes. Res. Microbiol. 2018, 169, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Stojković, V.; Noda-Garcia, L.; Tawfik, D.S.; Fujimori, D.G. Antibiotic resistance evolved via inactivation of a ribosomal RNA methylating enzyme. Nucleic Acids Res. 2016, 44, 8897–8907. [Google Scholar] [CrossRef]
- Tran, J.H.; Jacoby, G.A.; Hooper, D.C. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 2005, 49, 3050–3052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tran, J.H.; Jacoby, G.A.; Hooper, D.C. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 2005, 49, 118–125. [Google Scholar] [CrossRef]
- Kim, E.S.; Chen, C.; Braun, M.; Kim, H.Y.; Okumura, R.; Wang, Y.; Jacoby, G.A.; Hooper, D.C. Interactions between QnrB, QnrB mutants, and DNA gyrase. Antimicrob. Agents Chemother. 2015, 59, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Pachanon, R.; Koide, K.; Kongsoi, S.; Nakajima, C.; Kapalamula, T.F.; Suthienkul, O.; Suzuki, Y. Interaction of the plasmid-encoded quinolone resistance protein QnrB19 with Salmonella Typhimurium DNA gyrase. J. Infect. Chemother. 2020, 26, 1139–1145. [Google Scholar] [CrossRef]
- Su, W.; Kumar, V.; Ding, Y.; Ero, R.; Serra, A.; Lee, B.S.T.; Wong, A.S.W.; Shi, J.; Sze, S.K.; Yang, L.; et al. Ribosome protection by antibiotic resistance ATP-binding cassette protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5157–5162. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Sharkey, L.K.; Edwards, T.A.; O’Neill, A.J. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 2016, 7, e01975. [Google Scholar] [CrossRef]
- Ero, R.; Kumar, V.; Su, W.; Gao, Y.G. Ribosome protection by ABC-F proteins-Molecular mechanism and potential drug design. Protein Sci. 2019, 28, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Crowe-McAuliffe, C.; Murina, V.; Turnbull, K.J.; Kasari, M.; Mohamad, M.; Polte, C.; Takada, H.; Vaitkevicius, K.; Johansson, J.; Ignatova, Z.; et al. Structural basis of ABCF-mediated resistance to pleuromutilin, lincosamide, and streptogramin A antibiotics in Gram-positive pathogens. Nat. Commun. 2021, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- Connell, S.R.; Tracz, D.M.; Nierhaus, K.H.; Taylor, D.E. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob. Agents Chemother. 2003, 47, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Conibear, T.C.; Collins, S.L.; Webb, J.S. Role of mutation in Pseudomonas aeruginosa biofilm development. PLoS ONE 2009, 4, e6289. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- Toussaint, A.; Chandler, M. Prokaryote genome fluidity: Toward a system approach of the mobilome. Methods Mol. Biol. 2012, 804, 57–80. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, M.; Hasan, R.; Hossain, M.I.; Nabi, A.; Rahman, M.; Goessens, W.H.F.; Endtz, H.P.; Boehm, A.B.; Faruque, S.M. Environmental spread of New Delhi Metallo-β-Lactamase-1-producing multidrug-resistant bacteria in Dhaka, Bangladesh. Appl. Environ. Microbiol. 2017, 83, e00793-17. [Google Scholar] [CrossRef]
- Ribera, A.; Roca, I.; Ruiz, J.; Gibert, I.; Vila, J. Partial characterization of a transposon containing the tet(A) determinant in a clinical isolate of Acinetobacter baumannii. J. Antimicrob. Chemother. 2003, 52, 477–480. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, Y.; Liu, F.; Lv, N.; Yang, X.; Li, J.; Hu, Y.; Zhu, B. Distribution and molecular profiling of class 1 integrons in MDR Acinetobacter baumannii isolates and whole genome-based analysis of antibiotic resistance mechanisms in a representative strain. Microbiol. Res. 2014, 169, 811–816. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 2017, 8, e00543-17. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.C.; Coleman, D.C. Staphylococcal cassette chromosome mec: Recent advances and new insights. Int. J. Med. Microbiol. 2013, 303, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef]
- Iyer, R.; Moussa, S.H.; Durand-Réville, T.F.; Tommasi, R.; Miller, A. Acinetobacter baumannii OmpA is a selective antibiotic permeant porin. ACS Infect. Dis. 2018, 4, 373–381. [Google Scholar] [CrossRef]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Kwon, H.I.; Kim, S.; Oh, M.H.; Na, S.H.; Kim, Y.J.; Jeon, Y.H.; Lee, J.C. Outer membrane protein A contributes to antimicrobial resistance of Acinetobacter baumannii through the OmpA-like domain. J. Antimicrob. Chemother. 2017, 72, 3012–3015. [Google Scholar] [CrossRef]
- Sugawara, E.; Nikaido, H. OmpA is the principal nonspecific slow porin of Acinetobacter baumannii. J. Bacteriol. 2012, 194, 4089–4096. [Google Scholar] [CrossRef]
- Bhamidimarri, S.P.; Zahn, M.; Prajapati, J.D.; Schleberger, C.; Söderholm, S.; Hoover, J.; West, J.; Kleinekathöfer, U.; Bumann, D.; Winterhalter, M.; et al. A multidisciplinary approach toward identification of antibiotic scaffolds for Acinetobacter baumannii. Structure 2019, 27, 268–280.e266. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef] [PubMed]
- Na, S.H.; Jeon, H.; Oh, M.H.; Kim, Y.J.; Chu, M.; Lee, I.Y.; Lee, J.C. Therapeutic effects of inhibitor of ompA expression against carbapenem-resistant Acinetobacter baumannii strains. Int. J. Mol. Sci. 2021, 22, 12257. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef]
- Ziervogel, B.K.; Roux, B. The bind.ding of antibiotics in OmpF porin. Structure 2013, 21, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Kojima, S.; Nikaido, H. Klebsiella pneumoniae major porins OmpK35 and OmpK36 allow more efficient diffusion of β-Lactams than their Escherichia coli homologs OmpF and OmpC. J. Bacteriol. 2016, 198, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Moya-Torres, A.; Mulvey, M.R.; Kumar, A.; Oresnik, I.J.; Brassinga, A.K.C. The lack of OmpF, but not OmpC, contributes to increased antibiotic resistance in Serratia marcescens. Microbiology 2014, 160, 1882–1892. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.F.; Williams, B.J.; Blackwell, T.S.; Xie, C.M. Structure and function of OprD protein in Pseudomonas aeruginosa: From antibiotic resistance to novel therapies. Int. J. Med. Microbiol. 2012, 302, 63–68. [Google Scholar] [CrossRef]
- Thiolas, A.; Bornet, C.; Davin-Régli, A.; Pagès, J.M.; Bollet, C. Resistance to imipenem, cefepime, and cefpirome associated with mutation in Omp36 osmoporin of Enterobacter aerogenes. Biochem. Biophys. Res. Commun. 2004, 317, 851–856. [Google Scholar] [CrossRef]
- Lee, C.H.; Chu, C.; Liu, J.W.; Chen, Y.S.; Chiu, C.J.; Su, L.H. Collateral damage of flomoxef therapy: In vivo development of porin deficiency and acquisition of blaDHA-1 leading to ertapenem resistance in a clinical isolate of Klebsiella pneumoniae producing CTX-M-3 and SHV-5 beta-lactamases. J. Antimicrob. Chemother. 2007, 60, 410–413. [Google Scholar] [CrossRef]
- Pulzova, L.; Navratilova, L.; Comor, L. Alterations in outer membrane permeability favor drug-resistant phenotype of Klebsiella pneumoniae. Microb. Drug Resist. 2017, 23, 413–420. [Google Scholar] [CrossRef]
- Bowers, J.R.; Kitchel, B.; Driebe, E.M.; MacCannell, D.R.; Roe, C.; Lemmer, D.; de Man, T.; Rasheed, J.K.; Engelthaler, D.M.; Keim, P.; et al. Genomic analysis of the emergence and rapid global dissemination of the Clonal Group 258 Klebsiella pneumoniae Pandemic. PLoS ONE 2015, 10, e0133727. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Ohya, S.; Narita, T.; Katsuta, M.; Iijima, M.; Masuda, N.; Yasuda, H.; Trias, J.; Nikaido, H. Activity of the carbapenem panipenem and role of the OprD (D2) protein in its diffusion through the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 1993, 37, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Sugawara, E.; Nikaido, H.; Bezrukov, S.M. Pseudomonas aeruginosa porin OprF: Properties of the channel. J. Biol. Chem. 2006, 281, 16230–16237. [Google Scholar] [CrossRef]
- Bouffartigues, E.; Moscoso, J.A.; Duchesne, R.; Rosay, T.; Fito-Boncompte, L.; Gicquel, G.; Maillot, O.; Bénard, M.; Bazire, A.; Brenner-Weiss, G.; et al. The absence of the Pseudomonas aeruginosa OprF protein leads to increased biofilm formation through variation in c-di-GMP level. Front. Microbiol. 2015, 6, 630. [Google Scholar] [CrossRef]
- Gupta, N.; Limbago, B.M.; Patel, J.B.; Kallen, A.J. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin. Infect. Dis. 2011, 53, 60–67. [Google Scholar] [CrossRef]
- Tehrani, K.; Martin, N.I. β-lactam/β-lactamase inhibitor combinations: An update. Medchemcomm 2018, 9, 1439–1456. [Google Scholar] [CrossRef]
- Yang, W.; Moore, I.F.; Koteva, K.P.; Bareich, D.C.; Hughes, D.W.; Wright, G.D. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 2004, 279, 52346–52352. [Google Scholar] [CrossRef]
- Moore, I.F.; Hughes, D.W.; Wright, G.D. Tigecycline is modified by the flavin-dependent monooxygenase TetX. Biochemistry 2005, 44, 11829–11835. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Park, J.; Gasparrini, A.J.; Reck, M.R.; Symister, C.T.; Elliott, J.L.; Vogel, J.P.; Wencewicz, T.A.; Dantas, G.; Tolia, N.H. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat. Chem. Biol. 2017, 13, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-inactivating enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Jatzwauk, L.; Müller, E.; Nitschke, H.; Pfohl, K.; Slickers, P.; Reissig, A.; Ruppelt-Lorz, A.; Ehricht, R. Diversity of SCCmec elements in Staphylococcus aureus as observed in South-Eastern Germany. PLoS ONE 2016, 11, e0162654. [Google Scholar] [CrossRef] [PubMed]
- Guffey, A.A.; Loll, P.J. Regulation of Resistance in Vancomycin-Resistant Enterococci: The VanRS two-component system. Microorganisms 2021, 9, 2026. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E.; Arias, C.A.; Courvalin, P. Gene vanXYC encodes D,D -dipeptidase (VanX) and D,D-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol. Microbiol. 1999, 34, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Depardieu, F.; Cabanié, L.; Reynolds, P.; Courvalin, P. Requirement of the VanY and VanX D,D-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 1998, 30, 819–830. [Google Scholar] [CrossRef]
- Poole, K.; Srikumar, R. Multidrug efflux in Pseudomonas aeruginosa: Components, mechanisms and clinical significance. Curr. Top. Med. Chem. 2001, 1, 59–71. [Google Scholar] [CrossRef]
- Alvarez-Ortega, C.; Olivares, J.; Martínez, J.L. RND multidrug efflux pumps: What are they good for? Front. Microbiol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Rosner, J.L.; Martin, R.G. Reduction of cellular stress by TolC-dependent efflux pumps in Escherichia coli indicated by BaeSR and CpxARP activation of spy in efflux mutants. J. Bacteriol. 2013, 195, 1042–1050. [Google Scholar] [CrossRef]
- Tatsumi, R.; Wachi, M. TolC-dependent exclusion of porphyrins in Escherichia coli. J. Bacteriol. 2008, 190, 6228–6233. [Google Scholar] [CrossRef]
- Piddock, L.J. Multidrug-resistance efflux pumps—Not just for resistance. Nat. Rev. Microbiol. 2006, 4, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef]
- Baugh, S.; Ekanayaka, A.S.; Piddock, L.J.; Webber, M.A. Loss of or inhibition of all multidrug resistance efflux pumps of Salmonella enterica serovar Typhimurium results in impaired ability to form a biofilm. J. Antimicrob. Chemother. 2012, 67, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; Dos Santos Rodrigues, J.B.; Magnani, M.; de Souza, E.L.; de Siqueira-Júnior, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wang-Kan, X.; Blair, J.M.A.; Chirullo, B.; Betts, J.; La Ragione, R.M.; Ivens, A.; Ricci, V.; Opperman, T.J.; Piddock, L.J.V. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica Serovar Typhimurium. mBio 2017, 8, e00968-17. [Google Scholar] [CrossRef]
- Matsumura, K.; Furukawa, S.; Ogihara, H.; Morinaga, Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011, 16, 69–72. [Google Scholar] [CrossRef]
- Hassan, K.A.; Liu, Q.; Elbourne, L.D.H.; Ahmad, I.; Sharples, D.; Naidu, V.; Chan, C.L.; Li, L.; Harborne, S.P.D.; Pokhrel, A.; et al. Pacing across the membrane: The novel PACE family of efflux pumps is widespread in Gram-negative pathogens. Res. Microbiol. 2018, 169, 450–454. [Google Scholar] [CrossRef]
- Housseini, B.I.K.; Phan, G.; Broutin, I. Functional mechanism of the efflux pumps transcription regulators from Pseudomonas aeruginosa based on 3D structures. Front. Mol. BioSci. 2018, 5, 57. [Google Scholar] [CrossRef]
- Mousa, J.J.; Bruner, S.D. Structural and mechanistic diversity of multidrug transporters. Nat. Prod. Rep. 2016, 33, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Uniyal, A.; Tiwari, V. A comprehensive review on pharmacology of efflux pumps and their inhibitors in antibiotic resistance. Eur. J. Pharmacol. 2021, 903, 174151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, Physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Brennan, R.G. Structural mechanisms of multidrug recognition and regulation by bacterial multidrug transcription factors. Mol. Microbiol. 2002, 45, 885–893. [Google Scholar] [CrossRef]
- Takeuchi, K.; Imai, M.; Shimada, I. Conformational equilibrium defines the variable induction of the multidrug-binding transcriptional repressor QacR. Proc. Natl. Acad. Sci. USA 2019, 116, 19963–19972. [Google Scholar] [CrossRef]
- Wade, H. MD recognition by MDR gene regulators. Curr. Opin. Struct. Biol. 2010, 20, 489–496. [Google Scholar] [CrossRef]
- Bachas, S.; Eginton, C.; Gunio, D.; Wade, H. Structural contributions to multidrug recognition in the multidrug resistance (MDR) gene regulator, BmrR. Proc. Natl. Acad. Sci. USA 2011, 108, 11046–11051. [Google Scholar] [CrossRef]
- Ahmed, M.; Borsch, C.M.; Taylor, S.S.; Vázquez-Laslop, N.; Neyfakh, A.A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J. Biol. Chem. 1994, 269, 28506–28513. [Google Scholar] [CrossRef]
- Alguel, Y.; Meng, C.; Terán, W.; Krell, T.; Ramos, J.L.; Gallegos, M.T.; Zhang, X. Crystal structures of multidrug binding protein TtgR in complex with antibiotics and plant antimicrobials. J. Mol. Biol. 2007, 369, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.; Kisker, C.; Düvel, M.; Müller, A.; Tovar, K.; Hillen, W.; Saenger, W. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science 1994, 264, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural mechanisms of QacR induction and multidrug recognition. Science 2001, 294, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Terán, W.; Krell, T.; Ramos, J.L.; Gallegos, M.T. Effector-repressor interactions, binding of a single effector molecule to the operator-bound TtgR homodimer mediates derepression. J. Biol. Chem. 2006, 281, 7102–7109. [Google Scholar] [CrossRef] [PubMed]
- Hillen, W.; Berens, C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 1994, 48, 345–369. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, S.; Brown, M.H.; Roberts, N.J.; Paulsen, I.T.; Skurray, R.A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 1998, 273, 18665–18673. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Lewis, K.; Matin, A. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 1995, 177, 2328–2334. [Google Scholar] [CrossRef]
- Xiong, A.; Gottman, A.; Park, C.; Baetens, M.; Pandza, S.; Matin, A. The EmrR protein represses the Escherichia coli emrRAB multidrug resistance operon by directly binding to its promoter region. Antimicrob. Agents Chemother. 2000, 44, 2905–2907. [Google Scholar] [CrossRef][Green Version]
- Turner, A.K.; Eckert, S.E.; Turner, D.J.; Yasir, M.; Webber, M.A.; Charles, I.G.; Parkhill, J.; Wain, J. A whole-genome screen identifies Salmonella enterica serovar Typhi genes involved in fluoroquinolone susceptibility. J. Antimicrob. Chemother. 2020, 75, 2516–2525. [Google Scholar] [CrossRef]
- Chen, S.; Cui, S.; McDermott, P.F.; Zhao, S.; White, D.G.; Paulsen, I.; Meng, J. Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar typhimurium to fluoroquinolones and other antimicrobials. Antimicrob. Agents Chemother. 2007, 51, 535–542. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1995, 39, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.; Aras, R.; Hooper, D.C. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J. Bacteriol. 2000, 182, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.C.; Zhang, X.; Hooper, D.C. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J. Bacteriol. 2003, 185, 3127–3138. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.C.; Dunman, P.M.; Eidem, T.; Hooper, D.C. Transcriptional profiling analysis of the global regulator NorG, a GntR-like protein of Staphylococcus aureus. J. Bacteriol. 2011, 193, 6207–6214. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Thyagarajan, R.V.; Seo, S.M. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob. Agents Chemother. 2005, 49, 161–169. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Wang, Y.; Reedy, J.L.; Vyas, J.M.; Hooper, D.C. Staphylococcus aureus efflux pumps and tolerance to ciprofloxacin and chlorhexidine following induction by mupirocin. Antimicrob. Agents Chemother. 2021, 66, e0184521. [Google Scholar] [CrossRef]
- Zähner, D.; Zhou, X.; Chancey, S.T.; Pohl, J.; Shafer, W.M.; Stephens, D.S. Human antimicrobial peptide LL-37 induces MefE/Mel-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2010, 54, 3516–3519. [Google Scholar] [CrossRef]
- Skiada, A.; Markogiannakis, A.; Plachouras, D.; Daikos, G.L. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2011, 37, 187–193. [Google Scholar] [CrossRef]
- Puja, H.; Bolard, A.; Noguès, A.; Plésiat, P.; Jeannot, K. The efflux pump MexXY/OprM contributes to the tolerance and acquired resistance of Pseudomonas aeruginosa to colistin. Antimicrob. Agents Chemother. 2020, 64, e02033-02019. [Google Scholar] [CrossRef]
- Jeannot, K.; Sobel, M.L.; El Garch, F.; Poole, K.; Plésiat, P. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 2005, 187, 5341–5346. [Google Scholar] [CrossRef]
- Caughlan, R.E.; Sriram, S.; Daigle, D.M.; Woods, A.L.; Buco, J.; Peterson, R.L.; Dzink-Fox, J.; Walker, S.; Dean, C.R. Fmt bypass in Pseudomonas aeruginosa causes induction of MexXY efflux pump expression. Antimicrob. Agents Chemother. 2009, 53, 5015–5021. [Google Scholar] [CrossRef] [PubMed]
- El’Garch, F.; Jeannot, K.; Hocquet, D.; Llanes-Barakat, C.; Plésiat, P. Cumulative effects of several nonenzymatic mechanisms on the resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 2007, 51, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Westbrock-Wadman, S.; Sherman, D.R.; Hickey, M.J.; Coulter, S.N.; Zhu, Y.Q.; Warrener, P.; Nguyen, L.Y.; Shawar, R.M.; Folger, K.R.; Stover, C.K. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob. Agents Chemother. 1999, 43, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jin, Y.; Bian, T.; Li, K.; Sun, Z.; Cheng, Z.; Jin, S.; Wu, W. SuhB is a novel ribosome associated protein that regulates expression of MexXY by modulating ribosome stalling in Pseudomonas aeruginosa. Mol. Microbiol. 2015, 98, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ueda, A.; Kudo, M.; Matsuo, Y.; Fukushima, J.; Nakae, T.; Kaneko, T.; Ishigatsubo, Y. Role of MexZ and PA5471 in transcriptional regulation of mexXY in Pseudomonas aeruginosa. Microbiology 2009, 155, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Tetro, K.; Zhao, Q.; Neshat, S.; Heinrichs, D.E.; Bianco, N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 1996, 40, 2021–2028. [Google Scholar] [CrossRef]
- Lau, C.H.; Krahn, T.; Gilmour, C.; Mullen, E.; Poole, K. AmgRS-mediated envelope stress-inducible expression of the mexXY multidrug efflux operon of Pseudomonas aeruginosa. Microbiologyopen 2015, 4, 121–135. [Google Scholar] [CrossRef]
- Alguel, Y.; Lu, D.; Quade, N.; Sauter, S.; Zhang, X. Crystal structure of MexZ, a key repressor responsible for antibiotic resistance in Pseudomonas aeruginosa. J. Struct. Biol. 2010, 172, 305–310. [Google Scholar] [CrossRef]
- Jahandideh, S. Diversity in structural consequences of MexZ mutations in Pseudomonas aeruginosa. Chem. Biol. Drug Des. 2013, 81, 600–606. [Google Scholar] [CrossRef]
- Guénard, S.; Muller, C.; Monlezun, L.; Benas, P.; Broutin, I.; Jeannot, K.; Plésiat, P. Multiple mutations lead to MexXY-OprM-dependent aminoglycoside resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 221–228. [Google Scholar] [CrossRef]
- Gipson, K.S.; Nickerson, K.P.; Drenkard, E.; Llanos-Chea, A.; Dogiparthi, S.K.; Lanter, B.B.; Hibbler, R.M.; Yonker, L.M.; Hurley, B.P.; Faherty, C.S. The great ESKAPE: Exploring the crossroads of bile and antibiotic resistance in bacterial pathogens. Infect. Immun. 2020, 88, e00865-19. [Google Scholar] [CrossRef] [PubMed]
- Sistrunk, J.R.; Nickerson, K.P.; Chanin, R.B.; Rasko, D.A.; Faherty, C.S. Survival of the fittest: How bacterial pathogens utilize bile to enhance infection. Clin. Microbiol. Rev. 2016, 29, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Panigrahi, P.; Varshney, N.; Ramasamy, S.; Suresh, C.G. Structure and function of a highly active Bile Salt Hydrolase (BSH) from Enterococcus faecalis and post-translational processing of BSH enzymes. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 507–518. [Google Scholar] [CrossRef]
- Kristich, C.J.; Wells, C.L.; Dunny, G.M. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc. Natl. Acad. Sci. USA 2007, 104, 3508–3513. [Google Scholar] [CrossRef] [PubMed]
- Kristich, C.J.; Little, J.L.; Hall, C.L.; Hoff, J.S. Reciprocal regulation of cephalosporin resistance in Enterococcus faecalis. mBio 2011, 2, e00199-11. [Google Scholar] [CrossRef]
- Solheim, M.; Aakra, A.; Vebø, H.; Snipen, L.; Nes, I.F. Transcriptional responses of Enterococcus faecalis V583 to bovine bile and sodium dodecyl sulfate. Appl. Environ. Microbiol. 2007, 73, 5767–5774. [Google Scholar] [CrossRef]
- Reen, F.J.; Flynn, S.; Woods, D.F.; Dunphy, N.; Chróinín, M.N.; Mullane, D.; Stick, S.; Adams, C.; O’Gara, F. Bile signalling promotes chronic respiratory infections and antibiotic tolerance. Sci. Rep. 2016, 6, 29768. [Google Scholar] [CrossRef]
- Koskenniemi, K.; Laakso, K.; Koponen, J.; Kankainen, M.; Greco, D.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Surakka, A.; Salusjärvi, T.; et al. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell Proteom. 2011, 10, S1–S18. [Google Scholar] [CrossRef]
- Kus, J.V.; Gebremedhin, A.; Dang, V.; Tran, S.L.; Serbanescu, A.; Barnett Foster, D. Bile salts induce resistance to polymyxin in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 2011, 193, 4509–4515. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Tian, P.; Bao, T.; Li, L.; Zhao, X. The LiaFSR and BsrXRS systems contribute to bile salt resistance in Enterococcus faecium isolates. Front. Microbiol. 2019, 10, 1048. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Vaidyanathan, V.; Mondal, A.; Rajamohan, G. Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH-K2044. PLoS ONE 2012, 7, e33777. [Google Scholar] [CrossRef]
- Hirakawa, H.; Inazumi, Y.; Masaki, T.; Hirata, T.; Yamaguchi, A. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 2005, 55, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, E.; Giraud, E.; Baucheron, S.; Yamasaki, S.; Wiedemann, A.; Okamoto, K.; Takagi, T.; Yamaguchi, A.; Cloeckaert, A.; Nishino, K. Effects of indole on drug resistance and virulence of Salmonella enterica serovar Typhimurium revealed by genome-wide analyses. Gut Pathog. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, E.; Shirosaka, I.; Yamaguchi, A.; Nishino, K. Regulation of the AcrAB multidrug efflux pump in Salmonella enterica serovar Typhimurium in response to indole and paraquat. Microbiology 2011, 157, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Sato, K.; Shibayama, K.; Horii, T.; Iimuma, Y.; Arakawa, Y.; Ohta, M. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol. Lett. 1999, 179, 345–352. [Google Scholar] [CrossRef]
- Poole, K. Bacterial multidrug efflux pumps serve other functions. Microbe (Am. Soc. Microbiol.) 2008, 3, 179–185. [Google Scholar] [CrossRef]
- Li, R.; Han, Y.; Zhou, Y.; Du, Z.; Wu, H.; Wang, J.; Chen, Y. Tigecycline susceptibility and molecular resistance mechanisms among clinical Klebsiella pneumoniae strains isolated during non-tigecycline treatment. Microb. Drug Resist. 2017, 23, 139–146. [Google Scholar] [CrossRef]
- Fang, L.; Chen, Q.; Shi, K.; Li, X.; Shi, Q.; He, F.; Zhou, J.; Yu, Y.; Hua, X. Step-wise increase in Tigecycline resistance in Klebsiella pneumoniae associated with mutations in ramR, lon and rpsJ. PLoS ONE 2016, 11, e0165019. [Google Scholar] [CrossRef]
- Hentschke, M.; Wolters, M.; Sobottka, I.; Rohde, H.; Aepfelbacher, M. ramR mutations in clinical isolates of Klebsiella pneumoniae with reduced susceptibility to tigecycline. Antimicrob. Agents Chemother. 2010, 54, 2720–2723. [Google Scholar] [CrossRef]
- Campos, C.B.; Aepfelbacher, M.; Hentschke, M. Molecular analysis of the ramRA locus in clinical Klebsiella pneumoniae isolates with reduced susceptibility to tigecycline. New Microbiol. 2017, 40, 135–138. [Google Scholar]
- Abouzeed, Y.M.; Baucheron, S.; Cloeckaert, A. ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 2008, 52, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- De Majumdar, S.; Veleba, M.; Finn, S.; Fanning, S.; Schneiders, T. Elucidating the regulon of multidrug resistance regulator RarA in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Bratu, S.; Landman, D.; George, A.; Salvani, J.; Quale, J. Correlation of the expression of acrB and the regulatory genes marA, soxS and ramA with antimicrobial resistance in clinical isolates of Klebsiella pneumoniae endemic to New York City. J. Antimicrob. Chemother. 2009, 64, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Poza, M.; Aranda, J.; Latasa, C.; Medrano, F.J.; Tomás, M.; Romero, A.; Lasa, I.; Bou, G. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob. Agents Chemother. 2012, 56, 6256–6266. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, M.; Sandegren, L.; Andersson, D.I. Mechanisms and fitness costs of tigecycline resistance in Escherichia coli. J. Antimicrob. Chemother. 2013, 68, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, A.; Keeney, D.; Bradford, P.A. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 2007, 59, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.E.; Snesrud, E.C.; Onmus-Leone, F.; Kwak, Y.I.; Avilés, R.; Steele, E.D.; Sutter, D.E.; Waterman, P.E.; Lesho, E.P. IS5 element integration, a novel mechanism for rapid in vivo emergence of tigecycline nonsusceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2014, 58, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Olliver, A.; Vallé, M.; Chaslus-Dancla, E.; Cloeckaert, A. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob. Agents Chemother. 2005, 49, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Cao, L.; Gould, V.C.; Avison, M.B.; Poole, K. nalD encodes a second repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 8649–8654. [Google Scholar] [CrossRef]
- Liao, J.; Schurr, M.J.; Sauer, K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2013, 195, 3352–3363. [Google Scholar] [CrossRef]
- Tian, Z.X.; Yi, X.X.; Cho, A.; O’Gara, F.; Wang, Y.P. CpxR activates MexAB-OprM efflux pump expression and enhances antibiotic resistance in both laboratory and clinical nalB-type isolates of Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1005932. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Hirai, K. Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol. Lett. 1992, 76, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Epp, S.F.; Curty, L.K.; Pechère, J.C. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 1999, 181, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, C.; Zhang, X.; Wang, D.; Pan, X.; Liu, H.; Zhu, G.; Bai, F.; Cheng, Z.; Wu, W.; et al. A MexR mutation which confers aztreonam resistance to Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 659808. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Furlan, J.P.; Fernandes, A.F.; Stehling, E.G. Mutations in NalC induce MexAB-OprM overexpression resulting in high level of aztreonam resistance in environmental isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2016, 363, fnw166. [Google Scholar] [CrossRef]
- Suresh, M.; Nithya, N.; Jayasree, P.R.; Vimal, K.P.; Manish Kumar, P.R. Mutational analyses of regulatory genes, mexR, nalC, nalD and mexZ of mexAB-oprM and mexXY operons, in efflux pump hyperexpressing multidrug-resistant clinical isolates of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2018, 34, 83. [Google Scholar] [CrossRef]
- Aendekerk, S.; Diggle, S.P.; Song, Z.; Høiby, N.; Cornelis, P.; Williams, P.; Cámara, M. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 2005, 151, 1113–1125. [Google Scholar] [CrossRef]
- Sakhtah, H.; Koyama, L.; Zhang, Y.; Morales, D.K.; Fields, B.L.; Price-Whelan, A.; Hogan, D.A.; Shepard, K.; Dietrich, L.E. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc. Natl. Acad. Sci. USA 2016, 113, E3538–E3547. [Google Scholar] [CrossRef]
- Dietrich, L.E.; Price-Whelan, A.; Petersen, A.; Whiteley, M.; Newman, D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. [Google Scholar] [CrossRef]
- Duval, V.; Lister, I.M. MarA, SoxS and Rob of Escherichia coli—Global regulators of multidrug resistance, virulence and stress response. Int. J. Biotechnol. Wellness Ind. 2013, 2, 101–124. [Google Scholar] [CrossRef]
- Jiménez-Castellanos, J.C.; Wan Ahmad Kamil, W.N.; Cheung, C.H.; Tobin, M.S.; Brown, J.; Isaac, S.G.; Heesom, K.J.; Schneiders, T.; Avison, M.B. Comparative effects of overproducing the AraC-type transcriptional regulators MarA, SoxS, RarA and RamA on antimicrobial drug susceptibility in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2016, 71, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Yang, S.; Davidson, A.L.; Zechiedrich, E.L. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 2002, 43, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gu, R.; Su, C.C.; Routh, M.D.; Harris, K.C.; Jewell, E.S.; McDermott, G.; Yu, E.W. Crystal structure of the transcriptional regulator AcrR from Escherichia coli. J. Mol. Biol. 2007, 374, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.Y.; Bertenthal, D.; Nilles, M.L.; Bertrand, K.P.; Nikaido, H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 2003, 48, 1609–1619. [Google Scholar] [CrossRef]
- Prajapat, M.K.; Jain, K.; Saini, S. Control of MarRAB operon in Escherichia coli via autoactivation and autorepression. Biophys. J. 2015, 109, 1497–1508. [Google Scholar] [CrossRef]
- Vinué, L.; McMurry, L.M.; Levy, S.B. The 216-bp marB gene of the marRAB operon in Escherichia coli encodes a periplasmic protein which reduces the transcription rate of marA. FEMS Microbiol. Lett. 2013, 345, 49–55. [Google Scholar] [CrossRef]
- Sulavik, M.C.; Gambino, L.F.; Miller, P.F. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: Prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1995, 1, 436–446. [Google Scholar] [CrossRef]
- Randall, L.P.; Woodward, M.J. The multiple antibiotic resistance (mar) locus and its significance. Res. Vet. Sci. 2002, 72, 87–93. [Google Scholar] [CrossRef]
- Li, W.; Xue, M.; Yu, L.; Qi, K.; Ni, J.; Chen, X.; Deng, R.; Shang, F.; Xue, T. QseBC is involved in the biofilm formation and antibiotic resistance in Escherichia coli isolated from bovine mastitis. PeerJ 2020, 8, e8833. [Google Scholar] [CrossRef]
- Chubiz, L.M.; Rao, C.V. Role of the mar-sox-rob regulon in regulating outer membrane porin expression. J. Bacteriol. 2011, 193, 2252–2260. [Google Scholar] [CrossRef]
- Veleba, M.; Higgins, P.G.; Gonzalez, G.; Seifert, H.; Schneiders, T. Characterization of RarA, a novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2012, 56, 4450–4458. [Google Scholar] [CrossRef] [PubMed]
- Touati, D. Sensing and protecting against superoxide stress in Escherichia coli-how many ways are there to trigger soxRS response? Redox Rep. 2000, 5, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Nunoshiba, T.; Hidalgo, E.; Amábile Cuevas, C.F.; Demple, B. Two-stage control of an oxidative stress regulon: The Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J. Bacteriol. 1992, 174, 6054–6060. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Aslund, F.; Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 1998, 279, 1718–1721. [Google Scholar] [CrossRef]
- Anes, J.; Dever, K.; Eshwar, A.; Nguyen, S.; Cao, Y.; Sivasankaran, S.K.; Sakalauskaitė, S.; Lehner, A.; Devineau, S.; Daugelavičius, R.; et al. Analysis of the oxidative stress regulon identifies soxS as a genetic target for resistance reversal in multidrug-resistant Klebsiella pneumoniae. mBio 2021, 12, e0086721. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.V.; Babu, T.M.; Reddy, N.V.; Rajendra, W. Homology modeling, molecular dynamics, and virtual screening of NorA efflux pump inhibitors of Staphylococcus aureus. Drug Des. Devel. Ther. 2016, 10, 3237–3252. [Google Scholar] [CrossRef]
- Costa, S.S.; Sobkowiak, B.; Parreira, R.; Edgeworth, J.D.; Viveiros, M.; Clark, T.G.; Couto, I. Genetic diversity of norA, coding for a main efflux pump of Staphylococcus aureus. Front. Genet. 2018, 9, 710. [Google Scholar] [CrossRef]
- Villet, R.A.; Truong-Bolduc, Q.C.; Wang, Y.; Estabrooks, Z.; Medeiros, H.; Hooper, D.C. Regulation of expression of abcA and its response to environmental conditions. J. Bacteriol. 2014, 196, 1532–1539. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Hooper, D.C. The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and beta-lactams in Staphylococcus aureus. J. Bacteriol. 2007, 189, 2996–3005. [Google Scholar] [CrossRef]
- Schrader-Fischer, G.; Berger-Bächi, B. The AbcA transporter of Staphylococcus aureus affects cell autolysis. Antimicrob. Agents Chemother. 2001, 45, 407–412. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Duong, A.C.; Otto, M. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: Implications for diagnosis and pathogenesis. Microbes Infect. 2012, 14, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, H.; Rao, X.; Chen, W.; Wang, Z.; Hu, X. Phenol-soluble modulins: Novel virulence-associated peptides of Staphylococci. Future Microbiol. 2014, 9, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Hou, Y.; Soteyome, T.; Zeng, B.; Su, J.; Li, L.; Li, B.; Chen, D.; Li, Y.; et al. Transcriptomics study on Staphylococcus aureus Biofilm under low concentration of ampicillin. Front. Microbiol. 2018, 9, 2413. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, D.; Steitz, T.A.; Polikanov, Y.S.; Gagnon, M.G. Ribosome-targeting antibiotics: Modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 2018, 87, 451–478. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Mazel, D.; Baharoglu, Z. Deficiency in cytosine DNA methylation leads to high chaperonin expression and tolerance to aminoglycosides in Vibrio cholerae. PLoS Genet. 2021, 17, e1009748. [Google Scholar] [CrossRef]
- Murina, V.; Kasari, M.; Takada, H.; Hinnu, M.; Saha, C.K.; Grimshaw, J.W.; Seki, T.; Reith, M.; Putrinš, M.; Tenson, T.; et al. ABCF ATPases involved in protein synthesis, ribosome assembly and antibiotic resistance: Structural and functional diversification across the tree of life. J. Mol. Biol. 2019, 431, 3568–3590. [Google Scholar] [CrossRef]
- Fostier, C.R.; Monlezun, L.; Ousalem, F.; Singh, S.; Hunt, J.F.; Boël, G. ABC-F translation factors: From antibiotic resistance to immune response. FEBS Lett. 2021, 595, 675–706. [Google Scholar] [CrossRef]
- Murina, V.; Kasari, M.; Hauryliuk, V.; Atkinson, G.C. Antibiotic resistance ABCF proteins reset the peptidyl transferase centre of the ribosome to counter translational arrest. Nucleic Acids Res. 2018, 46, 3753–3763. [Google Scholar] [CrossRef]
- Sharkey, L.K.R.; O’Neill, A.J. Antibiotic resistance ABC-F proteins: Bringing target protection into the limelight. ACS Infect. Dis. 2018, 4, 239–246. [Google Scholar] [CrossRef]
- Eyraud, A.; Tattevin, P.; Chabelskaya, S.; Felden, B. A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res. 2014, 42, 4892–4905. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, R.; Tang, H.; Osei-Adjei, G.; Xu, S.; Zhang, Y.; Huang, X. A 3′ UTR-derived non-coding RNA RibS increases expression of cfa and promotes biofilm formation of Salmonella enterica serovar Typhi. Res. Microbiol. 2018, 169, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhu, W.; Chen, D.; Zhou, Y.; Lin, H. Small noncoding RNA sRNA0426 is involved in regulating biofilm formation in Streptococcus mutans. Microbiologyopen 2020, 9, e1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X.; Wang, H.; Fang, H.; Yan, Y.; Liu, L.; Chen, R.; Zhou, D.; Yang, R.; Han, Y. Plasmid pPCP1-derived sRNA HmsA promotes biofilm formation of Yersinia pestis. BMC Microbiol. 2016, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Förstner, K.U.; Cong, J.P.; Sharma, C.M.; Bassler, B.L. Differential RNA-seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc. Natl. Acad. Sci. USA 2015, 112, E766–E775. [Google Scholar] [CrossRef]
- Bordeau, V.; Felden, B. Curli synthesis and biofilm formation in enteric bacteria are controlled by a dynamic small RNA module made up of a pseudoknot assisted by an RNA chaperone. Nucleic Acids Res. 2014, 42, 4682–4696. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Thomason, M.K.; Havelund, J.; Valentin-Hansen, P.; Storz, G. Dual function of the McaS small RNA in controlling biofilm formation. Genes Dev. 2013, 27, 1132–1145. [Google Scholar] [CrossRef]
- Mann, B.; van Opijnen, T.; Wang, J.; Obert, C.; Wang, Y.D.; Carter, R.; McGoldrick, D.J.; Ridout, G.; Camilli, A.; Tuomanen, E.I.; et al. Control of virulence by small RNAs in Streptococcus pneumoniae. PLoS Pathog. 2012, 8, e1002788. [Google Scholar] [CrossRef]
- Danger, J.L.; Cao, T.N.; Cao, T.H.; Sarkar, P.; Treviño, J.; Pflughoeft, K.J.; Sumby, P. The small regulatory RNA FasX enhances group A Streptococcus virulence and inhibits pilus expression via serotype-specific targets. Mol. Microbiol. 2015, 96, 249–262. [Google Scholar] [CrossRef]
- Reinhart, A.A.; Powell, D.A.; Nguyen, A.T.; O’Neill, M.; Djapgne, L.; Wilks, A.; Ernst, R.K.; Oglesby-Sherrouse, A.G. The prrF-encoded small regulatory RNAs are required for iron homeostasis and virulence of Pseudomonas aeruginosa. Infect. Immun. 2015, 83, 863–875. [Google Scholar] [CrossRef]
- Michaux, C.; Hartke, A.; Martini, C.; Reiss, S.; Albrecht, D.; Budin-Verneuil, A.; Sanguinetti, M.; Engelmann, S.; Hain, T.; Verneuil, N.; et al. Involvement of Enterococcus faecalis small RNAs in stress response and virulence. Infect. Immun. 2014, 82, 3599–3611. [Google Scholar] [CrossRef]
- Koo, J.T.; Alleyne, T.M.; Schiano, C.A.; Jafari, N.; Lathem, W.W. Global discovery of small RNAs in Yersinia pseudotuberculosis identifies Yersinia-specific small, noncoding RNAs required for virulence. Proc. Natl. Acad. Sci. USA 2011, 108, E709–E717. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Vogel, J. A 3′ UTR-derived small RNA provides the regulatory noncoding arm of the inner membrane stress response. Mol. Cell 2016, 61, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.L.; Evans, A.D.; Guest, R.L.; Raivio, T.L. The Cpx envelope stress response regulates and is regulated by small noncoding RNAs. J. Bacteriol. 2014, 196, 4229–4238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, M.S.; Updegrove, T.B.; Gogol, E.B.; Shabalina, S.A.; Gross, C.A.; Storz, G. MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev. 2014, 28, 1620–1634. [Google Scholar] [CrossRef]
- Zhang, H.; Song, T.; Qin, C.; Xu, H.; Qiao, M. A novel non-coding RNA CsiR regulates the ciprofloxacin resistance in Proteus vulgaris by interacting with emrB mRNA. Int. J. Mol. Sci. 2021, 22, 10627. [Google Scholar] [CrossRef]
- Arenz, S.; Meydan, S.; Starosta, A.L.; Berninghausen, O.; Beckmann, R.; Vázquez-Laslop, N.; Wilson, D.N. Drug sensing by the ribosome induces translational arrest via active site perturbation. Mol. Cell 2014, 56, 446–452. [Google Scholar] [CrossRef]
- Schulthess, B.; Meier, S.; Homerova, D.; Goerke, C.; Wolz, C.; Kormanec, J.; Berger-Bächi, B.; Bischoff, M. Functional characterization of the sigmaB-dependent yabJ-spoVG operon in Staphylococcus aureus: Role in methicillin and glycopeptide resistance. Antimicrob. Agents Chemother. 2009, 53, 1832–1839. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Sun, B. SpoVG regulates cell wall metabolism and oxacillin resistance in methicillin-resistant Staphylococcus aureus strain N315. Antimicrob. Agents Chemother. 2016, 60, 3455–3461. [Google Scholar] [CrossRef]
- Groicher, K.H.; Firek, B.A.; Fujimoto, D.F.; Bayles, K.W. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 2000, 182, 1794–1801. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Shih, P.C.; Huang, C.T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Saipriya, K.; Swathi, C.H.; Ratnakar, K.S.; Sritharan, V. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2020, 128, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.K.B.; Ballard, J.D. Autoinducing peptide-based quorum signaling systems in Clostridioides difficile. Curr. Opin. Microbiol. 2021, 65, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ishii, E.; Eguchi, Y. Diversity in sensing and signaling of bacterial sensor histidine kinases. Biomolecules 2021, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Harjai, K.; Chhibber, S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: A chronicle through the perspective of quorum sensing. Environ. Microbiol. 2021, in press. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Ma, J.F.; Elkins, J.G.; McDermott, T.R.; Ochsner, U.A.; West, S.E.; Huang, C.T.; Fredericks, J.; Burnett, S.; Stewart, P.S.; et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 1999, 34, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, C.; Liu, J.; Liu, C.; Qiao, J. Vertical and horizontal quorum-sensing-based multicellular communications. Trends Microbiol. 2021, 29, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, S.L. Small RNA-based regulation of bacterial quorum sensing and biofilm formation. Microbiol. Spectr. 2018, 6, RWR-0017-2018. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Seif, Y.; Choudhary, K.S.; Dalldorf, C.; Poudel, S.; Monk, J.M.; Palsson, B.O. Pangenome analytics reveal two-component systems as conserved targets in ESKAPEE pathogens. mSystems 2021, 6, e00981-20. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; Sivaneson, M.; Filloux, A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 2011, 13, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.R.; Rather, P.N. Roles of two-component regulatory systems in antibiotic resistance. Future Microbiol. 2019, 14, 533–552. [Google Scholar] [CrossRef]
- Gooderham, W.J.; Hancock, R.E. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2009, 33, 279–294. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef]
- Rodrigue, A.; Quentin, Y.; Lazdunski, A.; Méjean, V.; Foglino, M. Two-component systems in Pseudomonas aeruginosa: Why so many? Trends Microbiol. 2000, 8, 498–504. [Google Scholar] [CrossRef]
- Mizuno, T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997, 4, 161–168. [Google Scholar] [CrossRef]
- Fabret, C.; Feher, V.A.; Hoch, J.A. Two-component signal transduction in Bacillus subtilis: How one organism sees its world. J. Bacteriol. 1999, 181, 1975–1983. [Google Scholar] [CrossRef]
- Haag, A.F.; Bagnoli, F. The role of two-component signal transduction systems in Staphylococcus aureus virulence regulation. Curr. Top. Microbiol. Immunol. 2017, 409, 145–198. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Bleul, L.; Francois, P.; Wolz, C. Two-component systems of S. aureus: Signaling and sensing mechanisms. Genes 2021, 13, 34. [Google Scholar] [CrossRef]
- Wu, S.; Lin, K.; Liu, Y.; Zhang, H.; Lei, L. Two-component signaling pathways modulate drug resistance of Staphylococcus aureus (Review). Biomed. Rep. 2020, 13, 5. [Google Scholar] [CrossRef]
- Ortet, P.; Whitworth, D.E.; Santaella, C.; Achouak, W.; Barakat, M. P2CS: Updates of the prokaryotic two-component systems database. Nucleic Acids Res. 2015, 43, D536–D541. [Google Scholar] [CrossRef]
- De Silva, P.M.; Kumar, A. Signal transduction proteins in Acinetobacter baumannii: Role in antibiotic resistance, virulence, and potential as drug targets. Front. Microbiol. 2019, 10, 49. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Li, Z.; Nair, S.K. Quorum sensing: How bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012, 21, 1403–1417. [Google Scholar] [CrossRef]
- Mull, R.W.; Harrington, A.; Sanchez, L.A.; Tal-Gan, Y. Cyclic peptides that govern signal transduction pathways: From prokaryotes to multi-cellular organisms. Curr. Top. Med. Chem. 2018, 18, 625–644. [Google Scholar] [CrossRef]
- Hense, B.A.; Schuster, M. Core principles of bacterial autoinducer systems. Microbiol. Mol. Biol. Rev. 2015, 79, 153–169. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial quorum sensing. Cell 2018, 174, 1328–1328.e1. [Google Scholar] [CrossRef]
- Kaplan, H.B.; Greenberg, E.P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 1985, 163, 1210–1214. [Google Scholar] [CrossRef]
- Pearson, J.P.; Van Delden, C.; Iglewski, B.H. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1999, 181, 1203–1210. [Google Scholar] [CrossRef]
- Mashburn, L.M.; Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005, 437, 422–425. [Google Scholar] [CrossRef]
- Evans, K.; Passador, L.; Srikumar, R.; Tsang, E.; Nezezon, J.; Poole, K. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1998, 180, 5443–5447. [Google Scholar] [CrossRef]
- Minagawa, S.; Inami, H.; Kato, T.; Sawada, S.; Yasuki, T.; Miyairi, S.; Horikawa, M.; Okuda, J.; Gotoh, N. RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 2012, 12, 70. [Google Scholar] [CrossRef]
- Köhler, T.; van Delden, C.; Curty, L.K.; Hamzehpour, M.M.; Pechere, J.C. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 2001, 183, 5213–5222. [Google Scholar] [CrossRef]
- Lamarche, M.G.; Déziel, E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline). PLoS ONE 2011, 6, e24310. [Google Scholar] [CrossRef]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef]
- Higgins, D.A.; Pomianek, M.E.; Kraml, C.M.; Taylor, R.K.; Semmelhack, M.F.; Bassler, B.L. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 2007, 450, 883–886. [Google Scholar] [CrossRef]
- Papenfort, K.; Silpe, J.E.; Schramma, K.R.; Cong, J.P.; Seyedsayamdost, M.R.; Bassler, B.L. A Vibrio cholerae autoinducer-receptor pair that controls biofilm formation. Nat. Chem. Biol. 2017, 13, 551–557. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Lenz, D.H.; Mok, K.C.; Lilley, B.N.; Kulkarni, R.V.; Wingreen, N.S.; Bassler, B.L. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 2004, 118, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; van Kessel, J.C.; Shao, Y.; Bassler, B.L. AphA and LuxR/HapR reciprocally control quorum sensing in Vibrios. Genes Dev. 2011, 25, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Frey, E.M.; Liu, Z.; Bishar, R.; Zhu, J. The virulence transcriptional activator AphA enhances biofilm formation by Vibrio cholerae by activating expression of the biofilm regulator VpsT. Infect. Immun. 2010, 78, 697–703. [Google Scholar] [CrossRef]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 2008, 190, 2527–2536. [Google Scholar] [CrossRef]
- Zhu, J.; Mekalanos, J.J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 2003, 5, 647–656. [Google Scholar] [CrossRef]
- Pompeani, A.J.; Irgon, J.J.; Berger, M.F.; Bulyk, M.L.; Wingreen, N.S.; Bassler, B.L. The Vibrio harveyi master quorum-sensing regulator, LuxR, a TetR-type protein is both an activator and a repressor: DNA recognition and binding specificity at target promoters. Mol. Microbiol. 2008, 70, 76–88. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006, 20, 2754–2767. [Google Scholar] [CrossRef]
- Ng, W.L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Ball, A.S.; Chaparian, R.R.; van Kessel, J.C. Quorum sensing gene regulation by LuxR/HapR master regulators in Vibrios. J. Bacteriol. 2017, 199, e00105-17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Gori, K.; Jespersen, L. AI-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp. Int. J. Food Microbiol. 2009, 135, 295–302. [Google Scholar] [CrossRef]
- Schauder, S.; Shokat, K.; Surette, M.G.; Bassler, B.L. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001, 41, 463–476. [Google Scholar] [CrossRef]
- Niazy, A.A. LuxS quorum sensing system and biofilm formation of oral microflora: A short review article. Saudi Dent. J. 2021, 33, 116–123. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Grenier, D.; Yi, L. Regulatory mechanisms of the LuxS/AI-2 system and bacterial resistance. Antimicrob. Agents Chemother. 2019, 63, e01186-19. [Google Scholar] [CrossRef]
- Yao, Y.; Martinez-Yamout, M.A.; Dickerson, T.J.; Brogan, A.P.; Wright, P.E.; Dyson, H.J. Structure of the Escherichia coli quorum sensing protein SdiA: Activation of the folding switch by acyl homoserine lactones. J Mol. Biol. 2006, 355, 262–273. [Google Scholar] [CrossRef]
- Styles, M.J.; Blackwell, H.E. Non-native autoinducer analogs capable of modulating the SdiA quorum sensing receptor in Salmonella enterica serovar Typhi.imurium. Beilstein J. Org. Chem. 2018, 14, 2651–2664. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 2020, 11, 5371. [Google Scholar] [CrossRef]
- Li, J.; Attila, C.; Wang, L.; Wood, T.K.; Valdes, J.J.; Bentley, W.E. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: Effects on small RNA and biofilm architecture. J. Bacteriol. 2007, 189, 6011–6020. [Google Scholar] [CrossRef]
- Xavier, K.B.; Bassler, B.L. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J. Bacteriol. 2005, 187, 238–248. [Google Scholar] [CrossRef]
- Ilangovan, A.; Fletcher, M.; Rampioni, G.; Pustelny, C.; Rumbaugh, K.; Heeb, S.; Cámara, M.; Truman, A.; Chhabra, S.R.; Emsley, J.; et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog. 2013, 9, e1003508. [Google Scholar] [CrossRef]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef]
- Passos da Silva, D.; Schofield, M.C.; Parsek, M.R.; Tseng, B.S. An update on the sociomicrobiology of quorum sensing in gram-negative biofilm development. Pathogens 2017, 6, 51. [Google Scholar] [CrossRef]
- Williams, P.; Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef]
- Schuster, M.; Greenberg, E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006, 296, 73–81. [Google Scholar] [CrossRef]
- Huang, H.; Shao, X.; Xie, Y.; Wang, T.; Zhang, Y.; Wang, X.; Deng, X. An integrated genomic regulatory network of virulence-related transcriptional factors in Pseudomonas aeruginosa. Nat. Commun. 2019, 10, 2931. [Google Scholar] [CrossRef]
- Brint, J.M.; Ohman, D.E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 1995, 177, 7155–7163. [Google Scholar] [CrossRef]
- McGrath, S.; Wade, D.S.; Pesci, E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 2004, 230, 27–34. [Google Scholar] [CrossRef]
- Sultan, M.; Arya, R.; Kim, K.K. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int. J. Mol. Sci. 2021, 22, 12152. [Google Scholar] [CrossRef]
- Rampioni, G.; Falcone, M.; Heeb, S.; Frangipani, E.; Fletcher, M.P.; Dubern, J.F.; Visca, P.; Leoni, L.; Cámara, M.; Williams, P. Unravelling the genome-wide contributions of specific 2-Alkyl-4-Quinolones and PqsE to quorum sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1006029. [Google Scholar] [CrossRef]
- García-Reyes, S.; Soberón-Chávez, G.; Cocotl-Yanez, M. The third quorum-sensing system of Pseudomonas aeruginosa: Pseudomonas quinolone signal and the enigmatic PqsE protein. J. Med. Microbiol. 2020, 69, 25–34. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Fan, J.; Cao, Z.; Ouyang, W.; Tong, N.; Hu, X.; Hu, J.; Li, P.; Feng, Z.; et al. Anti-biofilm effect of novel thiazole acid analogs against Pseudomonas aeruginosa through IQS pathways. Eur. J. Med. Chem. 2018, 145, 64–73. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Kong, K.F.; Jayawardena, S.R.; Leal, S.M.; Sautter, R.T.; Mathee, K. Co-regulation of b-lactam resistance, alginate production and quorum sensing in Pseudomonas aeruginosa. J. Med. Microbiol. 2011, 60, 147–156. [Google Scholar] [CrossRef]
- Kong, K.F.; Jayawardena, S.R.; Indulkar, S.D.; Del Puerto, A.; Koh, C.L.; Høiby, N.; Mathee, K. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB beta-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob. Agents Chemother. 2005, 49, 4567–4575. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Merighi, M.; Smith, R.; Narasimhan, G.; Lory, S.; Mathee, K. The regulatory repertoire of Pseudomonas aeruginosa AmpC b-lactamase regulator AmpR includes virulence genes. PLoS ONE 2012, 7, e34067. [Google Scholar] [CrossRef]
- George, E.A.; Novick, R.P.; Muir, T.W. Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J. Am. Chem. Soc. 2008, 130, 4914–4924. [Google Scholar] [CrossRef]
- Liu, Q.; Yeo, W.S.; Bae, T. The SaeRS two-component system of Staphylococcus aureus. Genes 2016, 7, 81. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, L.; Landwehr, C.; Lunsford, D.; Holmes, D.; Ji, Y. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J. Bacteriol. 2005, 187, 5486–5492. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Beenken, K.E.; Byrum, S.D.; Tackett, A.J.; Shaw, L.N.; Gimza, B.D.; Smeltzer, M.S. SarA plays a predominant role in controlling the production of extracellular proteases in the diverse clinical isolates of Staphylococcus aureus LAC and UAMS-1. Virulence 2020, 11, 1738–1762. [Google Scholar] [CrossRef]
- Wang, B.; Muir, T.W. Regulation of virulence in Staphylococcus aureus: Molecular mechanisms and remaining puzzles. Cell Chem. Biol. 2016, 23, 214–224. [Google Scholar] [CrossRef]
- Dufour, P.; Jarraud, S.; Vandenesch, F.; Greenland, T.; Novick, R.P.; Bes, M.; Etienne, J.; Lina, G. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 2002, 184, 1180–1186. [Google Scholar] [CrossRef]
- Fechter, P.; Caldelari, I.; Lioliou, E.; Romby, P. Novel aspects of RNA regulation in Staphylococcus aureus. FEBS Lett. 2014, 588, 2523–2529. [Google Scholar] [CrossRef]
- Morfeldt, E.; Taylor, D.; von Gabain, A.; Arvidson, S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995, 14, 4569–4577. [Google Scholar] [CrossRef]
- Gupta, R.K.; Luong, T.T.; Lee, C.Y. RNAIII of the Staphylococcus aureus agr system activates global regulator MgrA by stabilizing mRNA. Proc. Natl. Acad. Sci. USA 2015, 112, 14036–14041. [Google Scholar] [CrossRef]
- Benito, Y.; Kolb, F.A.; Romby, P.; Lina, G.; Etienne, J.; Vandenesch, F. Probing the structure of RNAIII, the Staphylococcus aureus agr regulatory RNA, and identification of the RNA domain involved in repression of protein A expression. Rna 2000, 6, 668–679. [Google Scholar] [CrossRef]
- Saïd-Salim, B.; Dunman, P.M.; McAleese, F.M.; Macapagal, D.; Murphy, E.; McNamara, P.J.; Arvidson, S.; Foster, T.J.; Projan, S.J.; Kreiswirth, B.N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003, 185, 610–619. [Google Scholar] [CrossRef]
- Reyes, D.; Andrey, D.O.; Monod, A.; Kelley, W.L.; Zhang, G.; Cheung, A.L. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 2011, 193, 6020–6031. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W.; Yang, J.; Guo, H.; Zhang, J.; Ji, Y. Contribution of coagulase and its regulator SaeRS to lethality of CA-MRSA 923 bacteremia. Pathogens 2021, 10, 1396. [Google Scholar] [CrossRef]
- Nygaard, T.K.; Pallister, K.B.; Ruzevich, P.; Griffith, S.; Vuong, C.; Voyich, J.M. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 2010, 201, 241–254. [Google Scholar] [CrossRef]
- Kawada-Matsuo, M.; Le, M.N.; Komatsuzawa, H. Antibacterial peptides resistance in Staphylococcus aureus: Various mechanisms and the association with pathogenicity. Genes 2021, 12, 1527. [Google Scholar] [CrossRef]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Matsuo, M.; Oogai, Y.; Kato, F.; Sugai, M.; Komatsuzawa, H. Growth-phase dependence of susceptibility to antimicrobial peptides in Staphylococcus aureus. Microbiology 2011, 157, 1786–1797. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Li, Y.; Patidar, R.; Yerex, K.; Ma, X.; Kumar, A.; Duan, K. Two component regulatory systems and antibiotic resistance in Gram-negative pathogens. Int. J. Mol. Sci. 2019, 20, 1781. [Google Scholar] [CrossRef]
- Dou, Y.; Song, F.; Guo, F.; Zhou, Z.; Zhu, C.; Xiang, J.; Huan, J. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol. Med. Rep. 2017, 15, 4061–4068. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Chua, K.L. The Burkholderia pseudomallei BpeAB-OprB efflux pump: Expression and impact on quorum sensing and virulence. J. Bacteriol. 2005, 187, 4707–4719. [Google Scholar] [CrossRef]
- Marchand, I.; Damier-Piolle, L.; Courvalin, P.; Lambert, T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 2004, 48, 3298–3304. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, F.; Zhu, L.; Felix, J.; Wu, D.; Wu, K.; Gutsche, I.; Wu, Y.; Hwang, P.M.; She, J.; et al. Proteolysis and multimerization regulate signaling along the two-component regulatory system AdeRS. iScience 2021, 24, 102476. [Google Scholar] [CrossRef]
- Richmond, G.E.; Evans, L.P.; Anderson, M.J.; Wand, M.E.; Bonney, L.C.; Ivens, A.; Chua, K.L.; Webber, M.A.; Sutton, J.M.; Peterson, M.L.; et al. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio 2016, 7, e00430-16. [Google Scholar] [CrossRef]
- Adams, F.G.; Stroeher, U.H.; Hassan, K.A.; Marri, S.; Brown, M.H. Resistance to pentamidine is mediated by AdeAB, regulated by AdeRS, and influenced by growth conditions in Acinetobacter baumannii ATCC 17978. PLoS ONE 2018, 13, e0197412. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Xue, T.; Sun, B. Modulation of cell wall synthesis and susceptibility to vancomycin by the two-component system AirSR in Staphylococcus aureus NCTC8325. BMC Microbiol. 2013, 13, 286. [Google Scholar] [CrossRef]
- Sun, F.; Ji, Q.; Jones, M.B.; Deng, X.; Liang, H.; Frank, B.; Telser, J.; Peterson, S.N.; Bae, T.; He, C. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in Staphylococcus aureus. J. Am. Chem. Soc. 2012, 134, 305–314. [Google Scholar] [CrossRef]
- Lau, C.H.; Fraud, S.; Jones, M.; Peterson, S.N.; Poole, K. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 2243–2251. [Google Scholar] [CrossRef]
- Poole, K.; Hay, T.; Gilmour, C.; Fruci, M. The aminoglycoside resistance-promoting AmgRS envelope stress-responsive two-component system in Pseudomonas aeruginosa is zinc-activated and protects cells from zinc-promoted membrane damage. Microbiology 2019, 165, 563–571. [Google Scholar] [CrossRef]
- Fruci, M.; Poole, K. Aminoglycoside-inducible expression of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: Involvement of the envelope stress-responsive AmgRS two-component system. PLoS ONE 2018, 13, e0205036. [Google Scholar] [CrossRef]
- Crosby, H.A.; Tiwari, N.; Kwiecinski, J.M.; Xu, Z.; Dykstra, A.; Jenul, C.; Fuentes, E.J.; Horswill, A.R. The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol. Microbiol. 2020, 113, 103–122. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, X.; Zhao, K.; Yan, Y.; Xu, T.; Wang, J.; Zheng, J.; Huang, W.; Shi, L.; Shang, Y.; et al. The role of ArlRS in regulating oxacillin susceptibility in methicillin-resistant Staphylococcus aureus indicates it is a potential target for antimicrobial resistance breakers. Emerg. Microbes Infect. 2019, 8, 503–515. [Google Scholar] [CrossRef]
- Jousselin, A.; Kelley, W.L.; Barras, C.; Lew, D.P.; Renzoni, A. The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 3283–3292. [Google Scholar] [CrossRef]
- Baranova, N.; Nikaido, H. The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 2002, 184, 4168–4176. [Google Scholar] [CrossRef]
- Nagakubo, S.; Nishino, K.; Hirata, T.; Yamaguchi, A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 2002, 184, 4161–4167. [Google Scholar] [CrossRef]
- Hirakawa, H.; Nishino, K.; Yamada, J.; Hirata, T.; Yamaguchi, A. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Antimicrob. Chemother. 2003, 52, 576–582. [Google Scholar] [CrossRef]
- Guerrero, P.; Collao, B.; Morales, E.H.; Calderón, I.L.; Ipinza, F.; Parra, S.; Saavedra, C.P.; Gil, F. Characterization of the BaeSR two-component system from Salmonella Typhimurium and its role in ciprofloxacin-induced mdtA expression. Arch. Microbiol. 2012, 194, 453–460. [Google Scholar] [CrossRef]
- Lin, M.F.; Lin, Y.Y.; Lan, C.Y. The role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in Acinetobacter baumannii. PLoS ONE 2015, 10, e0132843. [Google Scholar] [CrossRef]
- Lin, M.F.; Lin, Y.Y.; Yeh, H.W.; Lan, C.Y. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 2014, 14, 119. [Google Scholar] [CrossRef]
- Yu, L.; Li, W.; Xue, M.; Li, J.; Chen, X.; Ni, J.; Shang, F.; Xue, T. Regulatory role of the two-component system BasSR in the expression of the EmrD multidrug efflux in Escherichia coli. Microb. Drug Resist. 2020, 26, 1163–1173. [Google Scholar] [CrossRef]
- Kreamer, N.N.; Costa, F.; Newman, D.K. The ferrous iron-responsive BqsRS two-component system activates genes that promote cationic stress tolerance. mBio 2015, 6, e02549. [Google Scholar] [CrossRef]
- Hiron, A.; Falord, M.; Valle, J.; Débarbouillé, M.; Msadek, T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 2011, 81, 602–622. [Google Scholar] [CrossRef]
- Kawada-Matsuo, M.; Yoshida, Y.; Zendo, T.; Nagao, J.; Oogai, Y.; Nakamura, Y.; Sonomoto, K.; Nakamura, N.; Komatsuzawa, H. Three distinct two-component systems are involved in resistance to the class I bacteriocins, Nukacin ISK-1 and nisin A, in Staphylococcus aureus. PLoS ONE 2013, 8, e69455. [Google Scholar] [CrossRef]
- Guzmán Prieto, A.M.; Wijngaarden, J.; Braat, J.C.; Rogers, M.R.C.; Majoor, E.; Brouwer, E.C.; Zhang, X.; Bayjanov, J.R.; Bonten, M.J.M.; Willems, R.J.L.; et al. The two-component system ChtRS contributes to chlorhexidine tolerance in Enterococcus faecium. Antimicrob. Agents Chemother. 2017, 61, e02122-16. [Google Scholar] [CrossRef]
- Mascher, T.; Heintz, M.; Zähner, D.; Merai, M.; Hakenbeck, R. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J. Bacteriol. 2006, 188, 1959–1968. [Google Scholar] [CrossRef]
- Quach, D.; van Sorge, N.M.; Kristian, S.A.; Bryan, J.D.; Shelver, D.W.; Doran, K.S. The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J. Bacteriol. 2009, 191, 2023–2032. [Google Scholar] [CrossRef]
- He, L.Y.; Le, Y.J.; Guo, Z.; Li, S.; Yang, X.Y. The role and regulatory network of the CiaRH two-component system in Streptococcal species. Front. Microbiol. 2021, 12, 693858. [Google Scholar] [CrossRef]
- Nowicki, E.M.; O’Brien, J.P.; Brodbelt, J.S.; Trent, M.S. Extracellular zinc induces phosphoethanolamine addition to Pseudomonas aeruginosa lipid A via the ColRS two-component system. Mol. Microbiol. 2015, 97, 166–178. [Google Scholar] [CrossRef]
- Ducret, V.; Gonzalez, M.R.; Scrignari, T.; Perron, K. OprD repression upon metal treatment requires the RNA Chaperone Hfq in Pseudomonas aeruginosa. Genes 2016, 7, 82. [Google Scholar] [CrossRef]
- Caille, O.; Rossier, C.; Perron, K. A copper-activated two-component system interacts with zinc and imipenem resistance in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 4561–4568. [Google Scholar] [CrossRef]
- Fernández, L.; Jenssen, H.; Bains, M.; Wiegand, I.; Gooderham, W.J.; Hancock, R.E. The two-component system CprRS senses cationic peptides and triggers adaptive resistance in Pseudomonas aeruginosa independently of ParRS. Antimicrob. Agents Chemother. 2012, 56, 6212–6222. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, E.S.; Na, I.Y.; Kim, H.; Shin, D.; Ko, K.S. Development of colistin resistance in pmrA-, phoP-, parR- and cprR-inactivated mutants of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2014, 69, 2966–2971. [Google Scholar] [CrossRef]
- Audrain, B.; Ferrières, L.; Zairi, A.; Soubigou, G.; Dobson, C.; Coppée, J.Y.; Beloin, C.; Ghigo, J.M. Induction of the Cpx envelope stress pathway contributes to Escherichia coli tolerance to antimicrobial peptides. Appl. Environ. Microbiol. 2013, 79, 7770–7779. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Rajamohan, G. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob. Agents Chemother. 2013, 57, 4449–4462. [Google Scholar] [CrossRef]
- Raivio, T.L.; Leblanc, S.K.; Price, N.L. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 2013, 195, 2755–2767. [Google Scholar] [CrossRef]
- Weatherspoon-Griffin, N.; Yang, D.; Kong, W.; Hua, Z.; Shi, Y. The CpxR/CpxA two-component regulatory system up-regulates the multidrug resistance cascade to facilitate Escherichia coli resistance to a model antimicrobial peptide. J. Biol. Chem. 2014, 289, 32571–32582. [Google Scholar] [CrossRef]
- Weatherspoon-Griffin, N.; Zhao, G.; Kong, W.; Kong, Y.; Andrews-Polymenis, H.; McClelland, M.; Shi, Y. The CpxR/CpxA two-component system up-regulates two Tat-dependent peptidoglycan amidases to confer bacterial resistance to antimicrobial peptide. J. Biol. Chem. 2011, 286, 5529–5539. [Google Scholar] [CrossRef]
- Batchelor, E.; Walthers, D.; Kenney, L.J.; Goulian, M. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins ompF and ompC. J. Bacteriol. 2005, 187, 5723–5731. [Google Scholar] [CrossRef]
- Zamorano, L.; Moyà, B.; Juan, C.; Mulet, X.; Blázquez, J.; Oliver, A. The Pseudomonas aeruginosa CreBC two-component system plays a major role in the response to β-lactams, fitness, biofilm growth, and global regulation. Antimicrob. Agents Chemother. 2014, 58, 5084–5095. [Google Scholar] [CrossRef]
- Kellogg, S.L.; Kristich, C.J. Convergence of PASTA kinase and two-component signaling in response to cell wall stress in Enterococcus faecalis. J. Bacteriol. 2018, 200, e00086-18. [Google Scholar] [CrossRef]
- Kellogg, S.L.; Kristich, C.J. Functional dissection of the CroRS two-component system required for resistance to cell wall stressors in Enterococcus faecalis. J. Bacteriol. 2016, 198, 1326–1336. [Google Scholar] [CrossRef]
- Kellogg, S.L.; Little, J.L.; Hoff, J.S.; Kristich, C.J. Requirement of the CroRS two-component system for resistance to cell wall-targeting antimicrobials in Entero.ococcus faecium. Antimicrob. Agents Chemother. 2017, 61, e02461-16. [Google Scholar] [CrossRef]
- Comenge, Y.; Quintiliani, R., Jr.; Li, L.; Dubost, L.; Brouard, J.P.; Hugonnet, J.E.; Arthur, M. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 2003, 185, 7184–7192. [Google Scholar] [CrossRef]
- Dieppois, G.; Ducret, V.; Caille, O.; Perron, K. The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e38148. [Google Scholar] [CrossRef]
- Perron, K.; Caille, O.; Rossier, C.; Van Delden, C.; Dumas, J.L.; Köhler, T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 8761–8768. [Google Scholar] [CrossRef]
- Wang, D.; Chen, W.; Huang, S.; He, Y.; Liu, X.; Hu, Q.; Wei, T.; Sang, H.; Gan, J.; Chen, H. Structural basis of Zn(II) induced metal detoxification and antibiotic resistance by histidine kinase CzcS in Pseudomonas aeruginosa. PLoS Pathog. 2017, 13, e1006533. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 2001, 183, 1455–1458. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. EvgA of the two-component signal transduction system modulates production of the yhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 2002, 184, 2319–2323. [Google Scholar] [CrossRef]
- Kato, A.; Ohnishi, H.; Yamamoto, K.; Furuta, E.; Tanabe, H.; Utsumi, R. Transcription of emrKY is regulated by the EvgA-EvgS two-component system in Escherichia coli K-12. Biosci. Biotechnol. Biochem. 2000, 64, 1203–1209. [Google Scholar] [CrossRef]
- Eguchi, Y.; Oshima, T.; Mori, H.; Aono, R.; Yamamoto, K.; Ishihama, A.; Utsumi, R. Transcriptional regulation of drug efflux genes by EvgAS, a two-component system in Escherichia coli. Microbiology 2003, 149, 2819–2828. [Google Scholar] [CrossRef]
- Hirakawa, H.; Nishino, K.; Hirata, T.; Yamaguchi, A. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 2003, 185, 1851–1856. [Google Scholar] [CrossRef]
- Yang, S.J.; Bayer, A.S.; Mishra, N.N.; Meehl, M.; Ledala, N.; Yeaman, M.R.; Xiong, Y.Q.; Cheung, A.L. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 2012, 80, 74–81. [Google Scholar] [CrossRef]
- Cheung, A.L.; Bayer, A.S.; Yeaman, M.R.; Xiong, Y.Q.; Waring, A.J.; Memmi, G.; Donegan, N.; Chaili, S.; Yang, S.J. Site-specific mutation of the sensor kinase GraS in Staphylococcus aureus alters the adaptive response to distinct cationic antimicrobial peptides. Infect. Immun. 2014, 82, 5336–5345. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Xu, T.; Ge, H.; Zhou, F.; Zhu, X.; Li, X.; Qu, D.; Zheng, C.; Wu, Y.; et al. The role of graRS in regulating virulence and antimicrobial resistance in methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 727104. [Google Scholar] [CrossRef]
- Meehl, M.; Herbert, S.; Götz, F.; Cheung, A. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 2679–2689. [Google Scholar] [CrossRef]
- Kraus, D.; Herbert, S.; Kristian, S.A.; Khosravi, A.; Nizet, V.; Götz, F.; Peschel, A. The GraRS regulatory system controls Staphylococcus aureus susceptibility to antimicrobial host defenses. BMC Microbiol. 2008, 8, 85. [Google Scholar] [CrossRef]
- Reyes, J.; Panesso, D.; Tran, T.T.; Mishra, N.N.; Cruz, M.R.; Munita, J.M.; Singh, K.V.; Yeaman, M.R.; Murray, B.E.; Shamoo, Y.; et al. A liaR deletion restores susceptibility to daptomycin and antimicrobial peptides in multidrug-resistant Enterococcus faecalis. J. Infect. Dis. 2015, 211, 1317–1325. [Google Scholar] [CrossRef]
- Yang, S.J.; Xiong, Y.Q.; Yeaman, M.R.; Bayles, K.W.; Abdelhady, W.; Bayer, A.S. Role of the LytSR two-component regulatory system in adaptation to cationic antimicrobial peptides in Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3875–3882. [Google Scholar] [CrossRef]
- Brunskill, E.W.; Bayles, K.W. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 1996, 178, 5810–5812. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Utley-Drew, B.; Lee, S.Y.; Morrison-Williams, G.; O’Neill, A.J. Acquired nisin resistance in Staphylococcus aureus involves constitutive activation of an intrinsic peptide antibiotic detoxification module. mSphere 2018, 3, e00633-18. [Google Scholar] [CrossRef]
- Kolar, S.L.; Nagarajan, V.; Oszmiana, A.; Rivera, F.E.; Miller, H.K.; Davenport, J.E.; Riordan, J.T.; Potempa, J.; Barber, D.S.; Koziel, J.; et al. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 2011, 157, 2206–2219. [Google Scholar] [CrossRef]
- Fernández, L.; Gooderham, W.J.; Bains, M.; McPhee, J.B.; Wiegand, I.; Hancock, R.E. Adaptive resistance to the "last hope" antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob. Agents Chemother. 2010, 54, 3372–3382. [Google Scholar] [CrossRef]
- Wang, D.; Seeve, C.; Pierson, L.S., 3rd; Pierson, E.A. Transcriptome profiling reveals links between ParS/ParR, MexEF-OprN, and quorum sensing in the regulation of adaptation and virulence in Pseudomonas aeruginosa. BMC Genom. 2013, 14, 618. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Venkataramaiah, M.; Mondal, A.; Vaidyanathan, V.; Govil, T.; Rajamohan, G. Functional characterization of a novel outer membrane porin KpnO, regulated by PhoBR two-component system in Klebsiella pneumoniae NTUH-K2044. PLoS ONE 2012, 7, e41505. [Google Scholar] [CrossRef]
- Macfarlane, E.L.; Kwasnicka, A.; Ochs, M.M.; Hancock, R.E. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 1999, 34, 305–316. [Google Scholar] [CrossRef]
- McPhee, J.B.; Bains, M.; Winsor, G.; Lewenza, S.; Kwasnicka, A.; Brazas, M.D.; Brinkman, F.S.; Hancock, R.E. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 3995–4006. [Google Scholar] [CrossRef]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 544–553. [Google Scholar] [CrossRef]
- Macfarlane, E.L.A.; Kwasnicka, A.; Hancock, R.E.W. Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 2000, 146 Pt 10, 2543–2554. [Google Scholar] [CrossRef]
- Yang, B.; Liu, C.; Pan, X.; Fu, W.; Fan, Z.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Identification of novel PhoP-PhoQ regulated genes that contribute to polymyxin b tolerance in Pseudomonas aeruginosa. Microorganisms 2021, 9, 344. [Google Scholar] [CrossRef]
- Shi, Y.; Cromie, M.J.; Hsu, F.F.; Turk, J.; Groisman, E.A. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 2004, 53, 229–241. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Chen, Y.F.; Peng, H.L. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010, 17, 60. [Google Scholar] [CrossRef]
- Beceiro, A.; Llobet, E.; Aranda, J.; Bengoechea, J.A.; Doumith, M.; Hornsey, M.; Dhanji, H.; Chart, H.; Bou, G.; Livermore, D.M.; et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 2011, 55, 3370–3379. [Google Scholar] [CrossRef]
- Adams, M.D.; Nickel, G.C.; Bajaksouzian, S.; Lavender, H.; Murthy, A.R.; Jacobs, M.R.; Bonomo, R.A. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 2009, 53, 3628–3634. [Google Scholar] [CrossRef]
- Xiao, Y.; Nie, L.; Chen, H.; He, M.; Liang, Q.; Nie, H.; Chen, W.; Huang, Q. The two-component system TarR-TarS is regulated by c-di-GMP/FleQ and FliA and modulates antibiotic susceptibility in Pseudomonas putida. Environ. Microbiol. 2021, 23, 5239–5257. [Google Scholar] [CrossRef]
- Hughes, C.S.; Longo, E.; Phillips-Jones, M.K.; Hussain, R. Characterisation of the selective binding of antibiotics vancomycin and teicoplanin by the VanS receptor regulating type A vancomycin resistance in the enterococci. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Boyle-Vavra, S.; Ren, J.; Jarusiewicz, J.A.; Sharma, L.K.; Hoagland, D.T.; Yin, S.; Zhu, T.; Hevener, K.E.; Ojeda, I.; et al. Identification of small molecules exhibiting oxacillin synergy through a novel assay for inhibition of vraTSR expression in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02593-18. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Yin, S.; Jo, D.S.; Montgomery, C.P.; Daum, R.S. VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Gardete, S.; Wu, S.W.; Gill, S.; Tomasz, A. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 3424–3434. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Kuroda, H.; Oshima, T.; Takeuchi, F.; Mori, H.; Hiramatsu, K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 2003, 49, 807–821. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, Y.; Qian, L.; Ding, B.; Han, H.; Chen, H.; Bai, L.; Qu, D.; Wu, Y. The vancomycin resistance-associated regulatory system VraSR modulates biofilm formation of Staphylococcus epidermidis in an ica-dependent manner. mSphere 2021, 6, e0064121. [Google Scholar] [CrossRef]
- Howden, B.P.; McEvoy, C.R.; Allen, D.L.; Chua, K.; Gao, W.; Harrison, P.F.; Bell, J.; Coombs, G.; Bennett-Wood, V.; Porter, J.L.; et al. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog. 2011, 7, e1002359. [Google Scholar] [CrossRef]
- Poupel, O.; Proux, C.; Jagla, B.; Msadek, T.; Dubrac, S. SpdC, a novel virulence factor, controls histidine kinase activity in Staphylococcus aureus. PLoS Pathog. 2018, 14, e1006917. [Google Scholar] [CrossRef]
- Hu, Q.; Peng, H.; Rao, X. Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front. Microbiol. 2016, 7, 1601. [Google Scholar] [CrossRef]
- Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 2013, 4, 21. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating polymyxin resistance in Gram-negative bacteria: Roles of two-component systems PhoPQ and PmrAB. Future Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.S.; Jacobs, M.R.; Bonomo, R.A.; Adams, M.D. Transcriptome remodeling of Acinetobacter baumannii during infection and treatment. mBio 2017, 8, e02193-16. [Google Scholar] [CrossRef]
- Richards, S.M.; Strandberg, K.L.; Gunn, J.S. Salmonella-regulated lipopolysaccharide modifications. Subcell Biochem. 2010, 53, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Shprung, T.; Wani, N.A.; Wilmes, M.; Mangoni, M.L.; Bitler, A.; Shimoni, E.; Sahl, H.G.; Shai, Y. Opposing effects of PhoPQ and PmrAB on the properties of Salmonella enterica serovar Typhimurium: Implications on resistance to antimicrobial peptides. Biochemistry 2021, 60, 2943–2955. [Google Scholar] [CrossRef]
- Mahoney, T.F.; Silhavy, T.J. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J. Bacteriol. 2013, 195, 1869–1874. [Google Scholar] [CrossRef]
- Liu, X.; Omar, M.; Nagaraja, K.V.; Goyal, S.M.; Vidovic, S. Novel insight into the effects of CpxR on Salmonella enteritidis cells during the chlorhexidine treatment and non-stressful growing conditions. Int. J. Mol. Sci. 2021, 22, 8938. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, D.; Xin, S.; Zhou, D.; Li, T.; Tian, M.; Qi, J.; Ding, C.; Wang, S.; Yu, S. The CpxR regulates type VI secretion system 2 expression and facilitates the interbacterial competition activity and virulence of avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 40. [Google Scholar] [CrossRef]
- Ma, Q.; Wood, T.K. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ. Microbiol. 2009, 11, 2735–2746. [Google Scholar] [CrossRef]
- Dorel, C.; Lejeune, P.; Rodrigue, A. The Cpx system of Escherichia coli, a strategic signaling pathway for confronting adverse conditions and for settling biofilm communities? Res. Microbiol. 2006, 157, 306–314. [Google Scholar] [CrossRef]
- Raivio, T.L. Everything old is new again: An update on current research on the Cpx envelope stress response. Biochim. Biophys. Acta 2014, 1843, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Otto, K.; Silhavy, T.J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Laubacher, M.E.; Ades, S.E. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 2008, 190, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. At the nexus of antibiotics and metals: The impact of Cu and Zn on antibiotic activity and resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Cabas, M.; Ayala, J.A.; Raivio, T.L. The Cpx envelope stress response modifies peptidoglycan cross-linking via the L,D-transpeptidase LdtD and the novel protein YgaU. J. Bacteriol. 2015, 197, 603–614. [Google Scholar] [CrossRef]
- Hugonnet, J.E.; Mengin-Lecreulx, D.; Monton, A.; den Blaauwen, T.; Carbonnelle, E.; Veckerlé, C.; Brun, Y.V.; van Nieuwenhze, M.; Bouchier, C.; Tu, K.; et al. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife 2016, 5, e19469. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A.; Kempf, V.A.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 2005, 73, 8033–8038. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Tracing the origins of extracellular DNA in bacterial biofilms: Story of death and predation to community benefit. Biofouling 2021, 37, 1022–1039. [Google Scholar] [CrossRef]
- Das, T.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter 2011, 7, 2927–2935. [Google Scholar] [CrossRef]
- Qin, Z.; Ou, Y.; Yang, L.; Zhu, Y.; Tolker-Nielsen, T.; Molin, S.; Qu, D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 2007, 153, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Hussain, M.; Peters, G.; Götz, F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 1997, 24, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Christner, M.; Heinze, C.; Busch, M.; Franke, G.; Hentschke, M.; Bayard Dühring, S.; Büttner, H.; Kotasinska, M.; Wischnewski, V.; Kroll, G.; et al. sarA negatively regulates Staphylococcus epidermidis biofilm formation by modulating expression of 1 MDa extracellular matrix binding protein and autolysis-dependent release of eDNA. Mol. Microbiol. 2012, 86, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Mann, E.E.; Endres, J.L.; Weiss, E.C.; Cassat, J.E.; Smeltzer, M.S.; Bayles, K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb Perspect Biol. 2010, 2, a000398. [Google Scholar] [CrossRef]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, development, and cross-species interactions in biofilms. Front. Microbiol. 2021, 12, 757327. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Buzzo, J.R.; Devaraj, A.; Gloag, E.S.; Jurcisek, J.A.; Robledo-Avila, F.; Kesler, T.; Wilbanks, K.; Mashburn-Warren, L.; Balu, S.; Wickham, J.; et al. Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 2021, 184, 5740–5758. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Petrova, O.E.; Sauer, K. Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016, 30, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.R. Going beyond the control of quorum-sensing to combat biofilm infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Karatan, E.; Watnick, P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 2009, 73, 310–347. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef]
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Zhu, J.; Liu, W. Two-component signal transduction systems: A major strategy for connecting input stimuli to biofilm formation. Front. Microbiol. 2018, 9, 3279. [Google Scholar] [CrossRef]
- Poulin, M.B.; Kuperman, L.L. Regulation of biofilm exopolysaccharide production by cyclic di-guanosine monophosphate. Front. Microbiol. 2021, 12, 730980. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas aeruginosa biofilm formation and its control. Biologics 2021, 1, 19. [Google Scholar] [CrossRef]
- Yu, W.; Hallinen, K.M.; Wood, K.B. Interplay between antibiotic efficacy and drug-induced lysis underlies enhanced biofilm formation at subinhibitory drug concentrations. Antimicrob. Agents Chemother. 2018, 62, e01603-17. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Rachid, S.; Ohlsen, K.; Witte, W.; Hacker, J.; Ziebuhr, W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000, 44, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, I.; Barrow, P.A. Salmonella stress management and its relevance to behaviour during intestinal colonisation and infection. FEMS Microbiol. Rev. 2005, 29, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. mBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and Cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015, 11, e1004691. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, Y.; Zhan, Q.; Shang, Y.; Qu, D.; Yu, F. Subinhibitory concentrations of mupirocin stimulate Staphylococcus aureus biofilm formation by upregulating cidA. Antimicrob. Agents Chemother. 2020, 64, e01912-19. [Google Scholar] [CrossRef]
- Pérez-Martínez, I.; Haas, D. Azithromycin inhibits expression of the GacA-dependent small RNAs RsmY and RsmZ in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 3399–3405. [Google Scholar] [CrossRef]
- Imperi, F.; Leoni, L.; Visca, P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Elsheredy, A.; El-Soudany, I.; Elsherbini, E.; Metwally, D.; Ghazal, A. Effect of azithromycin and phenylalanine-arginine beta-naphthylamide on quorum sensing and virulence factors in clinical isolates of Pseudomonas aeruginosa. Iran. J. Microbiol. 2021, 13, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ryan Kaler, K.M.; Nix, J.C.; Schubot, F.D. RetS inhibits Pseudomonas aeruginosa biofilm formation by disrupting the canonical histidine kinase dimerization interface of GacS. J. Biol. Chem. 2021, 297, 101193. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Haagensen, J.A.; Rich, C.; Forestier, C. Characterization of type 2 quorum sensing in Klebsiella pneumoniae and relationship with biofilm formation. J. Bacteriol. 2005, 187, 2870–2880. [Google Scholar] [CrossRef]
- Irie, Y.; Parsek, M.R. Quorum sensing and microbial biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 67–84. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Bartels, D.J.; Volper, E.M.; Greenberg, E.P. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 1838–1850. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef]
- Hardie, K.R.; Heurlier, K. Establishing bacterial communities by ‘word of mouth’: LuxS and autoinducer 2 in biofilm development. Nat. Rev. Microbiol. 2008, 6, 635–643. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- De Kievit, T.R.; Gillis, R.; Marx, S.; Brown, C.; Iglewski, B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: Their role and expression patterns. Appl. Environ. Microbiol. 2001, 67, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.F.; Vuong, C.; Otto, M. Staphylococcus quorum sensing in biofilm formation and infection. Int. J. Med. Microbiol. 2006, 296, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Trappetti, C.; van der Maten, E.; Amin, Z.; Potter, A.J.; Chen, A.Y.; van Mourik, P.M.; Lawrence, A.J.; Paton, A.W.; Paton, J.C. Site of isolation determines biofilm formation and virulence phenotypes of Streptococcus pneumoniae serotype 3 clinical isolates. Infect. Immun. 2013, 81, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, A.; Brazel, E.B.; McLean, K.T.; Agnew, H.N.; Paton, J.C.; Trappetti, C. The role of luxS in the middle ear Streptococcus pneumoniae isolate 947. Pathogens 2022, 11, 216. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; Cepeda, M.L.; Widmer, K.; Dowd, S.E.; Soni, K.A.; Hume, M.E.; Zhu, J.; Pillai, S.D. Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonella enterica serovar Typhimurium. Foodborne Pathog. Dis. 2010, 7, 399–410. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. The primary physiological roles of Autoinducer 2 in Escherichia coli are chemotaxis and biofilm formation. Microorganisms 2021, 9, 386. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Xue, T.; Sun, B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef]
- Taga, M.E.; Miller, S.T.; Bassler, B.L. Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol. Microbiol. 2003, 50, 1411–1427. [Google Scholar] [CrossRef]
- Choi, J.; Shin, D.; Ryu, S. Implication of quorum sensing in Salmonella enterica serovar Typhimurium virulence: The luxS gene is necessary for expression of genes in pathogenicity island 1. Infect. Immun. 2007, 75, 4885–4890. [Google Scholar] [CrossRef]
- González Barrios, A.F.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.E.; Wood, T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 2006, 188, 305–316. [Google Scholar] [CrossRef]
- Laganenka, L.; Colin, R.; Sourjik, V. Chemotaxis towards autoinducer 2 mediates autoaggregation in Escherichia coli. Nat. Commun. 2016, 7, 12984. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, MB-0011-2014. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.C.; Sadykov, M.R.; Chaudhari, S.S.; Jones, J.; Endres, J.L.; Widhelm, T.J.; Ahn, J.S.; Jawa, R.S.; Zimmerman, M.C.; Bayles, K.W. A central role for carbon-overflow pathways in the modulation of bacterial cell death. PLoS Pathog. 2014, 10, e1004205. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Thomas, V.C.; Sadykov, M.R.; Bose, J.L.; Ahn, D.J.; Zimmerman, M.C.; Bayles, K.W. The LysR-type transcriptional regulator, CidR, regulates stationary phase cell death in Staphylococcus aureus. Mol. Microbiol. 2016, 101, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Hou, Z.; Li, J.; Luo, L.; Su, S.; Ye, Z.; Bai, Y.; Zhang, X.; Chen, G.; Li, Z.; et al. A new coumarin compound DCH combats methicillin-resistant Staphylococcus aureus biofilm by targeting arginine repressor. Sci. Adv. 2020, 6, eaay9597. [Google Scholar] [CrossRef]
- Beenken, K.E.; Dunman, P.M.; McAleese, F.; Macapagal, D.; Murphy, E.; Projan, S.J.; Blevins, J.S.; Smeltzer, M.S. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 4665–4684. [Google Scholar] [CrossRef]
- Burgui, S.; Gil, C.; Solano, C.; Lasa, I.; Valle, J. A systematic evaluation of the two-component systems network reveals that ArlRS is a key regulator of catheter colonization by Staphylococcus aureus. Front. Microbiol. 2018, 9, 342. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, Q.; Fang, B.; Sun, B. The ArlR-MgrA regulatory cascade regulates PIA-dependent and protein-mediated biofilm formation in Rbf-dependent and Rbf-independent pathways. Int. J. Med. Microbiol. 2019, 309, 85–96. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Merino, N.; Vergara-Irigaray, M.; Débarbouillé, M.; Penadés, J.R.; Lasa, I. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 2005, 187, 5318–5329. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Kratofil, R.M.; Parlet, C.P.; Surewaard, B.G.J.; Kubes, P.; Horswill, A.R. Staphylococcus aureus uses the ArlRS and MgrA cascade to regulate immune evasion during skin infection. Cell Rep. 2021, 36, 109462. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.L.; Lehman, M.K.; Fey, P.D.; Bayles, K.W. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS ONE 2012, 7, e42244. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, H.; Han, X.; Li, W.; Xue, M.; Qi, K.; Chen, X.; Ni, J.; Deng, R.; Shang, F.; et al. The two-component system, BasSR, is involved in the regulation of biofilm and virulence in avian pathogenic Escherichia coli. Avian Pathol. 2020, 49, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Sauer, K. SagS contributes to the motile-sessile switch and acts in concert with BfiSR to enable Pseudomonas aeruginosa biofilm formation. J. Bacteriol. 2011, 193, 6614–6628. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Sauer, K. The novel two-component regulatory system BfiSR regulates biofilm development by controlling the small RNA rsmZ through CafA. J. Bacteriol. 2010, 192, 5275–5288. [Google Scholar] [CrossRef]
- Petrova, O.E.; Sauer, K. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog. 2009, 5, e1000668. [Google Scholar] [CrossRef]
- Russo, T.A.; Manohar, A.; Beanan, J.M.; Olson, R.; MacDonald, U.; Graham, J.; Umland, T.C. The response regulator BfmR is a potential drug target for Acinetobacter baumannii. mSphere 2016, 1, e00082-16. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Flagler, M.J.; Dorsey, C.W.; Gaddy, J.A.; Actis, L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008, 154, 3398–3409. [Google Scholar] [CrossRef]
- Liou, M.L.; Soo, P.C.; Ling, S.R.; Kuo, H.Y.; Tang, C.Y.; Chang, K.C. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J. Microbiol. Immunol. Infect. 2014, 47, 275–281. [Google Scholar] [CrossRef]
- Petrova, O.E.; Schurr, J.R.; Schurr, M.J.; Sauer, K. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Mol. Microbiol. 2011, 81, 767–783. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Liu, J.; Chen, Y.; Zhou, X.; Ru, Y.; Zhu, J.; Liu, W. cAMP and c-di-GMP synergistically support biofilm maintenance through the direct interaction of their effectors. Nat. Commun. 2022, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Fan, J.; Duan, D.; Xu, L.; Wang, J.; Zhou, D.; Yang, H.; Li, B. Involvement of cAMP receptor protein in biofilm formation, fimbria production, capsular polysaccharide biosynthesis and lethality in mouse of Klebsiella pneumoniae serotype K1 causing pyogenic liver abscess. J. Med. Microbiol. 2017, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Panjaitan, N.S.D.; Horng, Y.T.; Cheng, S.W.; Chung, W.T.; Soo, P.C. EtcABC, a putative EII Complex, regulates Type 3 fimbriae via CRP-cAMP signaling in Klebsiella pneumoniae. Front. Microbiol. 2019, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.A.; Evans, M.L.; Greene, S.E.; Pinkner, J.S.; Hultgren, S.J.; Chapman, M.R. The Catabolite Repressor Protein-Cyclic AMP Complex regulates csgD and biofilm formation in uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Endres, J.L.; Chaudhari, S.S.; Zhang, X.; Prahlad, J.; Wang, S.Q.; Foley, L.A.; Luca, S.; Bose, J.L.; Thomas, V.C.; Bayles, K.W. The Staphylococcus aureus CidA and LrgA Proteins are functional holins involved in the transport of by-products of carbohydrate metabolism. mBio 2022, 13, e0282721. [Google Scholar] [CrossRef]
- Lee, V.T.; Matewish, J.M.; Kessler, J.L.; Hyodo, M.; Hayakawa, Y.; Lory, S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 2007, 65, 1474–1484. [Google Scholar] [CrossRef]
- Chambers, J.R.; Liao, J.; Schurr, M.J.; Sauer, K. BrlR from Pseudomonas aeruginosa is a c-di-GMP-responsive transcription factor. Mol. Microbiol. 2014, 92, 471–487. [Google Scholar] [CrossRef]
- Gupta, K.; Liao, J.; Petrova, O.E.; Cherny, K.E.; Sauer, K. Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa. Mol. Microbiol. 2014, 92, 488–506. [Google Scholar] [CrossRef]
- Boehm, A.; Steiner, S.; Zaehringer, F.; Casanova, A.; Hamburger, F.; Ritz, D.; Keck, W.; Ackermann, M.; Schirmer, T.; Jenal, U. Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 2009, 72, 1500–1516. [Google Scholar] [CrossRef]
- Nicastro, G.G.; Kaihami, G.H.; Pereira, T.O.; Meireles, D.A.; Groleau, M.C.; Déziel, E.; Baldini, R.L. Cyclic-di-GMP levels affect Pseudomonas aeruginosa fitness in the presence of imipenem. Environ. Microbiol. 2014, 16, 1321–1333. [Google Scholar] [CrossRef]
- Jiale, Z.; Jian, J.; Xinyi, T.; Haoji, X.; Xueqin, H.; Xiao, W. Design of a novel antimicrobial peptide 1018M targeted ppGpp to inhibit MRSA biofilm formation. AMB Express 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Rom, J.S.; Atwood, D.N.; Beenken, K.E.; Meeker, D.G.; Loughran, A.J.; Spencer, H.J.; Lantz, T.L.; Smeltzer, M.S. Impact of Staphylococcus aureus regulatory mutations that modulate biofilm formation in the USA300 strain LAC on virulence in a murine bacteremia model. Virulence 2017, 8, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Abrahante, J.E.; Chekabab, S.M.; Wu, X.; Korber, D.R.; Vidovic, S. Role of CpxR in biofilm development: Expression of key fimbrial, O-antigen and virulence operons of Salmonella Enteritidis. Int. J. Mol. Sci. 2019, 20, 5146. [Google Scholar] [CrossRef] [PubMed]
- Moya, B.; Dötsch, A.; Juan, C.; Blázquez, J.; Zamorano, L.; Haussler, S.; Oliver, A. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 2009, 5, e1000353. [Google Scholar] [CrossRef]
- Ogasawara, H.; Yamamoto, K.; Ishihama, A. Role of the biofilm master regulator CsgD in cross-regulation between biofilm formation and flagellar synthesis. J. Bacteriol. 2011, 193, 2587–2597. [Google Scholar] [CrossRef]
- Mika, F.; Hengge, R. Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol. 2014, 11, 494–507. [Google Scholar] [CrossRef]
- Gerstel, U.; Römling, U. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 2003, 154, 659–667. [Google Scholar] [CrossRef]
- Zakikhany, K.; Harrington, C.R.; Nimtz, M.; Hinton, J.C.; Römling, U. Unphosphorylated CsgD controls biofilm formation in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2010, 77, 771–786. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Hancock, L.E.; Perego, M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Macovei, L.; Ghosh, A.; Thomas, V.C.; Hancock, L.E.; Mahmood, S.; Zurek, L. Enterococcus faecalis with the gelatinase phenotype regulated by the fsr operon and with biofilm-forming capacity are common in the agricultural environment. Environ. Microbiol. 2009, 11, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.C.; Thurlow, L.R.; Boyle, D.; Hancock, L.E. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J. Bacteriol. 2008, 190, 5690–5698. [Google Scholar] [CrossRef] [PubMed]
- Chambonnier, G.; Roux, L.; Redelberger, D.; Fadel, F.; Filloux, A.; Sivaneson, M.; de Bentzmann, S.; Bordi, C. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genet. 2016, 12, e1006032. [Google Scholar] [CrossRef] [PubMed]
- Brencic, A.; McFarland, K.A.; McManus, H.R.; Castang, S.; Mogno, I.; Dove, S.L.; Lory, S. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 2009, 73, 434–445. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Kostoulias, X.; Khoo, C.; Aibinu, I.; Qu, Y.; Traven, A.; Peleg, A.Y. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J. Infect. Dis. 2014, 210, 46–55. [Google Scholar] [CrossRef]
- Parkins, M.D.; Ceri, H.; Storey, D.G. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 2001, 40, 1215–1226. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Roth, A.J.; Horswill, A.R. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS ONE 2010, 5, e10146. [Google Scholar] [CrossRef]
- Broder, U.N.; Jaeger, T.; Jenal, U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat. Microbiol. 2016, 2, 16184. [Google Scholar] [CrossRef]
- Gilbert, K.B.; Kim, T.H.; Gupta, R.; Greenberg, E.P.; Schuster, M. Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol. Microbiol. 2009, 73, 1072–1085. [Google Scholar] [CrossRef]
- Diggle, S.P.; Stacey, R.E.; Dodd, C.; Cámara, M.; Williams, P.; Winzer, K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol. 2006, 8, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qiu, S.; Jiang, Q.; Sun, H.; Xue, T.; Cai, G.; Sun, B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2017, 307, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.E.; Ludewick, H.P.; Kunkel, R.M.; Zähner, D.; Klugman, K.P. The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect. Immun. 2011, 79, 4050–4060. [Google Scholar] [CrossRef]
- Ju, X.; Li, J.; Zhu, M.; Lu, Z.; Lv, F.; Zhu, X.; Bie, X. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res. Int. 2018, 107, 385–393. [Google Scholar] [CrossRef]
- Moscoso, M.; García, E.; López, R. Biofilm formation by Streptococcus pneumoniae: Role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 2006, 188, 7785–7795. [Google Scholar] [CrossRef]
- Sharma-Kuinkel, B.K.; Mann, E.E.; Ahn, J.S.; Kuechenmeister, L.J.; Dunman, P.M.; Bayles, K.W. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J. Bacteriol. 2009, 191, 4767–4775. [Google Scholar] [CrossRef]
- Patton, T.G.; Yang, S.J.; Bayles, K.W. The role of proton motive force in expression of the Staphylococcus aureus cid and lrg operons. Mol. Microbiol. 2006, 59, 1395–1404. [Google Scholar] [CrossRef]
- Crosby, H.A.; Schlievert, P.M.; Merriman, J.A.; King, J.M.; Salgado-Pabón, W.; Horswill, A.R. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog. 2016, 12, e1005604. [Google Scholar] [CrossRef]
- Tatke, G.; Kumari, H.; Silva-Herzog, E.; Ramirez, L.; Mathee, K. Pseudomonas aeruginosa MifS-MifR two-component system is specific for α-ketoglutarate utilization. PLoS ONE 2015, 10, e0129629. [Google Scholar] [CrossRef]
- Hadjifrangiskou, M.; Kostakioti, M.; Chen, S.L.; Henderson, J.P.; Greene, S.E.; Hultgren, S.J. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol. Microbiol. 2011, 80, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Park, J.H.; Inouye, M. MqsR, a crucial regulator for quorum sensing and biofilm formation, is a GCU-specific mRNA interferase in Escherichia coli. J. Biol. Chem. 2009, 284, 28746–28753. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, W.; Zhang, Y.; Chen, L.; Zhang, Y.; Zheng, X.; Huang, X.; Ni, B. QseB mediates biofilm formation and invasion in Salmonella enterica serovar Typhi. Microb. Pathog. 2017, 104, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, L.; Zhao, J.; Shen, T.; Surette, M.G.; Shen, L.; Duan, K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol. Microbiol. 2013, 88, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.; Collie, E.S.; Free, P.D.; Livingston, S.P.; Mattick, J.S. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 1993, 7, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Kilmury, S.L.N.; Burrows, L.L. The Pseudomonas aeruginosa PilSR two-component system regulates both twitching and swimming motilities. mBio 2018, 9, e01310-18. [Google Scholar] [CrossRef]
- Izutsu, K.; Wada, A.; Wada, C. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 2001, 6, 665–676. [Google Scholar] [CrossRef]
- Kim, K.; Islam, M.; Jung, H.W.; Lim, D.; Kim, K.; Lee, S.G.; Park, C.; Lee, J.C.; Shin, M. ppGpp signaling plays a critical role in virulence of Acinetobacter baumannii. Virulence 2021, 12, 2122–2132. [Google Scholar] [CrossRef]
- Wood, T.K.; Song, S. Forming and waking dormant cells: The ppGpp ribosome dimerization persister model. Biofilm 2020, 2, 100018. [Google Scholar] [CrossRef]
- Salzer, A.; Keinhörster, D.; Kästle, C.; Kästle, B.; Wolz, C. Small alarmone synthetases RelP and RelQ of Staphylococcus aureus are involved in biofilm formation and maintenance under cell wall stress conditions. Front. Microbiol. 2020, 11, 575882. [Google Scholar] [CrossRef]
- Horvatek, P.; Salzer, A.; Hanna, A.M.F.; Gratani, F.L.; Keinhörster, D.; Korn, N.; Borisova, M.; Mayer, C.; Rejman, D.; Mäder, U.; et al. Inducible expression of (pp)pGpp synthetases in Staphylococcus aureus is associated with activation of stress response genes. PLoS Genet. 2020, 16, e1009282. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Kästle, B.; Gratani, F.L.; Goerke, C.; Wolz, C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 2014, 196, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Giraud, C.; Bernard, C.S.; Calderon, V.; Yang, L.; Filloux, A.; Molin, S.; Fichant, G.; Bordi, C.; de Bentzmann, S. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 2011, 13, 666–683. [Google Scholar] [CrossRef] [PubMed]
- de Bentzmann, S.; Giraud, C.; Bernard, C.S.; Calderon, V.; Ewald, F.; Plésiat, P.; Nguyen, C.; Grunwald, D.; Attree, I.; Jeannot, K.; et al. Unique biofilm signature, drug susceptibility and decreased virulence in Drosophila through the Pseudomonas aeruginosa two-component system PprAB. PLoS Pathog. 2012, 8, e1003052. [Google Scholar] [CrossRef]
- Cue, D.; Lei, M.G.; Lee, C.Y. Activation of sarX by Rbf is required for biofilm formation and icaADBC expression in Staphylococcus aureus. J. Bacteriol. 2013, 195, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.E.; Campbell, C.; Lowry, C.; O’Donnell, S.T.; Olson, M.E.; Lindgren, J.K.; Waters, E.M.; Fey, P.D.; O’Gara, J.P. AraC-type regulator Rbf controls the Staphylococcus epidermidis biofilm phenotype by negatively regulating the icaADBC repressor SarR. J. Bacteriol. 2016, 198, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Cue, D.; Lei, M.G.; Luong, T.T.; Kuechenmeister, L.; Dunman, P.M.; O’Donnell, S.; Rowe, S.; O’Gara, J.P.; Lee, C.Y. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J. Bacteriol. 2009, 191, 6363–6373. [Google Scholar] [CrossRef]
- Ferrières, L.; Clarke, D.J. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in.n response to growth on a solid surface. Mol. Microbiol. 2003, 50, 1665–1682. [Google Scholar] [CrossRef]
- Oropeza, R.; Salgado-Bravo, R.; Calva, E. Deletion analysis of RcsC reveals a novel signalling pathway controlling poly-N-acetylglucosamine synthesis and biofilm formation in Escherichia coli. Microbiology 2015, 161, 903–913. [Google Scholar] [CrossRef]
- Latasa, C.; García, B.; Echeverz, M.; Toledo-Arana, A.; Valle, J.; Campoy, S.; García-del Portillo, F.; Solano, C.; Lasa, I. Salmonella biofilm development depends on the phosphorylation status of RcsB. J. Bacteriol. 2012, 194, 3708–3722. [Google Scholar] [CrossRef]
- Wall, E.A.; Majdalani, N.; Gottesman, S. IgaA negatively regulates the Rcs phosphorelay via contact with the RcsD phosphotransfer protein. PLoS Genet. 2020, 16, e1008610. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; Ball, G.; Giraud, C.; Filloux, A. Expression of Pseudomonas aeruginosa CupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS ONE 2009, 4, e6018. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, G.G.; Boechat, A.L.; Abe, C.M.; Kaihami, G.H.; Baldini, R.L. Pseudomonas aeruginosa PA14 cupD transcription is activated by the RcsB response regulator, but repressed by its putative cognate sensor RcsC. FEMS Microbiol. Lett. 2009, 301, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, J.A.; Mikkelsen, H.; Heeb, S.; Williams, P.; Filloux, A. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ. Microbiol. 2011, 13, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Moustafa, D.A.; Stergioula, V.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The PqsE and RhlR proteins are an autoinducer synthase-receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, E9411–E9418. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moustafa, D.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017, 13, e1006504. [Google Scholar] [CrossRef]
- Kulasekara, H.D.; Ventre, I.; Kulasekara, B.R.; Lazdunski, A.; Filloux, A.; Lory, S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 2005, 55, 368–380. [Google Scholar] [CrossRef]
- Rao, F.; Yang, Y.; Qi, Y.; Liang, Z.X. Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: A study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J. Bacteriol. 2008, 190, 3622–3631. [Google Scholar] [CrossRef]
- Mootz, J.M.; Benson, M.A.; Heim, C.E.; Crosby, H.A.; Kavanaugh, J.S.; Dunman, P.M.; Kielian, T.; Torres, V.J.; Horswill, A.R. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol. Microbiol. 2015, 96, 388–404. [Google Scholar] [CrossRef]
- Hengge, R. The two-component network and the general stress sigma factor RpoS (sigma S) in Escherichia coli. Adv. Exp. Med. Biol. 2008, 631, 40–53. [Google Scholar] [CrossRef]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011, 65, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; McLean, R.J. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 1999, 65, 4285–4287. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; May, T.; Kawata, K.; Okabe, S. Significance of rpoS during maturation of Escherichia coli biofilms. Biotechnol. Bioeng. 2008, 99, 1462–1471. [Google Scholar] [CrossRef]
- Corona-Izquierdo, F.P.; Membrillo-Hernández, J. A mutation in rpoS enhances biofilm formation in Escherichia coli during exponential phase of growth. FEMS Microbiol. Lett. 2002, 211, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Uhlich, G.A.; Chen, C.Y.; Cottrell, B.J.; Hofmann, C.S.; Dudley, E.G.; Strobaugh, T.P.; Nguyen, L.H. Phage insertion in mlrA and variations in rpoS limit curli expression and biofilm formation in Escherichia coli serotype O157: H7. Microbiology 2013, 159, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Ono, T.; Viducic, D.; Kayama, S.; Mori, M.; Hirota, K.; Nemoto, K.; Miyake, Y. Role for rpoS gene of Pseudomonas aeruginosa in antibiotic tolerance. FEMS Microbiol. Lett. 2005, 242, 161–167. [Google Scholar] [CrossRef]
- Kuchma, S.L.; Connolly, J.P.; O’Toole, G.A. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1441–1454. [Google Scholar] [CrossRef]
- Benson, M.A.; Lilo, S.; Nygaard, T.; Voyich, J.M.; Torres, V.J. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J. Bacteriol. 2012, 194, 4355–4365. [Google Scholar] [CrossRef]
- Lou, Q.; Zhu, T.; Hu, J.; Ben, H.; Yang, J.; Yu, F.; Liu, J.; Wu, Y.; Fischer, A.; Francois, P.; et al. Role of the SaeRS two-component regulatory system in Staphylococcus epidermidis autolysis and biofilm formation. BMC Microbiol. 2011, 11, 146. [Google Scholar] [CrossRef]
- Petrova, O.E.; Gupta, K.; Liao, J.; Goodwine, J.S.; Sauer, K. Divide and conquer: The Pseudomonas aeruginosa two-component hybrid SagS enables biofilm formation and recalcitrance of biofilm cells to antimicrobial agents via distinct regulatory circuits. Environ. Microbiol. 2017, 19, 2005–2024. [Google Scholar] [CrossRef]
- Dingemans, J.; Poudyal, B.; Sondermann, H.; Sauer, K. The Yin and Yang of SagS: Distinct residues in the HmsP domain of SagS independently regulate biofilm formation and biofilm drug tolerance. mSphere 2018, 3, e00192-18. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, B.; Sauer, K. The ABC of biofilm drug tolerance: The MerR-like regulator BrlR is an activator of ABC transport systems, with PA1874-77 contributing to the tolerance of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother. 2018, 62, e01981-17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Marques, C.N.; Petrova, O.E.; Sauer, K. Antimicrobial tolerance of Pseudomonas aeruginosa biofilms is activated during an early developmental stage and requires the two-component hybrid SagS. J. Bacteriol. 2013, 195, 4975–4987. [Google Scholar] [CrossRef] [PubMed]
- Oriol, C.; Cengher, L.; Manna, A.C.; Mauro, T.; Pinel-Marie, M.L.; Felden, B.; Cheung, A.; Rouillon, A. Expanding the Staphylococcus aureus SarA regulon to small RNAs. mSystems 2021, 6, e0071321. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus virulence. Microbiol. Spectr. 2019, 7, GPP3-0031-2018. [Google Scholar] [CrossRef]
- Beenken, K.E.; Blevins, J.S.; Smeltzer, M.S. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003, 71, 4206–4211. [Google Scholar] [CrossRef]
- Tsang, L.H.; Cassat, J.E.; Shaw, L.N.; Beenken, K.E.; Smeltzer, M.S. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS ONE 2008, 3, e3361. [Google Scholar] [CrossRef]
- Hao, Z.; Guo, Y.; Rao, L.; Yu, J.; Zhan, Q.; Xu, Y.; Wang, B.; Wu, X.; Yu, F. Deletion of SarX decreases biofilm formation of Staphylococcus aureus in a Polysaccharide Intercellular Adhesin (PIA)-dependent manner by downregulating spa. Infect. Drug Resist. 2021, 14, 2241–2250. [Google Scholar] [CrossRef]
- Manna, A.C.; Cheung, A.L. Expression of SarX, a negative regulator of agr and exoprotein synthesis, is activated by MgrA in Staphylococcus aureus. J. Bacteriol. 2006, 188, 4288–4299. [Google Scholar] [CrossRef]
- Rowe, S.E.; Mahon, V.; Smith, S.G.; O’Gara, J.P. A novel role for SarX in Staphylococcus epidermidis biofilm regulation. Microbiology 2011, 157, 1042–1049. [Google Scholar] [CrossRef]
- Culler, H.F.; Couto, S.C.F.; Higa, J.S.; Ruiz, R.M.; Yang, M.J.; Bueris, V.; Franzolin, M.R.; Sircili, M.P. Role of SdiA on biofilm formation by atypical enteropathogenic Escherichia coli. Genes 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Ogasawara, H.; Yamada, K.; Shimura, M.; Kori, A.; Shimada, T.; Yamanaka, Y.; Yamamoto, K.; Ishihama, A. Screening of promoter-specific transcription factors: Multiple regulators for the sdiA gene involved in cell division control and quorum sensing. Microbiology 2013, 159, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Shimada, K.; Matsui, M.; Kitai, Y.; Igarashi, J.; Suga, H.; Ishihama, A. Roles of cell division control factor SdiA: Recognition of quorum sensing signals and modulation of transcription regulation targets. Genes Cells 2014, 19, 405–418. [Google Scholar] [CrossRef]
- Lauderdale, K.J.; Boles, B.R.; Cheung, A.L.; Horswill, A.R. Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 2009, 77, 1623–1635. [Google Scholar] [CrossRef]
- Chang, Y.M.; Jeng, W.Y.; Ko, T.P.; Yeh, Y.J.; Chen, C.K.; Wang, A.H. Structural study of TcaR and its complexes with multiple antibiotics from Staphylococcus epidermidis. Proc. Natl. Acad. Sci. USA 2010, 107, 8617–8622. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K.; Pier, D.B.; Goldmann, D.A.; Pier, G.B. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 2004, 186, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, M.; Tschierske, M.; Giachino, P.; Wada, A.; Berger-Bächi, B. Inactivation of a novel three-cistronic operon tcaR-tcaA-tcaB increases teicoplanin resistance in Staphylococcus aureus. Biochim. Biophys. Acta 2000, 1523, 135–139. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- Aline Dias da, P.; Nathalia Marins de, A.; Gabriel Guarany de, A.; Robson Francisco de, S.; Cristiane Rodrigues, G. The world of cyclic dinucleotides in bacterial behavior. Molecules 2020, 25, 2462. [Google Scholar] [CrossRef]
- Yoon, S.H.; Waters, C.M. The ever-expanding world of bacterial cyclic oligonucleotide second messengers. Curr. Opin. Microbiol. 2021, 60, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Waters, C.M. A tangled web: Regulatory connections between quorum sensing and cyclic Di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Lipchock, S.V.; Strobel, S.A. Structural and biochemical characterization of linear dinucleotide analogues bound to the c-di-GMP-I aptamer. Biochemistry 2012, 51, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Jacobs, H.M.; Matwichuk, M.; Wong, C.; Wozniak, D.J.; Parsek, M.R. The versatile Pseudomonas aeruginosa biofilm matrix protein CdrA promotes aggregation through different extracellular exopolysaccharide interactions. J. Bacteriol. 2020, 202, e00216-20. [Google Scholar] [CrossRef] [PubMed]
- Merighi, M.; Lee, V.T.; Hyodo, M.; Hayakawa, Y.; Lory, S. The second messenger bis-(3′–5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 876–895. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364, fnx120. [Google Scholar] [CrossRef]
- Bianco, C.M.; Fröhlich, K.S.; Vanderpool, C.K. Bacterial Cyclopropane Fatty Acid Synthase mRNA is targeted by activating and repressing small RNAs. J. Bacteriol. 2019, 201, e00461-19. [Google Scholar] [CrossRef]
- Schoenfelder, S.M.K.; Lange, C.; Prakash, S.A.; Marincola, G.; Lerch, M.F.; Wencker, F.D.R.; Förstner, K.U.; Sharma, C.M.; Ziebuhr, W. The small non-coding RNA RsaE influences extracellular matrix composition in Staphylococcus epidermidis biofilm communities. PLoS Pathog. 2019, 15, e1007618. [Google Scholar] [CrossRef]
- Lerch, M.F.; Schoenfelder, S.M.K.; Marincola, G.; Wencker, F.D.R.; Eckart, M.; Förstner, K.U.; Sharma, C.M.; Thormann, K.M.; Kucklick, M.; Engelmann, S.; et al. A non-coding RNA from the intercellular adhesion (ica) locus of Staphylococcus epidermidis controls polysaccharide intercellular adhesion (PIA)-mediated biofilm formation. Mol. Microbiol. 2019, 111, 1571–1591. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Romero, M.; Karna, S.L.; Chen, T.; Heeb, S.; Leung, K.P. RsmW, Pseudomonas aeruginosa small non-coding RsmA-binding RNA upregulated in biofilm versus planktonic growth conditions. BMC Microbiol. 2016, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, E.; Baysse, C.; Adams, C.; O’Gara, F. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 2006, 152, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, L.P.; Wood, T.E.; Howard, S.A.; Maggiorelli, F.; Nolan, L.M.; Wettstadt, S.; Filloux, A. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, 7707–7712. [Google Scholar] [CrossRef] [PubMed]
- Berk, V.; Fong, J.C.; Dempsey, G.T.; Develioglu, O.N.; Zhuang, X.; Liphardt, J.; Yildiz, F.H.; Chu, S. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science 2012, 337, 236–239. [Google Scholar] [CrossRef]
- Beyhan, S.; Bilecen, K.; Salama, S.R.; Casper-Lindley, C.; Yildiz, F.H. Regulation of rugosity and biofilm formation in Vibrio cholerae: Comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 2007, 189, 388–402. [Google Scholar] [CrossRef]
- Hsieh, M.L.; Waters, C.M.; Hinton, D.M. VpsR directly activates transcription of multiple biofilm genes in Vibrio cholerae. J. Bacteriol. 2020, 202, e00234-20. [Google Scholar] [CrossRef]
- Chakrabortty, T.; Roy Chowdhury, S.; Ghosh, B.; Sen, U. Crystal structure of VpsR revealed novel dimeric architecture and c-di-GMP binding site: Mechanistic implications in oligomerization, ATPase activity and DNA binding. J. Mol. Biol. 2022, 434, 167354. [Google Scholar] [CrossRef]
- Krasteva, P.V.; Fong, J.C.; Shikuma, N.J.; Beyhan, S.; Navarro, M.V.; Yildiz, F.H.; Sondermann, H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 2010, 327, 866–868. [Google Scholar] [CrossRef]
- Matter, L.B.; Ares, M.A.; Abundes-Gallegos, J.; Cedillo, M.L.; Yáñez, J.A.; Martínez-Laguna, Y.; De la Cruz, M.A.; Girón, J.A. The CpxRA stress response system regulates virulence features of avian pathogenic Escherichia coli. Environ. Microbiol. 2018, 20, 3363–3377. [Google Scholar] [CrossRef]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T. Biofilm development. Microbiol. Spectr. 2015, 3, Mb-0001-2014. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Kutty, S.K.; Tavallaie, R.; Ibugo, A.I.; Panchompoo, J.; Sehar, S.; Aldous, L.; Yeung, A.W.; Thomas, S.R.; Kumar, N.; et al. Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci. Rep. 2015, 5, 8398. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.W.; Griffin, J.L.; Welch, M. Quorum sensing is accompanied by global metabolic changes in the opportunistic human pathogen Pseudomonas aeruginosa. J. Bacteriol. 2015, 197, 2072–2082. [Google Scholar] [CrossRef]
- Liu, Z.; Hossain, S.S.; Morales Moreira, Z.; Haney, C.H. Putrescine and its metabolic precursor arginine promote biofilm and c-di-GMP synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2021, 204, Jb0029721. [Google Scholar] [CrossRef]
- Chen, G.; Gan, J.; Yang, C.; Zuo, Y.; Peng, J.; Li, M.; Huo, W.; Xie, Y.; Zhang, Y.; Wang, T.; et al. The SiaA/B/C/D signaling network regulates biofilm formation in Pseudomonas aeruginosa. EMBO J. 2020, 39, e103412. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 2010, 12, e11. [Google Scholar] [CrossRef]
- Yan, S.; Wu, G. Can biofilm be reversed through quorum sensing in Pseudomonas aeruginosa? Front. Microbiol. 2019, 10, 1582. [Google Scholar] [CrossRef]
- Rampioni, G.; Pustelny, C.; Fletcher, M.P.; Wright, V.J.; Bruce, M.; Rumbaugh, K.P.; Heeb, S.; Cámara, M.; Williams, P. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ. Microbiol. 2010, 12, 1659–1673. [Google Scholar] [CrossRef]
- Kay, E.; Humair, B.; Dénervaud, V.; Riedel, K.; Spahr, S.; Eberl, L.; Valverde, C.; Haas, D. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 6026–6033. [Google Scholar] [CrossRef]
- Bordi, C.; Lamy, M.C.; Ventre, I.; Termine, E.; Hachani, A.; Fillet, S.; Roche, B.; Bleves, S.; Méjean, V.; Lazdunski, A.; et al. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol. Microbiol. 2010, 76, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Colley, B.; Dederer, V.; Carnell, M.; Kjelleberg, S.; Rice, S.A.; Klebensberger, J. SiaA/D Interconnects c-di-GMP and RsmA signaling to coordinate cellular aggregation of Pseudomonas aeruginosa in response to environmental conditions. Front. Microbiol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.; Harrison, J.J.; Marques, L.L.; Foglia, G.R.; Stremick, C.A.; Storey, D.G.; Turner, R.J.; Olson, M.E.; Ceri, H. The GacS sensor kinase controls phenotypic reversion of small colony variants isolated from biofilms of Pseudomonas aeruginosa PA14. FEMS Microbiol. Ecol. 2007, 59, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Schurr, J.R.; Schurr, M.J.; Sauer, K. Microcolony formation by the opportunistic pathogen Pseudomonas aeruginosa requires pyruvate and pyruvate fermentation. Mol. Microbiol. 2012, 86, 819–835. [Google Scholar] [CrossRef]
- Katharios-Lanwermeyer, S.; O’Toole, G.A. Biofilm maintenance as an active process: Evidence that biofilms work hard to stay put. J. Bacteriol. 2022, 204, e0058721. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Valentini, M.; Filloux, A. Contribution of Cyclic di-GMP in the control of Type III and Type VI secretion in Pseudomonas aeruginosa. Methods Mol. Biol. 2017, 1657, 213–224. [Google Scholar] [CrossRef]
- Sivaneson, M.; Mikkelsen, H.; Ventre, I.; Bordi, C.; Filloux, A. Two-component regulatory systems in Pseudomonas aeruginosa: An intricate network mediating fimbrial and efflux pump gene expression. Mol. Microbiol. 2011, 79, 1353–1366. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Nguyen, T.H.; Otto, M. The staphylococcal exopolysaccharide PIA—Biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020, 18, 3324–3334. [Google Scholar] [CrossRef]
- O’Neill, E.; Pozzi, C.; Houston, P.; Humphreys, H.; Robinson, D.A.; Loughman, A.; Foster, T.J.; O’Gara, J.P. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 2008, 190, 3835–3850. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- McCourt, J.; O’Halloran, D.P.; McCarthy, H.; O’Gara, J.P.; Geoghegan, J.A. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol. Lett. 2014, 353, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Herman-Bausier, P.; El-Kirat-Chatel, S.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Staphylococcus aureus Fibronectin-Binding Protein A mediates cell-cell adhesion through low-affinity homophilic bonds. mBio 2015, 6, e00413-15. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Rigby, D.; Handley, P.; Foster, T.J. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 2007, 153, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Yonemoto, K.; Chiba, A.; Sugimoto, S.; Sato, C.; Saito, M.; Kinjo, Y.; Marumo, K.; Mizunoe, Y. Redundant and distinct roles of secreted protein Eap and cell wall-anchored protein SasG in biofilm formation and pathogenicity of Staphylococcus aureus. Infect. Immun. 2019, 87, e00894-18. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Speziale, P.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Zinc-dependent mechanical properties of Staphylococcus aureus biofilm-forming surface protein SasG. Proc. Natl. Acad. Sci. USA 2016, 113, 410–415. [Google Scholar] [CrossRef]
- Valle, J.; Fang, X.; Lasa, I. Revisiting Bap multidomain protein: More than sticking bacteria together. Front. Microbiol. 2020, 11, 613581. [Google Scholar] [CrossRef]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penades, J.R.; Lasa, I.; Valle, J. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016, 12, e1005711. [Google Scholar] [CrossRef]
- Merino, N.; Toledo-Arana, A.; Vergara-Irigaray, M.; Valle, J.; Solano, C.; Calvo, E.; Lopez, J.A.; Foster, T.J.; Penadés, J.R.; Lasa, I. Protein A-mediated multicellular behavior in Staphylococcus aureus. J. Bacteriol. 2009, 191, 832–843. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef]
- Yarwood, J.M.; McCormick, J.K.; Schlievert, P.M. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 2001, 183, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W. The biological role of death and lysis in biofilm development. Nat. Rev. Microbiol. 2007, 5, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, M.R.; Bayles, K.W. The control of death and lysis in staphylococcal biofilms: A coordination of physiological signals. Curr. Opin. Microbiol. 2012, 15, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Nistico, L.; Sambanthamoorthy, K.; Longwell, M.; Iannitelli, A.; Cellini, L.; Di Stefano, A.; Hall Stoodley, L.; Stoodley, P. Temporal expression of agrB, cidA, and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog. Dis. 2014, 70, 414–422. [Google Scholar] [CrossRef]
- Ranjit, D.K.; Endres, J.L.; Bayles, K.W. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 2011, 193, 2468–2476. [Google Scholar] [CrossRef]
- Balestrino, D.; Ghigo, J.M.; Charbonnel, N.; Haagensen, J.A.; Forestier, C. The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ. Microbiol. 2008, 10, 685–701. [Google Scholar] [CrossRef]
- Alcántar-Curiel, M.D.; Blackburn, D.; Saldaña, Z.; Gayosso-Vázquez, C.; Iovine, N.M.; De la Cruz, M.A.; Girón, J.A. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 2013, 4, 129–138. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.P.; Libori, M.F.; Tiracchia, V.; Salvia, A.; Varaldo, P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef]
- Chen, K.M.; Chiang, M.K.; Wang, M.; Ho, H.C.; Lu, M.C.; Lai, Y.C. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb. Pathog. 2014, 77, 89–99. [Google Scholar] [CrossRef]
- Peng, D.; Li, X.; Liu, P.; Zhou, X.; Luo, M.; Su, K.; Chen, S.; Zhang, Z.; He, Q.; Qiu, J.; et al. Transcriptional regulation of galF by RcsAB affects capsular polysaccharide formation in Klebsiella pneumoniae NTUH-K2044. Microbiol. Res. 2018, 216, 70–78. [Google Scholar] [CrossRef]
- Boddicker, J.D.; Anderson, R.A.; Jagnow, J.; Clegg, S. Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material. Infect. Immun. 2006, 74, 4590–4597. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Forestier, C. oxyR, a LysR-type regulator involved in Klebsiella pneumoniae mucosal and abiotic colonization. Infect. Immun. 2009, 77, 5449–5457. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. "It Takes a Village": Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Garnett, J.A.; Matthews, S. Interactions in bacterial biofilm development: A structural perspective. Curr. Protein Pept. Sci. 2012, 13, 739–755. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.; Wong, B.K.C.; Leung, P.H. Biofilm-induced antibiotic resistance in clinical Acinetobacter baumannii isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control. 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Dixon, L.; Benoit, M.R.; Brodie, E.L.; Keyhan, M.; Hu, P.; Ackerley, D.F.; Andersen, G.L.; Matin, A. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob. Agents Chemother. 2007, 51, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Vega, N.M.; Allison, K.R.; Samuels, A.N.; Klempner, M.S.; Collins, J.J. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 14420–14425. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320. [Google Scholar] [CrossRef]
- Johnson, L.; Horsman, S.R.; Charron-Mazenod, L.; Turnbull, A.L.; Mulcahy, H.; Surette, M.G.; Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.Y.; Smith, F.; Gimenez, M.R.; Yang, L.; Vejborg, R.M.; Fletcher, M.; Halliday, N.; Bleves, S.; Heeb, S.; Cámara, M.; et al. Disruption of the Pseudomonas aeruginosa Tat system perturbs PQS-dependent quorum sensing and biofilm maturation through lack of the Rieske cytochrome bc1 sub-unit. PLoS Pathog. 2021, 17, e1009425. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Eberly, A.R.; Werby, S.H.; Reasoner, S.A.; Brannon, J.R.; De, S.; Fitzgerald, M.J.; Huggins, M.M.; Clayton, D.B.; Cegelski, L.; et al. Respiratory heterogeneity shapes biofilm formation and host colonization in uropathogenic Escherichia coli. mBio 2019, 10, e02400-18. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Drescher, K.; Foster, K.R. Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 2016, 14, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Zhang, T.; Xu, R.; Pitts, B.; Walters, M.C.; Roe, F.; Kikhney, J.; Moter, A. Reaction-diffusion theory explains hypoxia and heterogeneous growth within microbial biofilms associated with chronic infections. NPJ Biofilms Microbiomes 2016, 2, 16012. [Google Scholar] [CrossRef]
- Walters, M.C., 3rd; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Chung, H.S.; Yao, Z.; Goehring, N.W.; Kishony, R.; Beckwith, J.; Kahne, D. Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc. Natl. Acad. Sci. USA 2009, 106, 21872–21877. [Google Scholar] [CrossRef]
- Martínez, J.L.; Rojo, F. Metabolic regulation of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 768–789. [Google Scholar] [CrossRef]
- Levin, B.R.; Rozen, D.E. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 2006, 4, 556–562. [Google Scholar] [CrossRef]
- Waters, E.M.; Rowe, S.E.; O’Gara, J.P.; Conlon, B.P. Convergence of Staphylococcus aureus persister and biofilm research: Can biofilms be defined as communities of adherent persister cells? PLoS Pathog. 2016, 12, e1006012. [Google Scholar] [CrossRef]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Bernier, S.P.; Lebeaux, D.; DeFrancesco, A.S.; Valomon, A.; Soubigou, G.; Coppée, J.Y.; Ghigo, J.M.; Beloin, C. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 2013, 9, e1003144. [Google Scholar] [CrossRef]
- Ma, D.; Mandell, J.B.; Donegan, N.P.; Cheung, A.L.; Ma, W.; Rothenberger, S.; Shanks, R.M.Q.; Richardson, A.R.; Urish, K.L. The toxin-antitoxin MazEF drives Staphylococcus aureus biofilm formation, antibiotic tolerance, and chronic infection. mBio 2019, 10, e01658-19. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Arora, G.; Singh, M.; Kidwai, S.; Narayan, O.P.; Singh, R. MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat. Commun. 2015, 6, 6059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Hoeflich, K.P.; Ikura, M.; Qing, G.; Inouye, M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 2003, 12, 913–923. [Google Scholar] [CrossRef]
- Al-Attar, S.; Yu, Y.; Pinkse, M.; Hoeser, J.; Friedrich, T.; Bald, D.; de Vries, S. Cytochrome bd displays significant quinol peroxidase activity. Sci. Rep. 2016, 6, 27631. [Google Scholar] [CrossRef]
- Beebout, C.J.; Sominsky, L.A.; Eberly, A.R.; Van Horn, G.T.; Hadjifrangiskou, M. Cytochrome bd promotes Escherichia coli biofilm antibiotic tolerance by regulating accumulation of noxious chemicals. NPJ Biofilms Microbiomes 2021, 7, 35. [Google Scholar] [CrossRef]
- Hall, C.W.; Hinz, A.J.; Gagnon, L.B.; Zhang, L.; Nadeau, J.P.; Copeland, S.; Saha, B.; Mah, T.F. Pseudomonas aeruginosa biofilm antibiotic resistance gene ndvB expression requires the RpoS stationary-phase sigma factor. Appl. Environ. Microbiol. 2018, 84, e02762-17. [Google Scholar] [CrossRef]
- Schellhorn, H.E. Elucidating the function of the RpoS regulon. Future Microbiol. 2014, 9, 497–507. [Google Scholar] [CrossRef]
- Lloyd, M.G.; Vossler, J.L.; Nomura, C.T.; Moffat, J.F. Blocking RpoN reduces virulence of Pseudomonas aeruginosa isolated from cystic fibrosis patients and increases antibiotic sensitivity in a laboratory strain. Sci. Rep. 2019, 9, 6677. [Google Scholar] [CrossRef]
- Bagge, N.; Hentzer, M.; Andersen, J.B.; Ciofu, O.; Givskov, M.; Høiby, N. Dynamics and spatial distribution of beta-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2004, 48, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Bagge, N.; Schuster, M.; Hentzer, M.; Ciofu, O.; Givskov, M.; Greenberg, E.P.; Høiby, N. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob. Agents Chemother. 2004, 48, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hinz, A.J.; Nadeau, J.P.; Mah, T.F. Pseudomonas aeruginosa tssC1 links type VI secretion and biofilm-specific antibiotic resistance. J. Bacteriol. 2011, 193, 5510–5513. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L.; Cagnazzo, J.; Phan, C.Q.; Barnes, A.M.; Dunny, G.M. Multiple roles for Enterococcus faecalis glycosyltransferases in biofilm-associated antibiotic resistance, cell envelope integrity, and conjugative transfer. Antimicrob. Agents Chemother. 2015, 59, 4094–4105. [Google Scholar] [CrossRef]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control. 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Bardbari, A.M.; Arabestani, M.R.; Karami, M.; Keramat, F.; Alikhani, M.Y.; Bagheri, K.P. Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb. Pathog. 2017, 108, 122–128. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, H.W.; Kim, S.M.; Kim, J. Proteomic analysis of Acinetobacter baumannii in biofilm and planktonic growth mode. J. Microbiol. 2009, 47, 728–735. [Google Scholar] [CrossRef]
- Gillis, R.J.; White, K.G.; Choi, K.H.; Wagner, V.E.; Schweizer, H.P.; Iglewski, B.H. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2005, 49, 3858–3867. [Google Scholar] [CrossRef]
- Zhang, L.; Mah, T.F. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Molin, S.; Tolker-Nielsen, T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr. Opin. Biotechnol 2003, 14, 255–261. [Google Scholar] [CrossRef]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.; Webber, M.A. Inhibition of multidrug efflux as a strategy to prevent biofilm formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in gram-negative (ESKAPEE) bacteria. Antibiotics 2019, 8, 229. [Google Scholar] [CrossRef]
- Dawan, J.; Li, Y.; Lu, F.; He, X.; Ahn, J. Role of efflux pump-mediated antibiotic resistance in quorum sensing-regulated biofilm formation by Salmonella Typhimurium. Pathogens 2022, 11, 147. [Google Scholar] [CrossRef]
- He, X.; Ahn, J. Differential gene expression in planktonic and biofilm cells of multiple antibiotic-resistant Salmonella Typhimurium and Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 325, 180–188. [Google Scholar] [CrossRef]
- Waite, R.D.; Papakonstantinopoulou, A.; Littler, E.; Curtis, M.A. Transcriptome analysis of Pseudomonas aeruginosa growth: Comparison of gene expression in planktonic cultures and developing and mature biofilms. J. Bacteriol. 2005, 187, 6571–6576. [Google Scholar] [CrossRef]
- Hirakata, Y.; Srikumar, R.; Poole, K.; Gotoh, N.; Suematsu, T.; Kohno, S.; Kamihira, S.; Hancock, R.E.; Speert, D.P. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 2002, 196, 109–118. [Google Scholar] [CrossRef]
- Buckley, A.M.; Webber, M.A.; Cooles, S.; Randall, L.P.; La Ragione, R.M.; Woodward, M.J.; Piddock, L.J. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol. 2006, 8, 847–856. [Google Scholar] [CrossRef]
- Subhadra, B.; Surendran, S.; Lim, B.R.; Yim, J.S.; Kim, D.H.; Woo, K.; Kim, H.J.; Oh, M.H.; Choi, C.H. Regulation of the AcrAB efflux system by the quorum-sensing regulator AnoR in Acinetobacter nosocomialis. J. Microbiol. 2020, 58, 507–518. [Google Scholar] [CrossRef]
- Subhadra, B.; Kim, J.; Kim, D.H.; Woo, K.; Oh, M.H.; Choi, C.H. Local repressor AcrR regulates AcrAB efflux pump required for biofilm formation and virulence in Acinetobacter nosocomialis. Front. Cell Infect. Microbiol. 2018, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Padilla, E.; Llobet, E.; Doménech-Sánchez, A.; Martínez-Martínez, L.; Bengoechea, J.A.; Albertí, S. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2010, 54, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bialek, S.; Lavigne, J.P.; Chevalier, J.; Marcon, E.; Leflon-Guibout, V.; Davin, A.; Moreau, R.; Pagès, J.M.; Nicolas-Chanoine, M.H. Membrane efflux and influx modulate both multidrug resistance and virulence of Klebsiella pneumoniae in a Caenorhabditis elegans model. Antimicrob. Agents Chemother. 2010, 54, 4373–4378. [Google Scholar] [CrossRef] [PubMed]
- Coudeyras, S.; Nakusi, L.; Charbonnel, N.; Forestier, C. A tripartite efflux pump involved in gastrointestinal colonization by Klebsiella pneumoniae confers a tolerance response to inorganic acid. Infect. Immun. 2008, 76, 4633–4641. [Google Scholar] [CrossRef]
- Singh, S.; Kalia, N.P.; Joshi, P.; Kumar, A.; Sharma, P.R.; Kumar, A.; Bharate, S.B.; Khan, I.A. Boeravinone B, A novel dual inhibitor of NorA bacterial efflux pump of Staphylococcus aureus and human P-Glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front. Microbiol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Sandle, T.; John, J.; Abuo-Rahma, G.E.A.; Hetta, H.F. A novel mechanism of action of ketoconazole: Inhibition of the NorA efflux pump system and biofilm formation in multidrug-resistant Staphylococcus aureus. Infect. Drug Resist. 2019, 12, 1703–1718. [Google Scholar] [CrossRef]
- Hirakata, Y.; Kondo, A.; Hoshino, K.; Yano, H.; Arai, K.; Hirotani, A.; Kunishima, H.; Yamamoto, N.; Hatta, M.; Kitagawa, M.; et al. Efflux pump inhibitors reduce the invasiveness of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2009, 34, 343–346. [Google Scholar] [CrossRef]
- Banerjee, S.; Sionov, R.V.; Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Anandamide alters the membrane properties, halts the cell division and prevents drug efflux in multidrug resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 8690. [Google Scholar] [CrossRef]
- Henson, K.E.; Yim, J.; Smith, J.R.; Sakoulas, G.; Rybak, M.J. β-Lactamase inhibitors enhance the synergy between β-Lactam antibiotics and daptomycin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e01564-16. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef]
- Annunziato, G. Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: A review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef]
- Silber, N.; Matos de Opitz, C.L.; Mayer, C.; Sass, P. Cell division protein FtsZ: From structure and mechanism to antibiotic target. Future Microbiol. 2020, 15, 801–831. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Sintim, H.O. Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Future Med. Chem. 2020, 12, 1253–1279. [Google Scholar] [CrossRef]
- Farha, M.A.; Leung, A.; Sewell, E.W.; D’Elia, M.A.; Allison, S.E.; Ejim, L.; Pereira, P.M.; Pinho, M.G.; Wright, G.D.; Brown, E.D. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to β-lactams. ACS Chem. Biol. 2013, 8, 226–233. [Google Scholar] [CrossRef]
- Munguia, J.; Nizet, V. Pharmacological targeting of the host-pathogen interaction: Alternatives to classical antibiotics to combat drug-resistant superbugs. Trends Pharmacol. Sci. 2017, 38, 473–488. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Phanstiel, O.t.; Brunel, J.M. Antibiotic adjuvants: Make antibiotics great again! J. Med. Chem. 2019, 62, 8665–8681. [Google Scholar] [CrossRef]
- Sharma, N.; Chhillar, A.K.; Dahiya, S.; Choudhary, P.; Punia, A.; Gulia, P. Antibiotic adjuvants: A promising approach to combat multidrug resistant bacteria. Curr. Drug Targets 2021, 22, 1334–1345. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef]
- Hussein, M.; Allobawi, R.; Levou, I.; Blaskovich, M.A.T.; Rao, G.G.; Li, J.; Velkov, T. Mechanisms underlying synergistic killing of polymyxin b in combination with cannabidiol against Acinetobacter baumannii: A metabolomic study. Pharmaceutics 2022, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissimo-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.L.; Ogasawara, T.M.C.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R.; et al. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef]
- Grimsey, E.M.; Piddock, L.J.V. Do phenothiazines possess antimicrobial and efflux inhibitory properties? FEMS Microbiol. Rev. 2019, 43, 577–590. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef]
- Vargiu, A.V.; Ruggerone, P.; Opperman, T.J.; Nguyen, S.T.; Nikaido, H. Molecular mechanism of MBX2319 inhibition of Escherichia coli AcrB multidrug efflux pump and comparison with other inhibitors. Antimicrob. Agents Chemother. 2014, 58, 6224–6234. [Google Scholar] [CrossRef]
- Hu, Y.; Coates, A. Zidovudine enhances activity of carbapenems against NDM-1-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2021, 76, 2302–2305. [Google Scholar] [CrossRef]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Baron, S.A.; Hadjadj, L.; Rolain, J.M. In vitro screening of a 1280 FDA-Approved drugs library against multidrug-resistant and extensively drug-resistant bacteria. Antibiotics 2022, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Jiménez-Mejías, M.E.; Sánchez-Encinales, V.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Pachón, J.; Smani, Y. Synergistic activity of niclosamide in combination with colistin against colistin-susceptible and colistin-resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 2018, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Domalaon, R.; De Silva, P.M.; Kumar, A.; Zhanel, G.G.; Schweizer, F. The Anthelmintic drug niclosamide synergizes with colistin and reverses colistin resistance in Gram-negative Bacilli. Antimicrob. Agents Chemother. 2019, 63, e02574-18. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Miró-Canturri, A.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. The anthelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2019, 54, 507–512. [Google Scholar] [CrossRef]
- Domalaon, R.; Okunnu, O.; Zhanel, G.G.; Schweizer, F. Synergistic combinations of anthelmintic salicylanilides oxyclozanide, rafoxanide, and closantel with colistin eradicates multidrug-resistant colistin-resistant Gram-negative bacilli. J. Antibiot. 2019, 72, 605–616. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.; Woertman, J.; Veldhuizen, E.J. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Shang, D.; Han, X.; Du, W.; Kou, Z.; Jiang, F. Trp-Containing antibacterial peptides impair quorum sensing and biofilm development in multidrug-resistant Pseudomonas aeruginosa and exhibit synergistic effects with antibiotics. Front. Microbiol. 2021, 12, 611009. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zhang, L.H. The roles of microbial cell-cell chemical communication systems in the modulation of antimicrobial resistance. Antibiotics 2020, 9, 779. [Google Scholar] [CrossRef]
- King, A.; Blackledge, M.S. Evaluation of small molecule kinase inhibitors as novel antimicrobial and antibiofilm agents. Chem. Biol. Drug Des. 2021, 98, 1038–1064. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through sRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, H.; Yu, H.; Dai, Q.; Xiong, J.; Sheng, H.; Qiu, J.; Jiang, L.; Peng, J.; He, X.; et al. Allicin inhibits Pseudomonas aeruginosa virulence by suppressing the rhl and pqs quorum-sensing systems. Can. J. Microbiol. 2019, 65, 563–574. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef]
- Liu, T.; Luo, J.; Bi, G.; Du, Z.; Kong, J.; Chen, Y. Antibacterial synergy between linezolid and baicalein against methicillin-resistant Staphylococcus aureus biofilm in vivo. Microb. Pathog. 2020, 147, 104411. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Q.; Wei, Z.; Yang, Q.; Guo, Z.; Shen, L.; Duan, K.; Chen, L. Baicalin represses type three secretion system of Pseudomonas aeruginosa through PQS System. Molecules 2021, 26, 1497. [Google Scholar] [CrossRef]
- Peng, L.Y.; Yuan, M.; Wu, Z.M.; Song, K.; Zhang, C.L.; An, Q.; Xia, F.; Yu, J.L.; Yi, P.F.; Fu, B.D.; et al. Anti-bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Sci. Rep. 2019, 9, 4063. [Google Scholar] [CrossRef]
- Abinaya, M.; Gayathri, M. Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P.aeruginosa. Bioorg. Chem. 2019, 87, 291–301. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Huang, F.; Yang, M.; He, D.; Deng, L. Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 1563–1573. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.D.; Hong, J.; Liu, C.; Zhang, X.L.; Zheng, J.P.; Xu, Y.J.; Ou, Z.Y.; Zheng, J.L.; Yu, D.J. Inhibitory effect of two traditional Chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.X.; Yu, J.H.; Xu, X.J.; Xu, X.F.; Zeng, T.; Lin, J.; Chen, W.M. Cajaninstilbene acid analogues as novel quorum sensing and biofilm inhibitors of Pseudomonas aeruginosa. Microb. Pathog. 2020, 148, 104414. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol prevents quorum sensing and biofilm formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-biofilm activity of cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Muthuramalingam, P.; Priya, A.; Suba, M.; Ramesh, M.; Karutha Pandian, S. Carvacrol targets SarA and CrtM of methicillin-resistant Staphylococcus aureus to mitigate biofilm formation and staphyloxanthin synthesis: An in vitro and in vivo approach. ACS Omega 2020, 5, 31100–31114. [Google Scholar] [CrossRef]
- Pesingi, P.V.; Singh, B.R.; Pesingi, P.K.; Bhardwaj, M.; Singh, S.V.; Kumawat, M.; Sinha, D.K.; Gandham, R.K. MexAB-OprM efflux pump of Pseudomonas aeruginosa offers resistance to carvacrol: A herbal antimicrobial agent. Front. Microbiol. 2019, 10, 2664. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Vazquez-Armenta, F.J.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Nazzaro, F.; Ayala-Zavala, J.F. Virulence of Pseudomonas aeruginosa exposed to carvacrol: Alterations of the quorum sensing at enzymatic and gene levels. J. Cell Commun. Signal. 2019, 13, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Jantaruk, P.; Pabuprapap, W.; Nakaew, A.; Kunthalert, D.; Suksamrarn, A. 4-methoxybenzalacetone, the cinnamic acid analog as a potential quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2021, 37, 153. [Google Scholar] [CrossRef] [PubMed]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of cinnamaldehyde on quorum sensing and biofilm susceptibility to antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Matinlinna, J.P.; Lévesque, C.M.; Neelakantan, P. Trans-Cinnamaldehyde attenuates Enterococcus faecalis virulence and inhibits biofilm formation. Antibiotics 2021, 10, 702. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef]
- D’Angelo, F.; Baldelli, V.; Halliday, N.; Pantalone, P.; Polticelli, F.; Fiscarelli, E.; Williams, P.; Visca, P.; Leoni, L.; Rampioni, G. Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e01296-18. [Google Scholar] [CrossRef] [PubMed]
- Bahari, S.; Zeighami, H.; Mirshahabi, H.; Roudashti, S.; Haghi, F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Shukla, G.; Parmar, P.; Patel, B.; Goswami, D.; Saraf, M. Exemplifying the next generation of antibiotic susceptibility intensifiers of phytochemicals by LasR-mediated quorum sensing inhibition. Sci. Rep. 2021, 11, 22421. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Parmar, P.; Rao, P.; Goswami, D.; Saraf, M. Twin peaks: Presenting the antagonistic molecular interplay of curcumin with LasR and LuxR quorum sensing pathways. Curr. Microbiol. 2020, 77, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Hu, P.; Huang, P.; Chen, W.M. Curcumin inhibits the Sortase A activity of the Streptococcus mutans UA159. Appl. Biochem. Biotechnol. 2013, 171, 396–402. [Google Scholar] [CrossRef]
- Wang, S.; Kim, M.C.; Kang, O.H.; Kwon, D.Y. The mechanism of bisdemethoxycurcumin enhances conventional antibiotics against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 7945. [Google Scholar] [CrossRef]
- El-Ganiny, A.M.; Gad, A.I.; El-Sayed, M.A.; Shaldam, M.A.; Abbas, H.A. The promising anti-virulence activity of candesartan, domperidone, and miconazole on Staphylococcus aureus. Braz. J. Microbiol. 2022, 53, 1–18. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Zhou, L.; Ji, H.; Zhao, L.; Yu, W.; Gong, Q. Falcarindiol isolated from Notopterygium incisum inhibits the quorum sensing of Pseudomonas aeruginosa. Molecules 2021, 26, 5896. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kadirvel, M.; Fanimarvasti, F.; Forbes, S.; McBain, A.; Gardiner, J.M.; Brown, G.D.; Freeman, S. Inhibition of quorum sensing and biofilm formation in Vibrio harveyi by 4-fluoro-DPD; a novel potent inhibitor of signalling. Chem. Commun. 2014, 50, 5000–5002. [Google Scholar] [CrossRef]
- Shukla, A.; Parmar, P.; Patel, B.; Goswami, D.; Saraf, M. Breaking bad: Better call gingerol for improving antibiotic susceptibility of Pseudomonas aeruginosa by inhibiting multiple quorum sensing pathways. Microbiol. Res. 2021, 252, 126863. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Byun, Y.; Park, H.D. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci. Rep. 2015, 5, 8656. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef]
- Christensen, L.D.; van Gennip, M.; Jakobsen, T.H.; Alhede, M.; Hougen, H.P.; Høiby, N.; Bjarnsholt, T.; Givskov, M. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 2012, 67, 1198–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Zhang, X.; Chen, L.; Xu, C.; Liu, S.; Cao, J.; Zheng, X.; Jia, H.; Chen, L.; et al. Combining colistin with furanone C-30 rescues colistin resistance of Gram-negative bacteria in vitro and in vivo. Microbiol. Spectr. 2021, 9, e0123121. [Google Scholar] [CrossRef]
- Ren, D.; Sims, J.J.; Wood, T.K. Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 2001, 3, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Bedzyk, L.A.; Ye, R.W.; Thomas, S.M.; Wood, T.K. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol. Bioeng. 2004, 88, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheng, W.; He, X.; Liu, Y.; Li, J.; Sun, J.; Li, J.; Wang, F.; Gao, Y. Association of furanone C-30 with biofilm formation & antibiotic resistance in Pseudomonas aeruginosa. Indian J. Med. Res. 2018, 147, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, A.; Macken, A.; Wennberg, A.C.; Grung, M.; Rundberget, J.T.; Fredriksen, L.; Scheie, A.A.; Benneche, T.; d’Auriac, M.A. Environmental fate and effects of novel quorum sensing inhibitors that can control biofilm formation. Chemosphere 2016, 164, 52–58. [Google Scholar] [CrossRef]
- Defoirdt, T.; Benneche, T.; Brackman, G.; Coenye, T.; Sorgeloos, P.; Scheie, A.A. A quorum sensing-disrupting brominated thiophenone with a promising therapeutic potential to treat luminescent vibriosis. PLoS ONE 2012, 7, e41788. [Google Scholar] [CrossRef]
- Lönn-Stensrud, J.; Naemi, A.O.; Benneche, T.; Petersen, F.C.; Scheie, A.A. Thiophenones inhibit Staphylococcus epidermidis biofilm formation at nontoxic concentrations. FEMS Immunol. Med. Microbiol. 2012, 65, 326–334. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Onyedibe, K.I.; Aryal, U.K.; Sintim, H.O. Proteomic analysis of bacterial response to a 4-hydroxybenzylidene indolinone compound, which re-sensitizes bacteria to traditional antibiotics. J. Proteom. 2019, 202, 103368. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Dayal, N.; Miller, J.; Sintim, H.O. Hydroxybenzylidene-indolinones, c-di-AMP synthase inhibitors, have antibacterial and anti-biofilm activities and also re-sensitize resistant bacteria to methicillin and vancomycin. RSC Adv. 2017, 7, 8288–8294. [Google Scholar] [CrossRef]
- Geng, Y.F.; Yang, C.; Zhang, Y.; Tao, S.N.; Mei, J.; Zhang, X.C.; Sun, Y.J.; Zhao, B.T. An innovative role for luteolin as a natural quorum sensing inhibitor in Pseudomonas aeruginosa. Life Sci. 2021, 274, 119325. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Q.; He, Q.; Chen, G. Metabolomic insights into the inhibition mechanism of methyl N-methylanthranilate: A novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. Int. J. Food Microbiol. 2021, 358, 109402. [Google Scholar] [CrossRef]
- Calfee, M.W.; Coleman, J.P.; Pesci, E.C. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2001, 98, 11633–11637. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, P.; Praveen Krishna, V.; Bharath, D.; Lavanya, R.; Vairaprakash, P.; Adline Princy, S. Staphylococcus aureus quorum regulator SarA targeted compound, 2-[(Methylamino)methyl]phenol inhibits biofilm and down-regulates virulence genes. Front. Microbiol. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Choi, H.; Hong, S.; Moon, H.R.; Lee, J.H. Antipathogenic compounds that are effective at very low concentrations and have both antibiofilm and antivirulence effects against Pseudomonas aeruginosa. Microbiol. Spectr. 2021, 9, e0024921. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015, 81, 774–782. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Feng, T.; Du, R.; Tian, X.; Wang, Y.; Zhang, X.H. Heterologous expression of the marine-derived quorum quenching enzyme MomL can expand the antibacterial spectrum of Bacillus brevis. Mar. Drugs 2019, 17, 128. [Google Scholar] [CrossRef]
- Hnamte, S.; Parasuraman, P.; Ranganathan, S.; Ampasala, D.R.; Reddy, D.; Kumavath, R.N.; Suchiang, K.; Mohanty, S.K.; Busi, S. Mosloflavone attenuates the quorum sensing controlled virulence phenotypes and biofilm formation in Pseudomonas aeruginosa PAO1: In vitro, in vivo and in silico approach. Microb. Pathog. 2019, 131, 128–134. [Google Scholar] [CrossRef]
- Imperi, F.; Massai, F.; Ramachandran Pillai, C.; Longo, F.; Zennaro, E.; Rampioni, G.; Visca, P.; Leoni, L. New life for an old drug: The anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef]
- Baldelli, V.; D’Angelo, F.; Pavoncello, V.; Fiscarelli, E.V.; Visca, P.; Rampioni, G.; Leoni, L. Identification of FDA-approved antivirulence drugs targeting the Pseudomonas aeruginosa quorum sensing effector protein PqsE. Virulence 2020, 11, 652–668. [Google Scholar] [CrossRef]
- Soukarieh, F.; Mashabi, A.; Richardson, W.; Oton, E.V.; Romero, M.; Roberston, S.N.; Grossman, S.; Sou, T.; Liu, R.; Halliday, N.; et al. Design and evaluation of new Quinazolin-4(3H)-one derived PqsR antagonists as quorum sensing quenchers in Pseudomonas aeruginosa. ACS Infect. Dis. 2021, 7, 2666–2685. [Google Scholar] [CrossRef]
- Yang, D.; Hao, S.; Zhao, L.; Shi, F.; Ye, G.; Zou, Y.; Song, X.; Li, L.; Yin, Z.; He, X.; et al. Paeonol attenuates quorum-sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 692474. [Google Scholar] [CrossRef]
- Wenderska, I.B.; Chong, M.; McNulty, J.; Wright, G.D.; Burrows, L.L. Palmitoyl-DL-carnitine is a multitarget inhibitor of Pseudomonas aeruginosa biofilm development. Chembiochem 2011, 12, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Narvi, S.S.; Agarwal, V. Exploring the impact of parthenolide as anti-quorum sensing and anti-biofilm agent against Pseudomonas aeruginosa. Life Sci. 2018, 199, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Diţu, L.M.; Banu, O.; Bleotu, C.; Drăcea, O.; Bucur, M.; Larion, C.; Israil, A.M.; Lazăr, V. Subinhibitory concentrations of phenyl lactic acid interfere with the expression of virulence factors in Staphylococcus aureus and Pseudomonas aeruginosa clinical strains. Roum Arch. Microbiol. Immunol. 2009, 68, 27–33. [Google Scholar] [PubMed]
- Furiga, A.; Lajoie, B.; El Hage, S.; Baziard, G.; Roques, C. Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by combining the drugs with a new quorum-sensing inhibitor. Antimicrob. Agents Chemother. 2015, 60, 1676–1686. [Google Scholar] [CrossRef]
- Grossman, S.; Soukarieh, F.; Richardson, W.; Liu, R.; Mashabi, A.; Emsley, J.; Williams, P.; Cámara, M.; Stocks, M.J. Novel quinazolinone inhibitors of the Pseudomonas aeruginosa quorum sensing transcriptional regulator PqsR. Eur. J. Med. Chem. 2020, 208, 112778. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of synergy between tetracycline and quercetin against antibiotic resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Zhou, J.W.; Chen, T.T.; Tan, X.J.; Sheng, J.Y.; Jia, A.Q. Can the quorum sensing inhibitor resveratrol function as an aminoglycoside antibiotic accelerant against Pseudomonas aeruginosa? Int. J. Antimicrob. Agents 2018, 52, 35–41. [Google Scholar] [CrossRef]
- Chen, T.; Sheng, J.; Fu, Y.; Li, M.; Wang, J.; Jia, A.Q. (1)H NMR-based global metabolic studies of Pseudomonas aeruginosa upon exposure of the quorum sensing inhibitor Resveratrol. J. Proteome Res. 2017, 16, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Tan, X.; Jiao, Y.; Liu, L.; Zhao, W.; Yang, S.; Jia, A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014, 4, 5467. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mikalauskas, A.; Parkins, M.D.; Poole, K. Rifampicin potentiation of aminoglycoside activity against cystic fibrosis isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2017, 72, 3349–3352. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Gilmour, C.; Farha, M.A.; Mullen, E.; Lau, C.H.; Brown, E.D. Potentiation of aminoglycoside activity in Pseudomonas aeruginosa by targeting the AmgRS envelope stress-responsive two-component system. Antimicrob. Agents Chemother. 2016, 60, 3509–3518. [Google Scholar] [CrossRef]
- Yadav, M.K.; Park, S.W.; Chae, S.W.; Song, J.J. Sinefungin, a natural nucleoside analogue of S-adenosylmethionine, inhibits Streptococcus pneumoniae biofilm growth. BioMed. Res. Int. 2014, 2014, 156987. [Google Scholar] [CrossRef]
- Mahdally, N.H.; George, R.F.; Kashef, M.T.; Al-Ghobashy, M.; Murad, F.E.; Attia, A.S. Staquorsin: A novel Staphylococcus aureus Agr-mediated quorum sensing inhibitor impairing virulence in vivo without notable resistance development. Front. Microbiol. 2021, 12, 700494. [Google Scholar] [CrossRef]
- Narendrakumar, L.; Theresa, M.; Krishnankutty Chandrika, S.; Thomas, S. Tryptanthrin, a potential biofilm inhibitor against toxigenic Vibrio cholerae, modulating the global quorum sensing regulator, LuxO. Biofouling 2019, 35, 1093–1103. [Google Scholar] [CrossRef]
- Shang, D.; Liu, Y.; Jiang, F.; Ji, F.; Wang, H.; Han, X. Synergistic antibacterial activity of designed Trp-containing antibacterial peptides in combination with antibiotics against multidrug-resistant Staphylococcus epidermidis. Front. Microbiol. 2019, 10, 2719. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated fatty acids affect quorum sensing communication system and inhibit motility and biofilm formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Kim, H.S.; Cha, E.; Ham, S.Y.; Park, J.H.; Nam, S.; Kwon, H.; Byun, Y.; Park, H.D. Linoleic acid inhibits Pseudomonas aeruginosa biofilm formation by activating diffusible signal factor-mediated quorum sensing. Biotechnol. Bioeng. 2021, 118, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lee, J.H.; Beyenal, H.; Lee, J. Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol. 2020, 28, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring oil and omega fatty acids inhibit Staphylococcus aureus biofilm formation and virulence. Front. Microbiol. 2018, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cheepurupalli, L.; Vigneswaran, S.; Singh Rathore, S.; Suma Mohan, S.; Ramakrishnan, J. In vitro and in silico investigation of caprylic acid effect on multi drug resistant (MDR) Klebsiella pneumoniae biofilm. J. Biomol. Struct. Dyn. 2020, 38, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Beavers, W.N.; Munneke, M.J.; Stackhouse, A.R.; Freiberg, J.A.; Skaar, E.P. Host polyunsaturated fatty acids potentiate aminoglycoside killing of Staphylococcus aureus. Microbiol. Spectr. 2022, 10, e0276721. [Google Scholar] [CrossRef]
- Beavers, W.N.; Monteith, A.J.; Amarnath, V.; Mernaugh, R.L.; Roberts, L.J., 2nd; Chazin, W.J.; Davies, S.S.; Skaar, E.P. Arachidonic acid kills Staphylococcus aureus through a lipid peroxidation mechanism. mBio 2019, 10, e01333-19. [Google Scholar] [CrossRef]
- Herndon, J.L.; Peters, R.E.; Hofer, R.N.; Simmons, T.B.; Symes, S.J.; Giles, D.K. Exogenous polyunsaturated fatty acids (PUFAs) promote changes in growth, phospholipid composition, membrane permeability and virulence phenotypes in Escherichia coli. BMC Microbiol. 2020, 20, 305. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.R. Unsaturated fatty acids control biofilm formation of Staphylococcus aureus and other Gram-positive bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Kumar, R.; Kumar, M.; Harjai, K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 2015, 102, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Chhibber, S.; Harjai, K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia 2013, 90, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Igarashi, M.; Okajima, T.; Kinoshita, N.; Umekita, M.; Sawa, R.; Inoue, K.; Watanabe, T.; Doi, A.; Martin, A.; et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J. Antibiot. 2010, 63, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145 Pt 2, 283–291. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, anti-quorum-sensing actions, and clinical trials of medicinal plant bioactive compounds against bacteria: A comprehensive review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hussain, F.; Negm, O.; Pavia, A.; Halliday, N.; Dubern, J.F.; Singh, S.; Muntaka, S.; Wheldon, L.; Luckett, J.; et al. Contribution of the alkylquinolone quorum-sensing system to the interaction of Pseudomonas aeruginosa with bronchial epithelial cells. Front. Microbiol. 2018, 9, 3018. [Google Scholar] [CrossRef]
- Bredenbruch, F.; Nimtz, M.; Wray, V.; Morr, M.; Müller, R.; Häussler, S. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J. Bacteriol. 2005, 187, 3630–3635. [Google Scholar] [CrossRef]
- Soheili, V.; Tajani, A.S.; Ghodsi, R.; Bazzaz, B.S.F. Anti-PqsR compounds as next-generation antibacterial agents against Pseudomonas aeruginosa: A review. Eur. J. Med. Chem. 2019, 172, 26–35. [Google Scholar] [CrossRef]
- Pustelny, C.; Albers, A.; Büldt-Karentzopoulos, K.; Parschat, K.; Chhabra, S.R.; Cámara, M.; Williams, P.; Fetzner, S. Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem. Biol. 2009, 16, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Lesic, B.; Lépine, F.; Déziel, E.; Zhang, J.; Zhang, Q.; Padfield, K.; Castonguay, M.H.; Milot, S.; Stachel, S.; Tzika, A.A.; et al. Inhibitors of pathogen intercellular signals as selective anti-infective compounds. PLoS Pathog. 2007, 3, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.P.; Maurer, C.K.; Zimmer, C.; Wagner, N.; Brengel, C.; de Jong, J.C.; Lucas, S.; Müsken, M.; Häussler, S.; Steinbach, A.; et al. Validation of PqsD as an anti-biofilm target in Pseudomonas aeruginosa by development of small-molecule inhibitors. J. Am. Chem. Soc. 2012, 134, 16143–16146. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Köte, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005, 151, 3873–3880. [Google Scholar] [CrossRef]
- El-Sayed, N.R.; Samir, R.; Jamil, M.A.-H.L.; Ramadan, M.A. Olive leaf extract modulates quorum sensing genes and biofilm formation in multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2020, 9, 526. [Google Scholar] [CrossRef]
- Song, Z.; Kong, K.F.; Wu, H.; Maricic, N.; Ramalingam, B.; Priestap, H.; Schneper, L.; Quirke, J.M.; Høiby, N.; Mathee, K. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine 2010, 17, 1040–1046. [Google Scholar] [CrossRef]
- Cordes, T.; Michelucci, A.; Hiller, K. Itaconic acid: The surprising role of an industrial compound as a mammalian antimicrobial metabolite. Annu. Rev. Nutr. 2015, 35, 451–473. [Google Scholar] [CrossRef]
- Ho, D.K.; De Rossi, C.; Loretz, B.; Murgia, X.; Lehr, C.M. Itaconic acid increases the efficacy of tobramycin against Pseudomonas aeruginosa biofilms. Pharmaceutics 2020, 12, 691. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Schneewind, O.; Missiakas, D. Sortases, surface proteins, and their roles in Staphylococcus aureus disease and vaccine development. Microbiol. Spectr. 2019, 7, PSIB-0004-2018. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G.; et al. 1,2,4-Oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem 2021, 209, 112892. [Google Scholar] [CrossRef] [PubMed]
- Wallock-Richards, D.J.; Marles-Wright, J.; Clarke, D.J.; Maitra, A.; Dodds, M.; Hanley, B.; Campopiano, D.J. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem. Commun. 2015, 51, 10483–10485. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Jing, S.; Dong, H.; Wang, D.; Wang, T. Astilbin inhibits the activity of Sortase A from Streptococcus mutans. Molecules 2019, 24, 465. [Google Scholar] [CrossRef]
- Nitulescu, G.; Margina, D.; Zanfirescu, A.; Olaru, O.T.; Nitulescu, G.M. Targeting bacterial sortases in search of anti-virulence therapies with low risk of resistance development. Pharmaceuticals 2021, 14, 415. [Google Scholar] [CrossRef]
- Alharthi, S.; Alavi, S.E.; Moyle, P.M.; Ziora, Z.M. Sortase A (SrtA) inhibitors as an alternative treatment for superbug infections. Drug Discov. Today 2021, 26, 2164–2172. [Google Scholar] [CrossRef]
- Thappeta, K.R.V.; Zhao, L.N.; Nge, C.E.; Crasta, S.; Leong, C.Y.; Ng, V.; Kanagasundaram, Y.; Fan, H.; Ng, S.B. In-silico identified new natural Sortase A inhibitors disrupt S. aureus biofilm formation. Int. J. Mol. Sci. 2020, 21, 8601. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Qu, H.; Wang, K.; Jing, S.; Guan, S.; Su, L.; Li, Q.; Wang, D. Taxifolin, an inhibitor of Sortase A, interferes with the adhesion of methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 686864. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Li, R.; Yuan, X.; Wei, J.; Zhang, X.; Cheng, G.; Wang, Z.A.; Du, Y. Synthesis and evaluation of a chitosan oligosaccharide-streptomycin conjugate against Pseudomonas aeruginosa biofilms. Mar. Drugs 2019, 17, 43. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan coupling makes microbial biofilms susceptible to antibiotics. Sci. Rep. 2013, 3, 3364. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Guo, J.; Zhao, Y.; Chen, J.; Meng, Q.; Qu, D.; Zheng, J.; Yu, Z.; Wu, Y.; Deng, Q. Clemastine inhibits the biofilm and hemolytic of Staphylococcus aureus through the GdpP Protein. Microbiol. Spectr. 2022, 10, e0054121. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.G.; Kashevarova, N.M.; Sidorov, R.Y.; Nesterova, L.Y.; Akhova, A.V.; Tsyganov, I.V.; Vaganov, V.Y.; Shipilovskikh, S.A.; Rubtsov, A.E.; Malkov, A.V. A synthetic diterpene analogue inhibits mycobacterial persistence and biofilm formation by targeting (p)ppGpp synthetases. Cell Chem. Biol. 2021, 28, 1420–1432.e1429. [Google Scholar] [CrossRef] [PubMed]
- Arita-Morioka, K.I.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef]
- Hengge, R. Targeting bacterial biofilms by the green tea polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef]
- Schneider-Rayman, M.; Steinberg, D.; Sionov, R.V.; Friedman, M.; Shalish, M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An in vitro study. BMC Oral Health 2021, 21, 447. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Xia, W.; Li, N.; Shan, H.; Lin, Y.; Yin, F.; Yu, X.; Zhou, Z. Gallium porphyrin and gallium nitrate reduce the high vancomycin tolerance of MRSA biofilms by promoting extracellular DNA-dependent biofilm dispersion. ACS Infect. Dis. 2021, 7, 2565–2582. [Google Scholar] [CrossRef]
- Kang, D.; Revtovich, A.V.; Deyanov, A.E.; Kirienko, N.V. Pyoverdine inhibitors and gallium nitrate synergistically affect Pseudomonas aeruginosa. mSphere 2021, 6, e0040121. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of gallium and gallium-based compounds as antimicrobial agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.P.; Hung, W.C.; Huang, C.Y.; Lin, Y.S.; Chan, M.Y.; Lu, P.L.; Lin, L.; Sheu, J.H. 5-Episinuleptolide decreases the expression of the extracellular matrix in early biofilm formation of multi-drug resistant Acinetobacter baumannii. Mar. Drugs 2016, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Thirunanasambandham, R. 5-Hydroxymethylfurfural inhibits Acinetobacter baumannii biofilms: An in vitro study. Arch. Microbiol. 2021, 203, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Tang, Z.; Li, T.; Wang, J. Combination of kaempferol and azithromycin attenuates Staphylococcus aureus-induced osteomyelitis via anti-biofilm effects and by inhibiting the phosphorylation of ERK1/2 and SAPK. Pathog. Dis. 2021, 79, ftab048. [Google Scholar] [CrossRef]
- Sivaranjani, M.; Srinivasan, R.; Aravindraja, C.; Karutha Pandian, S.; Veera Ravi, A. Inhibitory effect of α-mangostin on Acinetobacter baumannii biofilms—An in vitro study. Biofouling 2018, 34, 579–593. [Google Scholar] [CrossRef]
- She, P.; Wang, Y.; Luo, Z.; Chen, L.; Tan, R.; Wang, Y.; Wu, Y. Meloxicam inhibits biofilm formation and enhances antimicrobial agents efficacy by Pseudomonas aeruginosa. Microbiologyopen 2018, 7, e00545. [Google Scholar] [CrossRef]
- Soheili, V.; Bazzaz, B.S.; Abdollahpour, N.; Hadizadeh, F. Investigation of Pseudomonas aeruginosa quorum-sensing signaling system for identifying multiple inhibitors using molecular docking and structural analysis methodology. Microb. Pathog. 2015, 89, 73–78. [Google Scholar] [CrossRef]
- Guan, X.N.; Zhang, T.; Yang, T.; Dong, Z.; Yang, S.; Lan, L.; Gan, J.; Yang, C.G. Covalent Sortase A inhibitor ML346 prevents Staphylococcus aureus infection of Galleria mellonella. RSC Med. Chem. 2022, 13, 138–149. [Google Scholar] [CrossRef]
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S.K. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front. Microbiol. 2019, 10, 2027. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, S.; Qu, H.; Wang, K.; Jin, Y.; Ding, Y.; Yang, L.; Yu, H.; Shi, Y.; Li, Q.; et al. Orientin mediates protection against MRSA-induced pneumonia by inhibiting Sortase A. Virulence 2021, 12, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Go, Y.Y.; Chae, S.W.; Song, J.J. The small molecule DAM inhibitor, pyrimidinedione, disrupts Streptococcus pneumoniae biofilm growth in vitro. PLoS ONE 2015, 10, e0139238. [Google Scholar] [CrossRef] [PubMed]
- Abirami, G.; Durgadevi, R.; Velmurugan, P.; Ravi, A.V. Gene expressing analysis indicates the role of Pyrogallol as a novel antibiofilm and antivirulence agent against Acinetobacter baumannii. Arch. Microbiol. 2021, 203, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Payne, D.E.; Martin, N.R.; Parzych, K.R.; Rickard, A.H.; Underwood, A.; Boles, B.R. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 2013, 81, 496–504. [Google Scholar] [CrossRef]
- Kang, X.; Ma, Q.; Wang, G.; Li, N.; Mao, Y.; Wang, X.; Wang, Y.; Wang, G. Potential mechanisms of quercetin influence the ClfB protein during biofilm formation of Staphylococcus aureus. Front. Pharmacol. 2022, 13, 825489. [Google Scholar] [CrossRef]
- Lidor, O.; Al-Quntar, A.; Pesci, E.C.; Steinberg, D. Mechanistic analysis of a synthetic inhibitor of the Pseudomonas aeruginosa LasI quorum-sensing signal synthase. Sci. Rep. 2015, 5, 16569. [Google Scholar] [CrossRef]
- Feldman, M.; Al-Quntar, A.; Polacheck, I.; Friedman, M.; Steinberg, D. Therapeutic potential of thiazolidinedione-8 as an antibiofilm agent against Candida albicans. PLoS ONE 2014, 9, e93225. [Google Scholar] [CrossRef] [PubMed]
- Stegenga, M.E.; Florquin, S.; de Vos, A.F.; van der Poll, T. The thiazolidinedione ciglitazone reduces bacterial outgrowth and early inflammation during Streptococcus pneumoniae pneumonia in mice. Crit. Care Med. 2009, 37, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Al Quntar, A.A.; Enk, C.D.; Karalic, I.; Nelis, H.J.; Van Calenbergh, S.; Srebnik, M.; Coenye, T. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi. Bioorg. Med. Chem. 2013, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zuo, R.; González Barrios, A.F.; Bedzyk, L.A.; Eldridge, G.R.; Pasmore, M.E.; Wood, T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005, 71, 4022–4034. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes-asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Nait Chabane, Y.; Mlouka, M.B.; Alexandre, S.; Nicol, M.; Marti, S.; Pestel-Caron, M.; Vila, J.; Jouenne, T.; Dé, E. Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol. 2014, 14, 62. [Google Scholar] [CrossRef]
- Hung, D.T.; Shakhnovich, E.A.; Pierson, E.; Mekalanos, J.J. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 2005, 310, 670–674. [Google Scholar] [CrossRef]
- Oh, M.H.; Choi, C.H. Role of LuxIR Homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J. Microbiol. Biotechnol. 2015, 25, 1390–1400. [Google Scholar] [CrossRef]
- Kim, H.R.; Shin, D.S.; Jang, H.I.; Eom, Y.B. Anti-biofilm and anti-virulence effects of zerumbone against Acinetobacter baumannii. Microbiology 2020, 166, 717–726. [Google Scholar] [CrossRef]
- Monteiro, K.L.C.; de Aquino, T.M.; Mendonça Junior, F.J.B. An update on Staphylococcus aureus NorA efflux pump inhibitors. Curr. Top. Med. Chem. 2020, 20, 2168–2185. [Google Scholar] [CrossRef]
- Wang, Y.; Venter, H.; Ma, S. Efflux pump inhibitors: A novel approach to combat efflux-mediated drug resistance in bacteria. Curr. Drug Targets 2016, 17, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Waditzer, M.; Bucar, F. Flavonoids as inhibitors of bacterial efflux pumps. Molecules 2021, 26, 6904. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.M.F.; Cardoso, D.S.P.; Ferreira, M.J.U. Overcoming multidrug resistance: Flavonoid and terpenoid nitrogen-containing derivatives as ABC transporter modulators. Molecules 2020, 25, 3364. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Sharma, A.; Akhter, J.; Pathania, R. The small molecule IITR08027 restores the antibacterial activity of fluoroquinolones against multidrug-resistant Acinetobacter baumannii by efflux inhibition. Int. J. Antimicrob. Agents 2017, 50, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Grimsey, E.M.; Fais, C.; Marshall, R.L.; Ricci, V.; Ciusa, M.L.; Stone, J.W.; Ivens, A.; Malloci, G.; Ruggerone, P.; Vargiu, A.V.; et al. Chlorpromazine and Amitriptyline are substrates and inhibitors of the AcrB multidrug efflux pump. mBio 2020, 11, e00465-20. [Google Scholar] [CrossRef]
- Tegos, G.P.; Haynes, M.; Strouse, J.J.; Khan, M.M.; Bologa, C.G.; Oprea, T.I.; Sklar, L.A. Microbial efflux pump inhibition: Tactics and strategies. Curr. Pharm. Des. 2011, 17, 1291–1302. [Google Scholar] [CrossRef]
- Fernebro, J. Fighting bacterial infections-future treatment options. Drug Resist. Updat. 2011, 14, 125–139. [Google Scholar] [CrossRef]
- Malléa, M.; Mahamoud, A.; Chevalier, J.; Alibert-Franco, S.; Brouant, P.; Barbe, J.; Pagès, J.M. Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem. J. 2003, 376, 801–805. [Google Scholar] [CrossRef]
- Tambat, R.; Jangra, M.; Mahey, N.; Chandal, N.; Kaur, M.; Chaudhary, S.; Verma, D.K.; Thakur, K.G.; Raje, M.; Jachak, S.; et al. Microbe-derived indole metabolite demonstrates potent multidrug efflux pump inhibition in Staphylococcus aureus. Front. Microbiol. 2019, 10, 2153. [Google Scholar] [CrossRef]
- Fontaine, F.; Héquet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef]
- Giorgini, G.; Mangiaterra, G.; Cedraro, N.; Laudadio, E.; Sabbatini, G.; Cantarini, M.; Minnelli, C.; Mobbili, G.; Frangipani, E.; Biavasco, F.; et al. Berberine derivatives as Pseudomonas aeruginosa MexXY-OprM inhibitors: Activity and in silico insights. Molecules 2021, 26, 6644. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Cedraro, N.; Mangiaterra, G.; Citterio, B.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F.; Galeazzi, R. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: In silico and in vitro studies. J. Nat. Prod. 2019, 82, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Barbosa, C.R.; Scherf, J.R.; de Freitas, T.S.; de Menezes, I.R.A.; Pereira, R.L.S.; Dos Santos, J.F.S.; de Jesus, S.S.P.; Lopes, T.P.; de Sousa Silveira, Z.; de Morais Oliveira-Tintino, C.D.; et al. Effect of carvacrol and thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus aureus strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Voravuthikunchai, S.P.; Coote, P.J. Steroidal alkaloids and conessine from the medicinal plant Holarrhena antidysenterica restore antibiotic efficacy in a Galleria mellonella model of multidrug-resistant Pseudomonas aeruginosa infection. BMC Complement. Altern. Med. 2018, 18, 285. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakayama, K.; Ohtsuka, M.; Kuru, N.; Yokomizo, Y.; Sakamoto, A.; Takemura, M.; Hoshino, K.; Kanda, H.; Nitanai, H.; et al. MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 7: Highly soluble and in vivo active quaternary ammonium analogue D13-9001, a potential preclinical candidate. Bioorg. Med. Chem. 2007, 15, 7087–7097. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2020, 15, e0231583. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696. [Google Scholar] [CrossRef]
- Muniz, D.F.; Dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.; da Costa, R.H.S.; de Morais Oliveira-Tintino, C.D.; et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gaur, R.; Sharma, A.; Akther, J.; Saini, M.; Bhakuni, R.S.; Pathania, R. A novel bi-functional chalcone inhibits multi-drug resistant Staphylococcus aureus and potentiates the activity of fluoroquinolones. Bioorg. Chem. 2019, 83, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Opperman, T.J.; Kwasny, S.M.; Kim, H.S.; Nguyen, S.T.; Houseweart, C.; D’Souza, S.; Walker, G.C.; Peet, N.P.; Nikaido, H.; Bowlin, T.L. Characterization of a novel pyranopyridine inhibitor of the AcrAB efflux pump of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Gilmour, C.; Farha, M.A.; Parkins, M.D.; Klinoski, R.; Brown, E.D. Meropenem potentiation of aminoglycoside activity against Pseudomonas aeruginosa: Involvement of the MexXY-OprM multidrug efflux system. J. Antimicrob. Chemother. 2018, 73, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Baiomy, A.A.; Shaker, G.H.; Abbas, H.A. Sensitizing multi drug resistant Staphylococcus aureus isolated from surgical site infections to antimicrobials by efflux pump inhibitors. Afr. Health Sci. 2020, 20, 1632–1645. [Google Scholar] [CrossRef]
- Thamilselvan, G.; Sarveswari, H.B.; Vasudevan, S.; Stanley, A.; Shanmugam, K.; Vairaprakash, P.; Solomon, A.P. Development of an antibiotic resistance breaker to resensitize drug-resistant Staphylococcus aureus: In silico and in vitro approach. Front. Cell Infect. Microbiol. 2021, 11, 700198. [Google Scholar] [CrossRef]
- Kinana, A.D.; Vargiu, A.V.; May, T.; Nikaido, H. Aminoacyl β-naphthylamides as substrates and modulators of AcrB multidrug efflux pump. Proc. Natl. Acad. Sci. USA 2016, 113, 1405–1410. [Google Scholar] [CrossRef]
- Sabatini, S.; Kaatz, G.W.; Rossolini, G.M.; Brandini, D.; Fravolini, A. From phenothiazine to 3-phenyl-1,4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J. Med. Chem. 2008, 51, 4321–4330. [Google Scholar] [CrossRef]
- Bailey, A.M.; Paulsen, I.T.; Piddock, L.J. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob. Agents Chemother. 2008, 52, 3604–3611. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Mortensen, I.; Nissen, B. Membrane stabilizers inhibit potassium efflux from Staphylococcus aureus strain No. U2275. Biochim. Biophys. Acta 1982, 685, 379–382. [Google Scholar] [CrossRef]
- Kaczor, A.; Witek, K.; Podlewska, S.; Sinou, V.; Czekajewska, J.; Żesławska, E.; Doroz-Płonka, A.; Lubelska, A.; Latacz, G.; Nitek, W.; et al. Molecular insights into an antibiotic enhancer action of new morpholine-containing 5-arylideneimidazolones in the fight against MDR bacteria. Int. J. Mol. Sci. 2021, 22, 2062. [Google Scholar] [CrossRef]
- Casalone, E.; Vignolini, T.; Braconi, L.; Gardini, L.; Capitanio, M.; Pavone, F.S.; Giovannelli, L.; Dei, S.; Teodori, E. Characterization of substituted piperazines able to reverse MDR in Escherichia coli strains overexpressing resistance-nodulation-cell division (RND) efflux pumps. J. Antimicrob. Chemother. 2021, 77, 413–424. [Google Scholar] [CrossRef]
- Sangwan, P.L.; Koul, J.L.; Koul, S.; Reddy, M.V.; Thota, N.; Khan, I.A.; Kumar, A.; Kalia, N.P.; Qazi, G.N. Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorg. Med. Chem. 2008, 16, 9847–9857. [Google Scholar] [CrossRef]
- Mahey, N.; Tambat, R.; Chandal, N.; Verma, D.K.; Thakur, K.G.; Nandanwar, H. Repurposing approved drugs as fluoroquinolone potentiators to overcome efflux pump resistance in Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0095121. [Google Scholar] [CrossRef]
- Neyfakh, A.A.; Borsch, C.M.; Kaatz, G.W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 1993, 37, 128–129. [Google Scholar] [CrossRef]
- Gibbons, S.; Udo, E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 2000, 14, 139–140. [Google Scholar] [CrossRef]
- Parai, D.; Banerjee, M.; Dey, P.; Chakraborty, A.; Islam, E.; Mukherjee, S.K. Effect of reserpine on Pseudomonas aeruginosa quorum sensing mediated virulence factors and biofilm formation. Biofouling 2018, 34, 320–334. [Google Scholar] [CrossRef]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.K. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef]
- Singkham-In, U.; Higgins, P.G.; Wannigama, D.L.; Hongsing, P.; Chatsuwan, T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS ONE 2020, 15, e0243082. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol controls Escherichia coli growth by inhibiting the AcrAB-TolC efflux pump. FEMS Microbiol. Lett. 2019, 366, fnz030. [Google Scholar] [CrossRef]
- Vestergaard, M.; Roshanak, S.; Ingmer, H. Targeting the ATP synthase in Staphylococcus aureus small colony variants, Streptococcus pyogenes and pathogenic fungi. Antibiotics 2021, 10, 376. [Google Scholar] [CrossRef]
- Liu, L.; Beck, C.; Nøhr-Meldgaard, K.; Peschel, A.; Kretschmer, D.; Ingmer, H.; Vestergaard, M. Inhibition of the ATP synthase sensitizes Staphylococcus aureus towards human antimicrobial peptides. Sci. Rep. 2020, 10, 11391. [Google Scholar] [CrossRef]
- Vestergaard, M.; Nøhr-Meldgaard, K.; Bojer, M.S.; Krogsgård Nielsen, C.; Meyer, R.L.; Slavetinsky, C.; Peschel, A.; Ingmer, H. Inhibition of the ATP synthase eliminates the intrinsic resistance of Staphylococcus aureus towards polymyxins. mBio 2017, 8, e01114-17. [Google Scholar] [CrossRef]
- Pereira, P.S.; Lima, M.; Neto, P.P.M.; Oliveira-Tintino, C.D.M.; Tintino, S.R.; Menezes, I.R.A.; de Oliveira, J.F.; Marchand, P.; Coutinho, H.D.M.; Rodrigues, M.D.D.; et al. Thiazolidinedione and thiazole derivatives potentiate norfloxacin activity against NorA efflux pump over expression in Staphylococcus aureus 1199B strains. Bioorg. Med. Chem. 2019, 27, 3797–3804. [Google Scholar] [CrossRef]
- Feldman, M.; Ginsburg, I.; Al-Quntar, A.; Steinberg, D. Thiazolidinedione-8 alters symbiotic relationship in C. albicans-S. mutans dual species biofilm. Front. Microbiol. 2016, 7, 140. [Google Scholar] [CrossRef]
- Froes, T.Q.; Chaves, B.T.; Mendes, M.S.; Ximenes, R.M.; da Silva, I.M.; da Silva, P.B.G.; de Albuquerque, J.F.C.; Castilho, M.S. Synthesis and biological evaluation of thiazolidinedione derivatives with high ligand efficiency to P. aeruginosa PhzS. J. Enzyme Inhib. Med. Chem. 2021, 36, 1217–1229. [Google Scholar] [CrossRef]
- Smith, E.C.; Kaatz, G.W.; Seo, S.M.; Wareham, N.; Williamson, E.M.; Gibbons, S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 4480–4483. [Google Scholar] [CrossRef]
- Piddock, L.J.; Garvey, M.I.; Rahman, M.M.; Gibbons, S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1215–1223. [Google Scholar] [CrossRef]
- Gupta, S.; Cohen, K.A.; Winglee, K.; Maiga, M.; Diarra, B.; Bishai, W.R. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 574–576. [Google Scholar] [CrossRef]
- Viveiros, M.; Amaral, L. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 2001, 17, 225–228. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef]
- Brown, S.; Xia, G.; Luhachack, L.G.; Campbell, J.; Meredith, T.C.; Chen, C.; Winstel, V.; Gekeler, C.; Irazoqui, J.E.; Peschel, A.; et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 18909–18914. [Google Scholar] [CrossRef]
- Campbell, J.; Singh, A.K.; Santa Maria, J.P., Jr.; Kim, Y.; Brown, S.; Swoboda, J.G.; Mylonakis, E.; Wilkinson, B.J.; Walker, S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011, 6, 106–116. [Google Scholar] [CrossRef]
- Wang, H.; Gill, C.J.; Lee, S.H.; Mann, P.; Zuck, P.; Meredith, T.C.; Murgolo, N.; She, X.; Kales, S.; Liang, L.; et al. Discovery of wall teichoic acid inhibitors as potential anti-MRSA β-lactam combination agents. Chem. Biol. 2013, 20, 272–284. [Google Scholar] [CrossRef]
- Kohler, T.; Weidenmaier, C.; Peschel, A. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J. Bacteriol. 2009, 191, 4482–4484. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kulauzovic, E.; Kohler, T.; Thumm, G.; Stoll, H.; Götz, F.; Peschel, A. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int. J. Med. Microbiol. 2008, 298, 505–513. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A.; Xiong, Y.Q.; Kristian, S.A.; Dietz, K.; Yeaman, M.R.; Bayer, A.S. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 2005, 191, 1771–1777. [Google Scholar] [CrossRef]
- van Dalen, R.; Peschel, A.; van Sorge, N.M. Wall teichoic acid in Staphylococcus aureus host interaction. Trends Microbiol. 2020, 28, 985–998. [Google Scholar] [CrossRef]
- Frankel, M.B.; Schneewind, O. Determinants of murein hydrolase targeting to cross-wall of Staphylococcus aureus peptidoglycan. J. Biol. Chem. 2012, 287, 10460–10471. [Google Scholar] [CrossRef]
- Schlag, M.; Biswas, R.; Krismer, B.; Kohler, T.; Zoll, S.; Yu, W.; Schwarz, H.; Peschel, A.; Götz, F. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 2010, 75, 864–873. [Google Scholar] [CrossRef]
- Kajimura, J.; Fujiwara, T.; Yamada, S.; Suzawa, Y.; Nishida, T.; Oyamada, Y.; Hayashi, I.; Yamagishi, J.; Komatsuzawa, H.; Sugai, M. Identification and molecular characterization of an N-acetylmuramyl-L-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 2005, 58, 1087–1101. [Google Scholar] [CrossRef]
- Tiwari, K.B.; Gatto, C.; Walker, S.; Wilkinson, B.J. Exposure of Staphylococcus aureus to Targocil blocks translocation of the major autolysin Atl across the membrane, resulting in a significant decrease in autolysis. Antimicrob. Agents Chemother. 2018, 62, e00323-18. [Google Scholar] [CrossRef]
- Thalsø-Madsen, I.; Torrubia, F.R.; Xu, L.; Petersen, A.; Jensen, C.; Frees, D. The Sle1 cell wall amidase is essential for β-Lactam resistance in community-acquired methicillin-resistant Staphylococcus aureus USA300. Antimicrob. Agents Chemother. 2019, 64, e01931-19. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Qin, J.; Cheng, S.; Yeo, W.S.; He, L.; Ma, X.; Liu, X.; Li, M.; Bae, T. The ATP-dependent protease ClpP inhibits biofilm formation by regulating Agr and cell wall hydrolase Sle1 in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2017, 7, 181. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef]
- Mann, P.A.; Müller, A.; Wolff, K.A.; Fischmann, T.; Wang, H.; Reed, P.; Hou, Y.; Li, W.; Müller, C.E.; Xiao, J.; et al. Chemical genetic analysis and functional characterization of Staphylococcal wall teichoic acid 2-epimerases reveals unconventional antibiotic drug targets. PLoS Pathog. 2016, 12, e1005585. [Google Scholar] [CrossRef]
- Reichmann, N.T.; Cassona, C.P.; Gründling, A. Revised mechanism of D-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 2013, 159, 1868–1877. [Google Scholar] [CrossRef]
- Coupri, D.; Verneuil, N.; Hartke, A.; Liebaut, A.; Lequeux, T.; Pfund, E.; Budin-Verneuil, A. Inhibition of D-alanylation of teichoic acids overcomes resistance of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2021, 76, 2778–2786. [Google Scholar] [CrossRef]
- Kovács, M.; Halfmann, A.; Fedtke, I.; Heintz, M.; Peschel, A.; Vollmer, W.; Hakenbeck, R.; Brückner, R. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 5797–5805. [Google Scholar] [CrossRef]
- Mechler, L.; Bonetti, E.J.; Reichert, S.; Flötenmeyer, M.; Schrenzel, J.; Bertram, R.; François, P.; Götz, F. Daptomycin tolerance in the Staphylococcus aureus pitA6 mutant is due to upregulation of the dlt operon. Antimicrob. Agents Chemother. 2016, 60, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Campbell, J.; Swoboda, J.G.; Cuny, G.D.; Walker, S. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2010, 20, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Swoboda, J.G.; Campbell, J.; Walker, S.; Gilmore, M.S. In vitro antimicrobial activity of wall teichoic acid biosynthesis inhibitors against Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2011, 55, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Singh, A.K.; Swoboda, J.G.; Gilmore, M.S.; Wilkinson, B.J.; Walker, S. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 1810–1820. [Google Scholar] [CrossRef]
- Farha, M.A.; Czarny, T.L.; Myers, C.L.; Worrall, L.J.; French, S.; Conrady, D.G.; Wang, Y.; Oldfield, E.; Strynadka, N.C.; Brown, E.D. Antagonism screen for inhibitors of bacterial cell wall biogenesis uncovers an inhibitor of undecaprenyl diphosphate synthase. Proc. Natl. Acad. Sci. USA 2015, 112, 11048–11053. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Onyedibe, K.I.; Sintim, H.O. Lipoteichoic acid biosynthesis inhibitors as potent inhibitors of S. aureus and E. faecalis growth and biofilm formation. Molecules 2020, 25, 2277. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, H.; Labroli, M.; Koseoglu, S.; Zuck, P.; Mayhood, T.; Gill, C.; Mann, P.; Sher, X.; Ha, S.; et al. TarO-specific inhibitors of wall teichoic acid biosynthesis restore β-lactam efficacy against methicillin-resistant Staphylococci. Sci. Transl. Med. 2016, 8, 329ra32. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Singh, A.K.; Drolia, R.; Bai, X.; Tenguria, S.; Bhunia, A.K. Tunicamycin mediated inhibition of wall teichoic acid affects Staphylococcus aureus and Listeria monocytogenes cell morphology, biofilm formation and virulence. Front. Microbiol. 2018, 9, 1352. [Google Scholar] [CrossRef]
- Pinho, M.G.; Filipe, S.R.; de Lencastre, H.; Tomasz, A. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 2001, 183, 6525–6531. [Google Scholar] [CrossRef]
- Hamilton, S.M.; Alexander, J.A.N.; Choo, E.J.; Basuino, L.; da Costa, T.M.; Severin, A.; Chung, M.; Aedo, S.; Strynadka, N.C.J.; Tomasz, A.; et al. High-level resistance of Staphylococcus aureus to β-Lactam antibiotics mediated by Penicillin-Binding Protein 4 (PBP4). Antimicrob. Agents Chemother. 2017, 61, e02727-16. [Google Scholar] [CrossRef]
- Lee, S.H.; Jarantow, L.W.; Wang, H.; Sillaots, S.; Cheng, H.; Meredith, T.C.; Thompson, J.; Roemer, T. Antagonism of chemical genetic interaction networks resensitize MRSA to β-lactam antibiotics. Chem. Biol. 2011, 18, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.G.; Errington, J. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol. Microbiol. 2003, 50, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.H.; Rojas-Altuve, A.; Llarrull, L.I.; Carrasco-López, C.; Kumarasiri, M.; Lastochkin, E.; Fishovitz, J.; Dawley, M.; Hesek, D.; Lee, M.; et al. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc. Natl. Acad. Sci. USA 2013, 110, 16808–16813. [Google Scholar] [CrossRef] [PubMed]
- Acebrón, I.; Chang, M.; Mobashery, S.; Hermoso, J.A. The allosteric site for the nascent cell wall in Penicillin-Binding Protein 2a: An Achilles’ heel of methicillin-resistant Staphylococcus aureus. Curr. Med. Chem. 2015, 22, 1678–1686. [Google Scholar] [CrossRef]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell 2017, 171, 1354–1367.e1320. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Makris, G.C.; Matthaiou, D.K.; Rafailidis, P.I. Statins for infection and sepsis: A systematic review of the clinical evidence. J. Antimicrob. Chemother. 2008, 61, 774–785. [Google Scholar] [CrossRef]
- Tralhão, A.F.; Cés de Souza-Dantas, V.; Salluh, J.I.; Póvoa, P.M. Impact of statins in outcomes of septic patients: A systematic review. Postgrad. Med. 2014, 126, 45–58. [Google Scholar] [CrossRef]
- Almog, Y.; Shefer, A.; Novack, V.; Maimon, N.; Barski, L.; Eizinger, M.; Friger, M.; Zeller, L.; Danon, A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 2004, 110, 880–885. [Google Scholar] [CrossRef]
- López-Cortés, L.E.; Gálvez-Acebal, J.; Del Toro, M.D.; Velasco, C.; de Cueto, M.; Caballero, F.J.; Muniain, M.A.; Pascual, A.; Rodríguez-Baño, J. Effect of statin therapy in the outcome of bloodstream infections due to Staphylococcus aureus: A prospective cohort study. PLoS ONE 2013, 8, e82958. [Google Scholar] [CrossRef]
- Casiraghi, A.; Suigo, L.; Valoti, E.; Straniero, V. Targeting bacterial cell division: A binding site-centered approach to the most promising inhibitors of the essential protein FtsZ. Antibiotics 2020, 9, 69. [Google Scholar] [CrossRef]
- Barrows, J.M.; Goley, E.D. FtsZ dynamics in bacterial division: What, how, and why? Curr. Opin. Cell Biol. 2021, 68, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.; Therien, A.G.; Lu, J.; Lee, S.H.; Caron, A.; Gill, C.J.; Lebeau-Jacob, C.; Benton-Perdomo, L.; Monteiro, J.M.; Pereira, P.M.; et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci. Transl. Med. 2012, 4, 126ra135. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P.; Anderson, D.E.; Osawa, M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 2010, 74, 504–528. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-González, E.; Kaul, M.; Parhi, A.K.; LaVoie, E.J.; Pilch, D.S. β-Lactam antibiotics with a high affinity for PBP2 act synergistically with the FtsZ-targeting agent TXA707 against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e00863-17. [Google Scholar] [CrossRef]
- Ferrer-González, E.; Huh, H.; Al-Tameemi, H.M.; Boyd, J.M.; Lee, S.H.; Pilch, D.S. Impact of FtsZ inhibition on the localization of the penicillin binding proteins in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2021, 203, e0020421. [Google Scholar] [CrossRef]
- Kaul, M.; Mark, L.; Parhi, A.K.; LaVoie, E.J.; Pilch, D.S. Combining the FtsZ-targeting prodrug TXA709 and the Cephalosporin Cefdinir confers synergy and reduces the frequency of resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 4290–4296. [Google Scholar] [CrossRef]
- Sun, N.; Ban, L.; Li, M.; Fang, Z.; Li, X.; Yao, W.; Pan, J.; Lu, Y.; Liu, Z.; Wong, W.L. Probing the benzofuroquinolinium derivative as a potent antibacterial agent through the inhibition of FtsZ activity. J. Pharmacol. Sci. 2018, 138, 83–85. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, L.; Kang, G.; Wang, P.; Yin, H.; Huang, H. A potential combination therapy of berberine hydrochloride with antibiotics against multidrug-resistant Acinetobacter baumannii. Front. Cell Infect. Microbiol. 2021, 11, 660431. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Wang, S.; Kang, O.H.; Kwon, D.Y. Trans-cinnamaldehyde exhibits synergy with conventional antibiotic against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 2752. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of trans-cinnamaldehyde on methicillin-resistant Staphylococcus aureus biofilm formation: Metabolic activity assessment and analysis of the biofilm-associated genes expression. Int. J. Mol. Sci. 2019, 21, 102. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Grużewska, A.; Lohinau, D. The effects of selected phytochemicals on biofilm formed by five methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ban, L.; Li, Y.; Yuan, W.; Liu, Z.; Liu, T.; Li, X.; Wong, K.Y.; Lu, Y.; Sun, N.; et al. A quinoline-based FtsZ inhibitor for the study of antimicrobial activity and synergistic effects with β-lactam antibiotics. J. Pharmacol. Sci. 2018, 137, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.J.; Stokes, N.R.; Ure, R.; Galbraith, G.; Bennett, J.M.; Brown, D.R.; Baker, P.J.; Barynin, V.V.; Rice, D.W.; Sedelnikova, S.E.; et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 2008, 321, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.M.; Schaffner-Barbero, C.; Huecas, S.; Alonso, D.; Lopez-Rodriguez, M.L.; Ruiz-Avila, L.B.; Núñez-Ramírez, R.; Llorca, O.; Martín-Galiano, A.J. The antibacterial cell division inhibitor PC190723 is an FtsZ polymer-stabilizing agent that induces filament assembly and condensation. J. Biol. Chem. 2010, 285, 14239–14246. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.Y.; Sun, N.; Leung, Y.C.; Wong, K.Y. Antimicrobial activity of a quinuclidine-based FtsZ inhibitor and its synergistic potential with β-lactam antibiotics. J. Antibiot. 2015, 68, 253–258. [Google Scholar] [CrossRef]



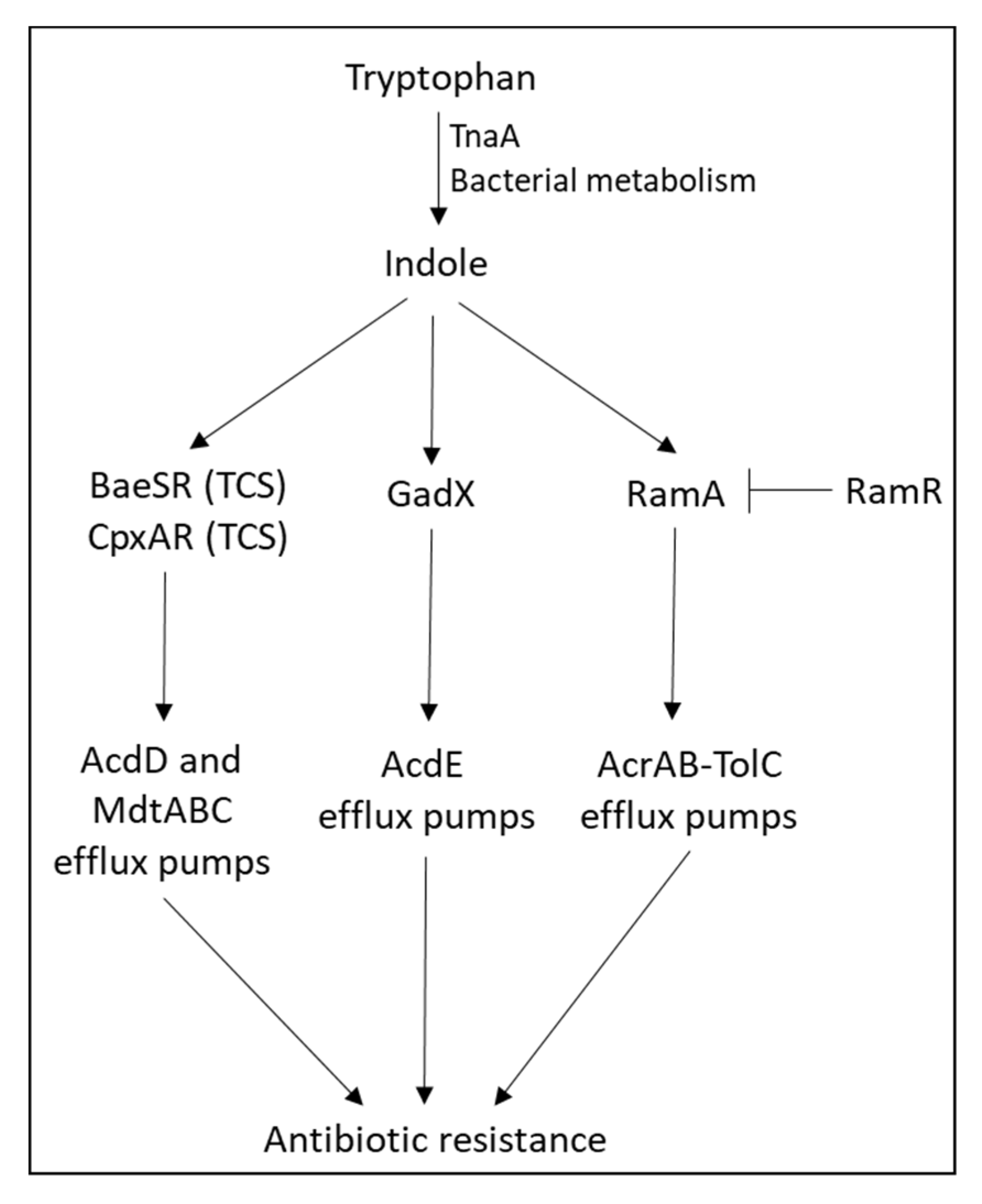


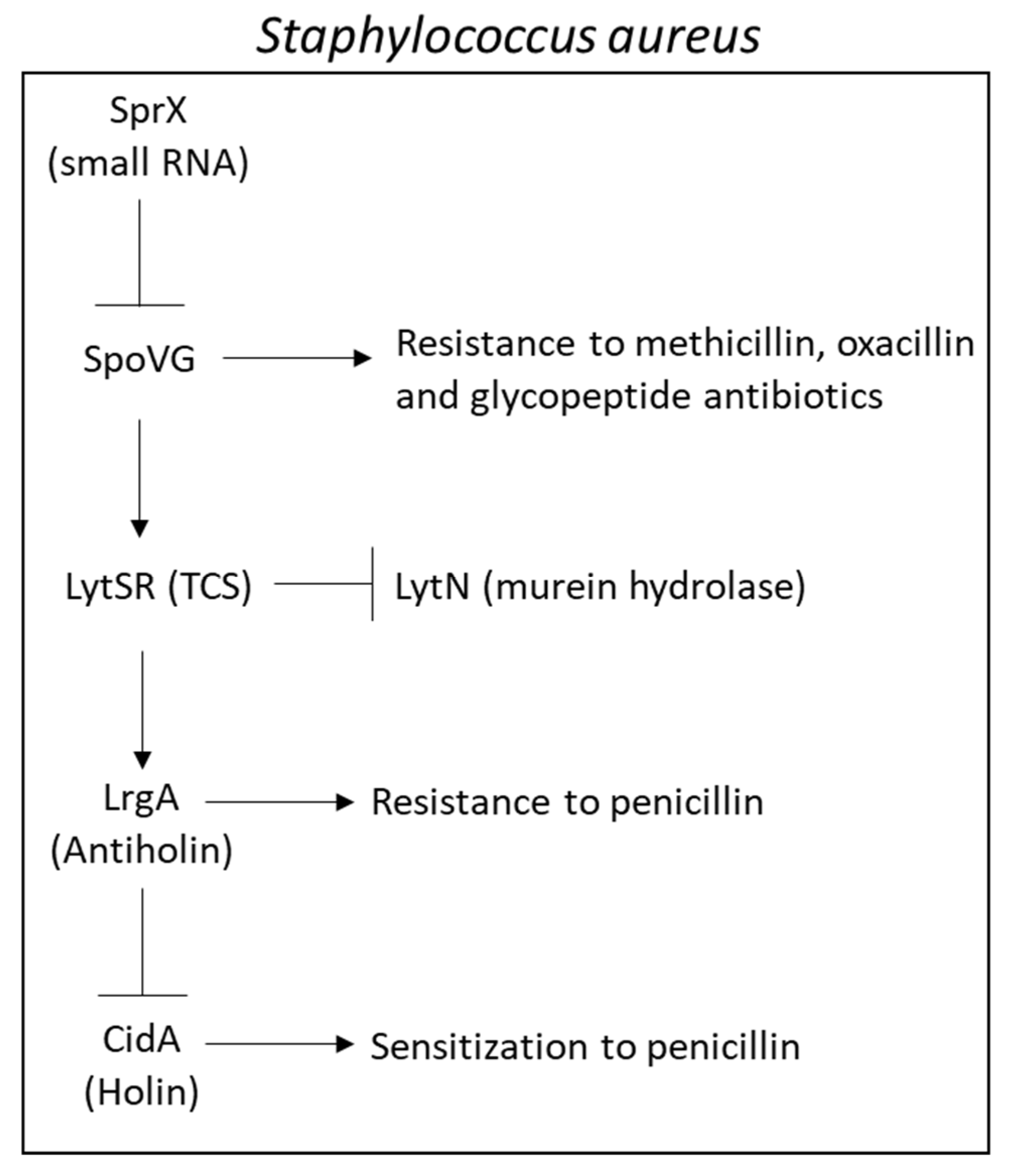
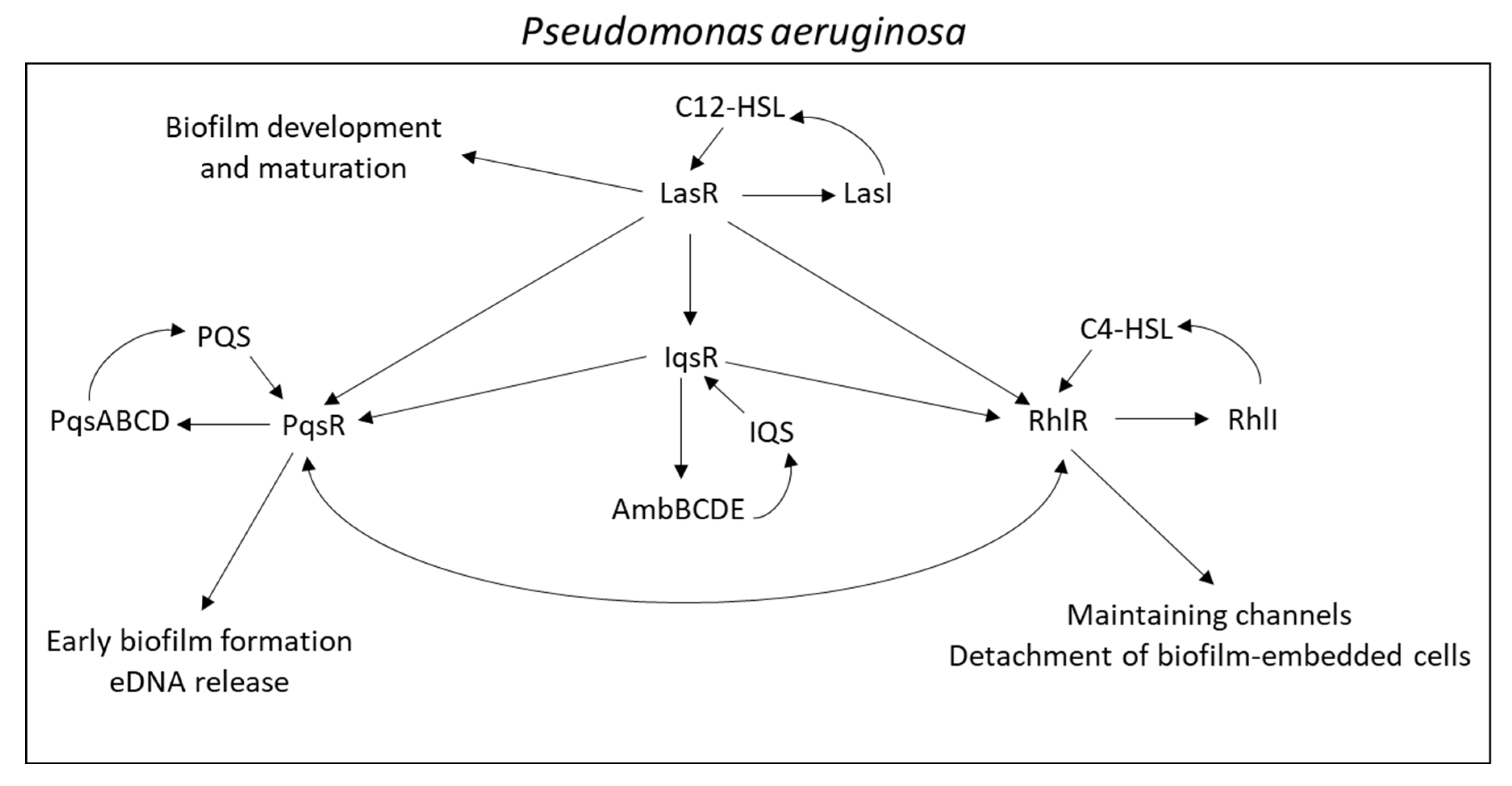

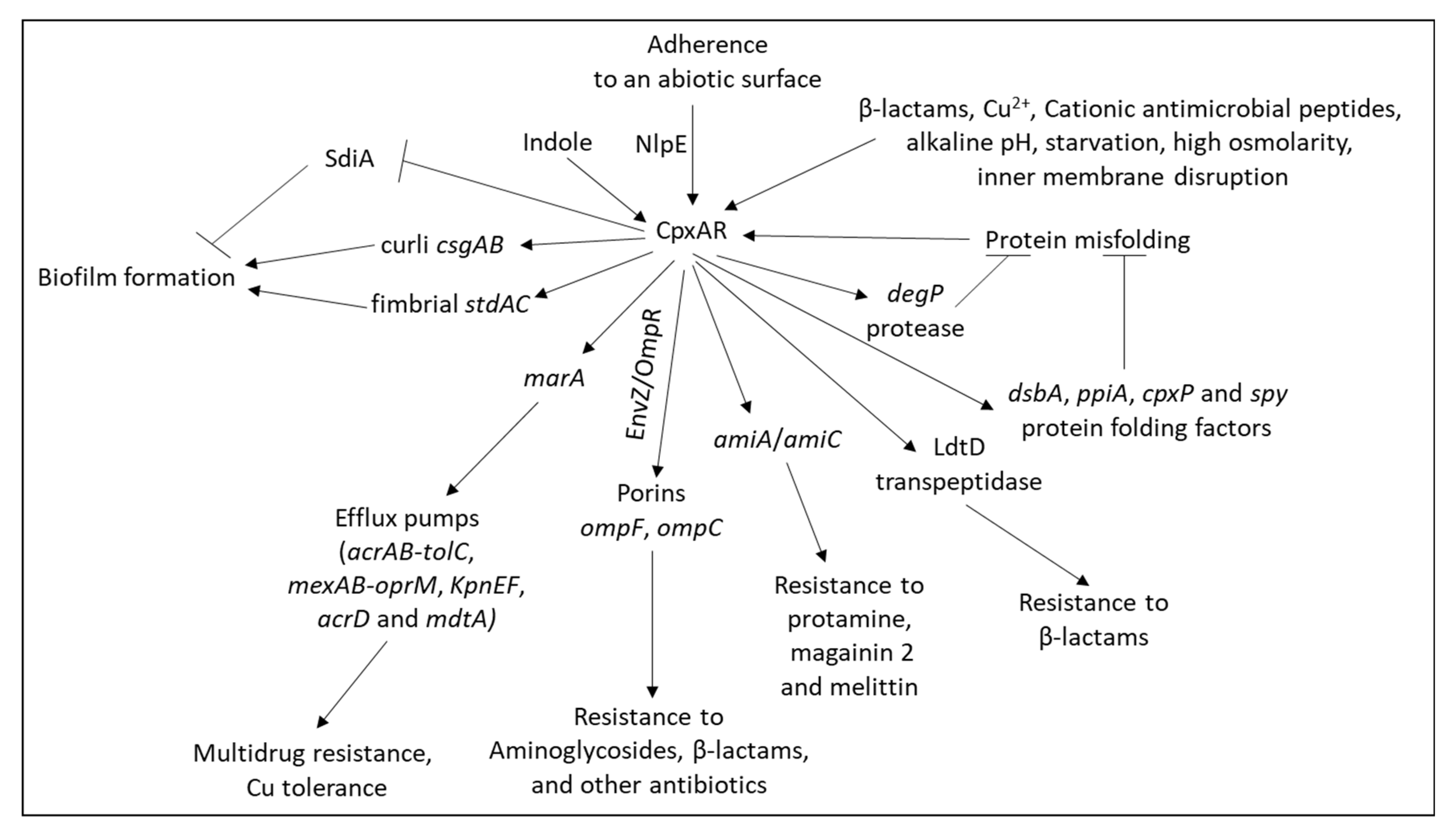
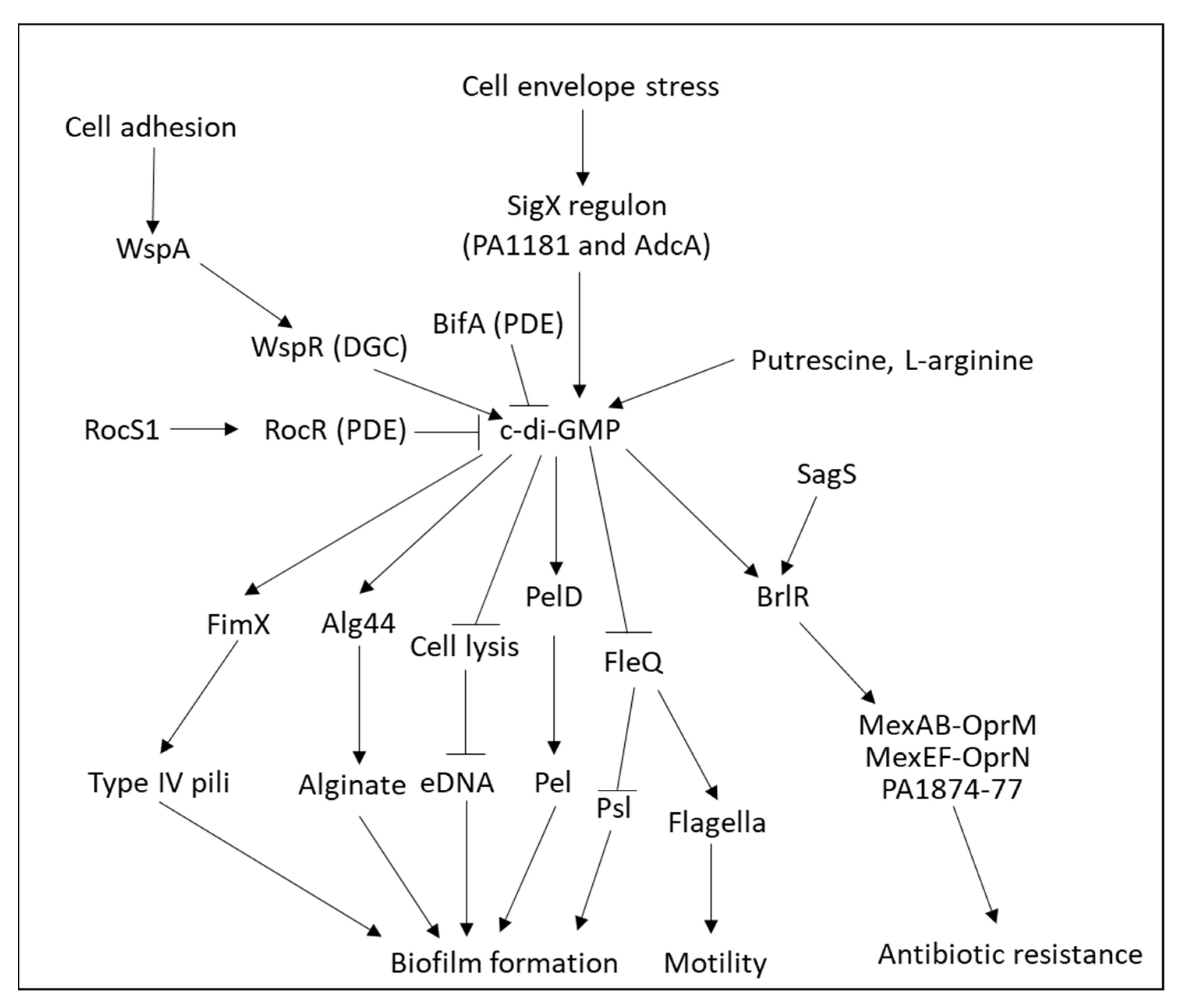

| TCS Involved in Antibiotic Resistance | Function | Bacterial Species | References |
|---|---|---|---|
| AdeRS |
| Acinetobacter baumannii | [226,389,390,391,392] |
| AirSR (YhcSR) |
| Staphylococcus aureus | [393,394] |
| AmgRS |
| Pseudomonas aeruginosa | [52,197,395,396,397] |
| ApsRS |
| Staphylococcus aureus | [381,382,383] |
| ArlRS |
| Staphylococcus aureus | [182,398,399,400] |
| BaeSR |
| Escherichia coli, Salmonella typhimurium, Acinetobacter baumannii | [401,402,403,404,405,406] |
| BasSR |
| Escherichia coli | [407] |
| BqsRS |
| Pseudomonas aeruginosa | [408] |
| BraRS |
| Staphylococcus aureus | [409,410] |
| ChtRS |
| Enterococcus faecium | [411] |
| CiaRH |
| Streptococcus pneumoniae | [412,413,414] |
| ColRS |
| Pseudomonas aeruginosa | [415] |
| CopRS |
| Pseudomonas aeruginosa | [416,417] |
| CprRS |
| Pseudomonas aeruginosa | [418,419] |
| CpxAR |
| Escherichia coli, Klebsiella pneumoniae, Salmonella typhimurium and other Gram-negative bacteria | [420,421,422,423,424,425] |
| CreBC |
| Pseudomonas aeruginosa | [426] |
| CroRS |
| Enterococcus faecium | [427,428,429,430] |
| CzcRS |
| Pseudomonas aeruginosa | [416,431,432,433] |
| EvgAS |
| Escherichia coli | [434,435,436,437,438] |
| GraRS (ApsRS) |
| Staphylococcus aureus | [410,439,440,441,442,443] |
| LiaFSR |
| Enterococcus faecium, Enterococcus faecalis | [210,444] |
| LrgAB |
| Staphylococcus aureus | [289] |
| LytSR |
| Staphylococcus aureus | [445,446] |
| NsaRS |
| Staphylococcus aureus | [447,448] |
| ParRS |
| Pseudomonas aeruginosa | [66,189,419,449,450] |
| PhoBR |
| Klebsiella pneumoniae | [451] |
| PhoPQ and PmrAB |
| Pseudomonas aeruginosa, Salmonella enterica, Klebsiella pneumoniae, Acinetobacter baumannii | [91,419,452,453,454,455,456,457,458,459,460] |
| QseBC |
| Escherichia coli | [249] |
| TarRS |
| Pseudomonas aeruginosa | [461] |
| VanRS |
| Enterococcus faecium | [462] |
| VraTSR |
| Staphylococcus aureus | [463,464,465,466,467] |
| WalKR (YycFG, VicRK, MicAB) |
| Staphylococcus aureus | [468,469,470] |
| Compound | Effects on Bacteria | References |
|---|---|---|
| Ajoene |
| [823,824] |
| Allicin |
| [825] |
| Baicalin (5,6,7- trihydroxyflavone) and 3,5,7- Trihydroxyflavone |
| [531,826,827,828,829,830] |
| Berberine |
| [831,832] |
| Betulin and betulinic acid |
| [833] |
| meta- Bromothiolactone |
| [834] |
| Cajaninstilbene acid analogs |
| [835] |
| Cannabigerol (CBG) and Cannabidiol (CBD) |
| [803,804,805,836,837,838,839] |
| Carvacrol |
| [819,840,841,842] |
| Cinnamic acid |
| [843,844] |
| Cinnamaldehyde |
| [845,846,847,848,849,850,851] |
| Clofoctol |
| [852] |
| Clotrimazole and miconazole |
| [852] |
| Curcumin |
| [853,854,855,856,857,858,859] |
| Domperidone |
| [860] |
| Falcarindol |
| [861] |
| Flavonoids |
| [862,863,864,865] |
| 4-Fluoro- 5-hydroxypentane-2,3-dione (F-DPD) |
| [866] |
| Gingerol |
| [867,868] |
| Halogenated furanones |
| [869,870,871,872,873,874] |
| Halogenated thiophenones |
| [875,876,877] |
| Hamamelitannin |
| [531] |
| 4- Hydroxybenzylidene indolinone |
| [878,879] |
| Luteolin |
| [880] |
| Methyl anthranilate |
| [881,882] |
| 2-[(Methylamino) methyl]phenol |
| [883] |
| MHY1383 and MHY1387 |
| [884] |
| MomL |
| [885,886] |
| Mosloflavone |
| [887] |
| Niclosamide |
| [888] |
| Nitrofurazone and erythromycin estolate |
| [889] |
| Oritavancin |
| [399] |
| An Oxoquinazolin derivate |
| [890] |
| Paeonol |
| [891] |
| Palmitoyl- DL-carnitine |
| [892] |
| Parthenolide |
| [893] |
| Phenyl lactic acid |
| [894] |
| N-(2-Pyrimidyl) butanamide |
| [895] |
| Quinazolinone analogs |
| [351,896] |
| Quercetin |
| [862,867,897,898,899,900] |
| Resveratrol(3,5,4′- trihydroxystilbene) |
| [901,902,903,904] |
| Rifampicin |
| [905,906] |
| Sesamin and Sesamolin lignans |
| [818] |
| Sinefungin |
| [907] |
| Staquorsin |
| [908] |
| Tryptanthrin |
| [909] |
| Tryptophan-containing antibacterial peptides |
| [820,910] |
| Unsaturated fatty acids |
| [911,912,913,914,915,916,917,918,919,920,921] |
| Zingerone |
| [867,922,923] |
| Walkmycin B |
| [924] |
| Compound | Effects on Bacteria | References |
|---|---|---|
| Astilbin |
| [945] |
| Trans-Chalcone |
| [944] |
| Chitosan |
| [950,951,952] |
| Clemastine |
| [953] |
| Compound 62520 (5-fluoro-1-((1R,3S)- 3-(hyroxymethyl)- 1,3-dihydroisobenzofuran- 1-yl)pyrimidine- 2,4-(1H,3H)-dione) |
| [122] |
| DMNP—A diterpene analog |
| [954] |
| Epigallocatechin- 3-gallate (EGCG) |
| [955,956,957] |
| Eugenol/carvacrol |
| [958] |
| Gallium nitrate |
| [959,960,961,962,963] |
| 5-Episinuleptolide |
| [964] |
| Flavonoids |
| [156] |
| 5-Hydroxymethylfurfural |
| [965] |
| Kaempferol |
| [966,967] |
| α-Mangostin |
| [968] |
| Meloxicam |
| [969,970] |
| ML346 |
| [971] |
| Myrtenol |
| [972,973] |
| Orientin |
| [974] |
| 1,2,4- Oxadiazole derivatives |
| [943] |
| Pyrimidinedione |
| [975] |
| Pyrogallol |
| [976] |
| Quercetin and tannic acid |
| [977,978,979,980] |
| Taxifolin |
| [949] |
| Thiazolidinediones (e.g., ciglitazone, TZD-C8, and thiazolidinedione-8) |
| [981,982,983,984] |
| Ursolic acid and Asiatic acid |
| [903,985,986] |
| Virstatin (4- [N-(1,8-naphthalimide)]- n-butyric acid) |
| [987,988,989] |
| Zerumbone |
| [990] |
| Compound | Effects on Bacteria | References |
|---|---|---|
| Alkylaminoquinolines |
| [999] |
| 2-(2-Aminophenyl) indole |
| [1000] |
| 6-(Aryl)alkoxypyridine- 3-boronic acid derivatives |
| [1001] |
| Arylpiperazines such as 1-(1-naphthylmethyl)-piperazine (NMP) |
| [810] |
| Berberine |
| [1002,1003] |
| Boeravinone B |
| [786] |
| Boronic acid |
| [1001] |
| Capsaicin |
| [1004] |
| Carvacrol |
| [1005] |
| Conessine |
| [1006,1007] |
| D13-9001(A 4-oxo- 4H-pyrido [1,2-a] pyrimidine derivative) |
| [810,1008] |
| Doxorubicin |
| [810] |
| Endocannabinoids |
| [789,1009,1010] |
| Eugenol derivatives |
| [1011] |
| IITR08027 |
| [995] |
| Isoliquiritigenin derivates (e.g., IMRG4) |
| [1012] |
| Ketoconazole |
| [787] |
| MBX2319 (A pyranopyridine) |
| [810,1013] |
| Meropenem |
| [1014] |
| Metformin |
| [1015] |
| Minocycline |
| [810] |
| 1-(1-Naphthylmethyl) piperazine |
| [153] |
| Nilotinib |
| [154] |
| 5-Nitro-2-(3-phenylpropoxy)pyridine |
| [1016] |
| Phenylalanine arginyl β-naphthylamide (PAβN) |
| [45,153,155,810,1017] |
| Phenothiazines such as chlorpromazine, thioridazine and amitriptyline |
| [153,155,774,996,1018,1019,1020] |
| 4-Phenylbenzylidene derivatives |
| [1021] |
| Piperazine derivatives |
| [1022] |
| Piperine analogs |
| [1023] |
| Raloxifene and pyrvinium |
| [1024] |
| Reserpine |
| [1025,1026,1027,1028] |
| Resveratrol |
| [1029,1030,1031,1032,1033] |
| Thiazolidinedione derivatives |
| [981,982,984,1034,1035,1036] |
| Thymol |
| [1005] |
| Totarol |
| [1037] |
| Trimethoprim |
| [1038] |
| Verapamil |
| [1015,1039] |
| Compound | Effects on Bacteria | References |
|---|---|---|
| A benzofuroquinolinium derivative |
| [1088] |
| Berberine |
| [1089,1090] |
| Cinnamaldehyde |
| [1091,1092,1093,1094] |
| 1-Methylquinolinium iodide derivative |
| [1095] |
| PC190723 |
| [1083,1096,1097] |
| Quinuclidine 1 |
| [1098] |
| TXA707 |
| [1085] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. https://doi.org/10.3390/microorganisms10061239
Sionov RV, Steinberg D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms. 2022; 10(6):1239. https://doi.org/10.3390/microorganisms10061239
Chicago/Turabian StyleSionov, Ronit Vogt, and Doron Steinberg. 2022. "Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria" Microorganisms 10, no. 6: 1239. https://doi.org/10.3390/microorganisms10061239
APA StyleSionov, R. V., & Steinberg, D. (2022). Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms, 10(6), 1239. https://doi.org/10.3390/microorganisms10061239






