Ingenious Action of Vibrio cholerae Neuraminidase Recruiting Additional GM1 Cholera Toxin Receptors for Primary Human Colon Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Primary Human Colon Epithelial Cells
2.2. Extraction of Lipids and Isolation of Gangliosides from Primary Human Colon Epithelial Cells
2.3. Choleragenoid, Antibodies, Neuraminidases and Ganglioside Reference
2.4. Thin-Layer Chromatography and Detection of GM1 Using Choleragenoid Combined with Neuraminidase Treatment
2.5. Structural Characterization of Gangliosides by Electrospray Ionization Mass Spectrometry
3. Results
3.1. Action of Bacterial Neuraminidases on Reference Ganglio-Series Gangliosides with GM1-Core
3.2. TLC Overlay Detection of Choleragenoid-Binding GM1 Using Reference Gangliosides from Human Brain
3.3. Mass Spectrometric Characterization of Choleragenoid-Binding GM1 Lipoforms in Human Brain Gangliosides
3.4. TLC Overlay Detection of Choleragenoid-Binding GM1 in Ganglioside Preparations from pHCoEpiCs
3.5. Mass Spectrometric Characterization of Choleragenoid-Binding GM1 Lipoforms in Ganglioside Preparations of pHCoEpiCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, S.; Mandal, M.D.; Pal, N.K. Cholera: A great global concern. Asian Pac. Trop. Med. 2011, 4, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.B.; LaRocque, R.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Ramamurthy, T.; Nandy, R.K.; Mukhopadhyay, A.K.; Dutta, S.; Mutreja, A.; Okamoto, K.; Miyoshi, S.I.; Nair, G.B.; Ghosh, A. Virulence regulation and innate host response in the pathogenicity of Vibrio cholerae. Front. Cell. Infect. Microbiol. 2020, 10, 572096. [Google Scholar] [CrossRef]
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera toxin B: One subunit with many pharmaceutical applications. Toxins 2015, 7, 974–996. [Google Scholar] [CrossRef] [Green Version]
- Spangler, B.D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 1992, 56, 622–647. [Google Scholar] [CrossRef]
- Zhang, R.G.; Westbrook, M.L.; Westbrook, E.M.; Scott, D.L.; Otwinowski, Z.; Maulik, P.R.; Reed, R.A.; Shipley, G.G. The 2.4 Å crystal structure of cholera toxin B subunit pentamer: Choleragenoid. J. Mol. Biol. 1995, 251, 550–562. [Google Scholar] [CrossRef]
- Zhang, R.G.; Scott, D.L.; Westbrook, M.L.; Nance, S.; Spangler, B.D.; Shipley, G.G.; Westbrook, E.M. The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 1995, 251, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Lencer, W.I.; Saslowsky, D. Raft trafficking of AB5 subunit bacterial toxins. Biochim. Biophys. Acta 2005, 1746, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Chinnapen, D.J.; Chinnapen, H.; Saslowsky, D.; Lencer, W.I. Rafting with cholera toxin: Endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol. Lett. 2007, 266, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Wernick, N.L.; Chinnapen, D.J.; Cho, J.A.; Lencer, W.I. Cholera toxin: An intracellular journey into the cytosol by way of the endoplasmic reticuluim. Toxins 2010, 2, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Lencer, W.I.; Tsai, B. The intracellular voyage of cholera toxin: Going retro. Trends Biochem. Sci. 2003, 28, 639–645. [Google Scholar] [CrossRef]
- Sánchez, J.; Holmgren, J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008, 65, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Saslowsky, D.E.; te Welscher, Y.M.; Chinnapen, D.J.; Wagner, J.S.; Wan, J.; Kern, E.; Lencer, W.I. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J. Biol. Chem. 2013, 288, 25804–25809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, R.; Wiegandt, H. Die Konstitution der Ganglio-N-tetraose und des Gangliosids GI. Chem. Ber. 1963, 96, 866–880. [Google Scholar] [CrossRef]
- Chester, M.A. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids—recommendations 1997. Eur. J. Biochem. 1998, 257, 293–298. [Google Scholar] [CrossRef]
- Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Toffano, G.; Secchieri, C.; Sonnino, S. GM1 ganglioside: Past studies and future potential. Mol. Neurobiol. 2016, 53, 1824–1842. [Google Scholar] [CrossRef]
- Svennerholm, S. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef]
- Svennerholm, L. The gangliosides. J. Lipid Res. 1964, 5, 145–155. [Google Scholar] [CrossRef]
- Holmgren, J.; Lönnroth, I.; Svennerholm, L. Tissue receptor for cholera exotoxin: Postulated structure from studies with GM1 ganglioside and related glycolipids. Infect. Immun. 1973, 8, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Fishman, P.H. Role of membrane gangliosides in the binding and action of bacterial toxins. J. Membr. Biol. 1982, 69, 85–97. [Google Scholar] [CrossRef]
- Holmgren, J.; Lönnroth, I.; Månsson, J.E.; Svennerholm, L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc. Natl. Acad. Sci. USA 1975, 72, 2520–2524. [Google Scholar] [CrossRef] [Green Version]
- Detzner, J.; Krojnewski, E.; Pohlentz, G.; Steil, D.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Shiga toxin (Stx)-binding glycosphingolipids of primary human renal cortical epithelial cells (pHRCEpiCs) and Stx-mediated cytotoxicity. Toxins 2021, 13, 139. [Google Scholar] [CrossRef]
- Detzner, J.; Klein, A.L.; Pohlentz, G.; Krojnewski, E.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Primary human renal proximal tubular epithelial cells (pHRPTEpiCs): Shiga toxin (Stx) glycosphingolipid receptors, Stx susceptibility, and interaction with membrane microdomains. Toxins 2021, 13, 529. [Google Scholar] [CrossRef]
- Detzner, J.; Püttmann, C.; Pohlentz, G.; Humpf, H.-U.; Mellmann, A.; Karch, H.; Müthing, J. Primary human colon epithelial cells (pHCoEpiCs) do express the Shiga toxin (Stx) receptor glycosphingolipids Gb3Cer and Gb4Cer and are largely refractory but not resistant towards Stx. Int. J. Mol. Sci. 2021, 22, 10002. [Google Scholar] [CrossRef] [PubMed]
- Müthing, J.; Egge, H.; Kniep, B.; Mühlradt, P.F. Structural characterization of gangliosides from murine T lymphocytes. Eur. J. Biochem. 1987, 163, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Müthing, J.; Pörtner, A.; Jäger, V. Ganglioside alterations in YAC-1 cells cultivated in serum-supplemented and serum-free growth medium. Glycoconj. J. 1992, 9, 265–273. [Google Scholar] [CrossRef]
- Müthing, J. High-resolution thin-layer chromatography of gangliosides. J. Chromatogr. A 1996, 720, 3–25. [Google Scholar] [CrossRef]
- Detzner, J.; Pohlentz, G.; Müthing, J. Thin-layer chromatography in structure and recognition studies of Shiga toxin glycosphingolipid receptors. Methods Mol. Biol. 2021, 2291, 229–252. [Google Scholar] [CrossRef] [PubMed]
- Radsak, K.; Schwarzmann, G.; Wiegandt, H. Studies on the cell association of exogenously added sialo-glycolipids. Hoppe Seylers Z. Physiol. Chem. 1982, 363, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Saqr, H.E.; Pearl, D.K.; Yates, A.J. A review and predictive models of ganglioside uptake by biological membranes. J. Neurochem. 1993, 61, 395–411. [Google Scholar] [CrossRef]
- Schwarzmann, G. Uptake and metabolism of exogenous glycosphingolipids by cultured cells. Semin. Cell Dev. Biol. 2001, 12, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef] [Green Version]
- Müthing, J.; Distler, U. Advances on the compositional analysis of glycosphingolipids combining thin-layer chromatography with mass spectrometry. Mass Spectrom. Rev. 2010, 29, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Sonnino, S.; Chigorno, V. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim. Biophys. Acta 2000, 1469, 63–77. [Google Scholar] [CrossRef]
- Lunghi, G.; Fazzari, M.; Di Biase, E.; Mauri, L.; Chiricozzi, E.; Sonnino, S. The structure of gangliosides hides a code for determining neuronal functions. FEBS Open Bio 2021, 11, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.N.; Colsch, B.; Egan, T.; Lewis, E.K.; Schultz, J.A.; Woods, A.S. Gangliosides’ analysis by MALDI-ion mobility MS. Analyst 2011, 136, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Gangliosides of the vertebrate nervous system. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef] [Green Version]
- Berenson, C.S.; Nawar, H.F.; Kruzel, R.L.; Mandell, L.M.; Connell, T.D. Ganglioside-binding specificities of E. coli enterotoxin LT-IIc: Importance of long-chain fatty acyl ceramide. Glycobiology 2013, 23, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, H.; Römer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Badizadegan, K.; Wolf, A.A.; Rodighiero, C.; Jobling, M.; Hirst, T.R.; Holmes, R.K.; Lencwer, W.I. Floating cholera toxin into epithelial cells: Functional association with caveolae-like detergent-insoluble membrane microdomains. Int. J. Med. Microbiol. 2020, 290, 403–408. [Google Scholar] [CrossRef]
- Kabbani, A.M.; Raghunathan, K.; Lencer, W.I.; Kenworthy, A.K.; Kelly, C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 14978–14986. [Google Scholar] [CrossRef]
- Chinnapen, D.J.; Hsieh, W.T.; te Welscher, Y.M.; Saslowsky, D.; Kaoutzani, L.; Brandsma, E.; D’Auria, L.; Park, H.; Wagner, J.S.; Drake, K.R.; et al. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell 2012, 23, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltwisch, J.; Kettling, H.; Vens-Cappell, S.; Wiegelmann, M.; Müthing, J.; Dreisewerd, K. Mass spectrometry imaging with laser-induced postionization. Science 2015, 348, 211–215. [Google Scholar] [CrossRef]
- Niehaus, M.; Soltwisch, J.; Belov, M.E.; Dreisewerd, K. Transmission-mode MALDI-2 mass spectrometry imaging of cells and tissues at subcellular resolution. Nat. Methods 2019, 16, 925–931. [Google Scholar] [CrossRef]
- Dreisewerd, K.; Bien, T.; Soltwisch, J. MALDI-2 and t-MALDI-2 mass spectrometry. Methods Mol. Biol. 2022, 2437, 21–40. [Google Scholar] [CrossRef]
- Capolupo, L.; Khven, I.; Lederer, A.R.; Mazzeo, L.; Glousker, G.; Ho, S.; Russo, F.; Paz Montoya, J.; Bhandari, D.R.; Bowman, A.P.; et al. Sphingolipids control dermal fibroblast heterogeneity. Science 2022, 376, eabh1623. [Google Scholar] [CrossRef] [PubMed]
- Kennworthy, A.K.; Schmieder, S.S.; Raghunathan, K.; Tiwari, A.; Wang, T.; Kelly, C.V.; Lencer, W.I. Cholera toxin as a probe for membrane biology. Toxins 2021, 13, 543. [Google Scholar] [CrossRef]
- Dreisewerd, K.; Yew, J.Y. Mass spectrometry imaging goes three dimensional. Nat. Methods 2017, 14, 1139–1140. [Google Scholar] [CrossRef]
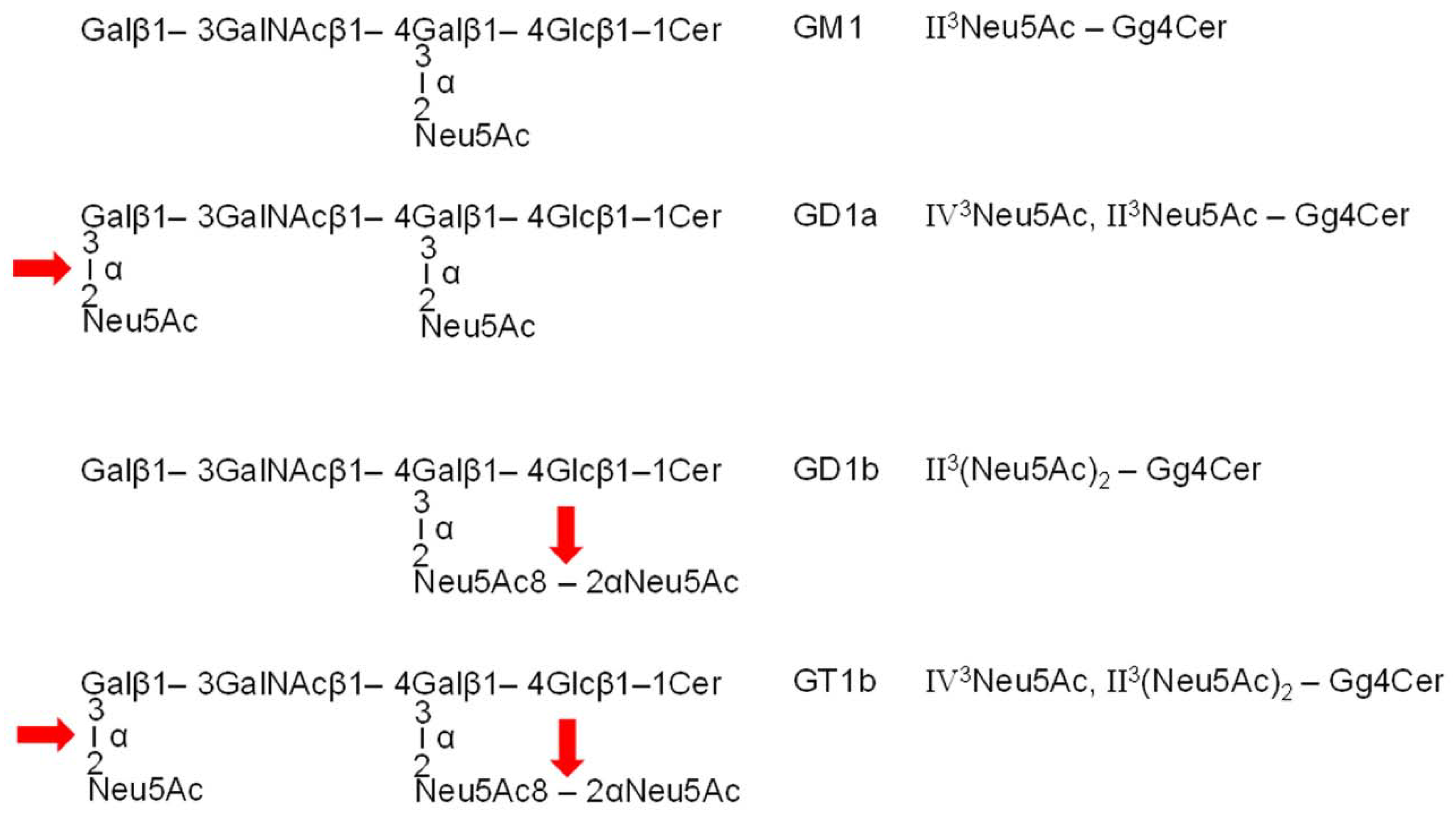
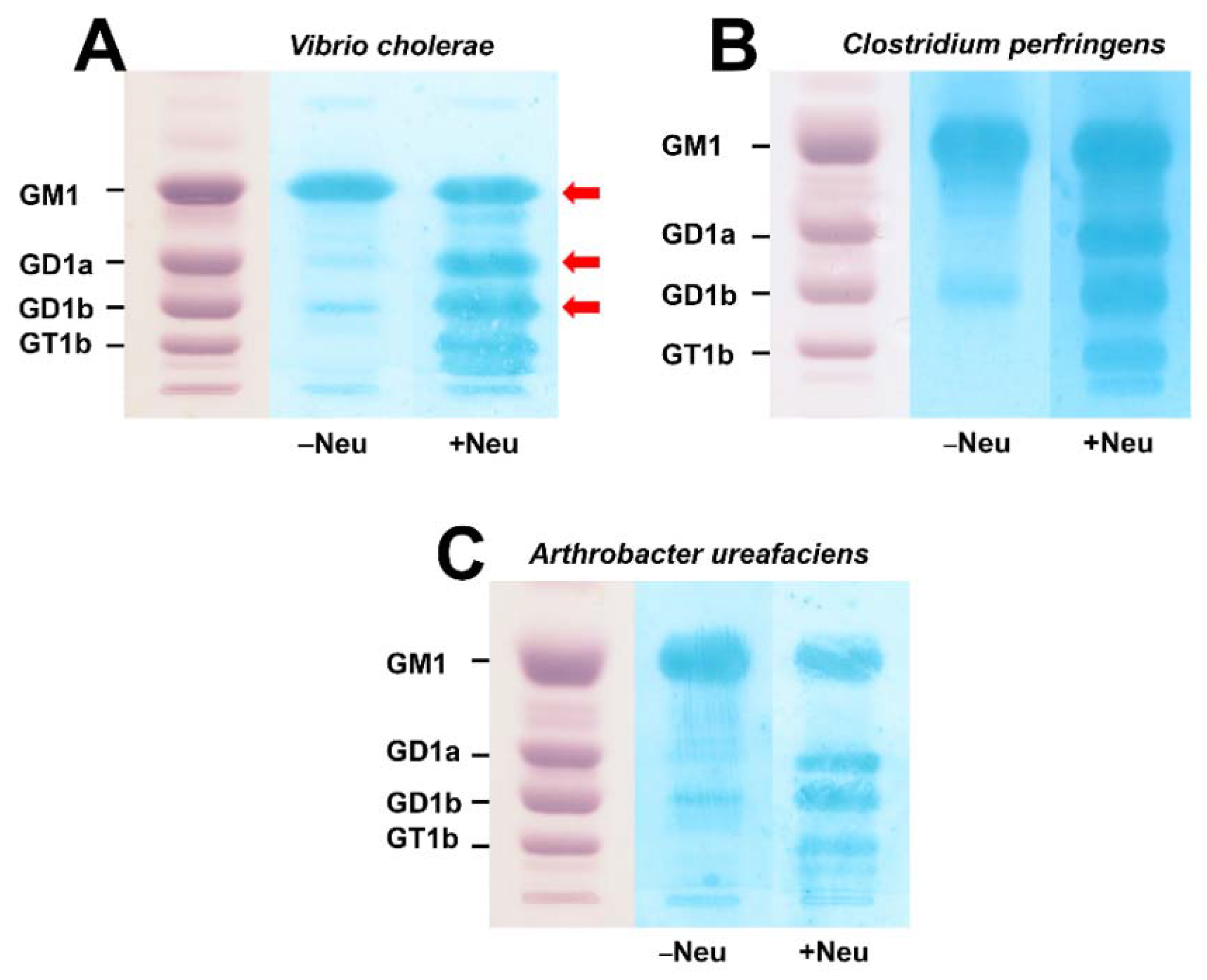



| TLC Band | Ceramide | Formula | m/zexp4 | m/zcalc4 |
|---|---|---|---|---|
| GM1 band 2 | ||||
| GM1 | d18:1, C18:0 | II3Neu5Ac-Gg4Cer | 1544.87 | 1544.8688 |
| GM1 | d20:1, C18:0 | II3Neu5Ac-Gg4Cer | 1572.90 | 1572.9001 |
| GD1a band 3 | ||||
| GM1 | d18:1, C18:0 | II3Neu5Ac-Gg4Cer | 1544.87 | 1544.8688 |
| GM1 | d20:1, C18:0 | II3Neu5Ac-Gg4Cer | 1572.90 | 1572.9001 |
| GD1a | d18:1, C18:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1857.95 | 1857.9462 |
| GD1a | d20:1, C18:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1885.99 | 1885.9775 |
| GD1b band 3 | ||||
| GM1 | d18:1, C18:0 | II3Neu5Ac-Gg4Cer | 1544.85 | 1544.8688 |
| GM1 | d20:1, C18:0 | II3Neu5Ac-Gg4Cer | 1572.90 | 1572.9001 |
| GD1b | d18:1, C18:0 | II3(Neu5Ac)2-Gg4Cer | 1857.95 | 1857.9462 |
| GD1b | d20:1, C18:0 | II3(Neu5Ac)2-Gg4Cer | 1885.99 | 1885.9775 |
| GD1b | d20:1, C18:0 | II3(Neu5Ac)2-Gg4Cer | 1907.96 | 1907.9594 |
| TLC Band | Ceramide | Formula | m/zexp4 | m/zcalc4 |
|---|---|---|---|---|
| Band 1 2 | ||||
| GM1 | d18:1, C14:0 | II3Neu5Ac-Gg4Cer | 1488.81 | 1488.8062 |
| GM1 | d18:1, C16:0 | II3Neu5Ac-Gg4Cer | 1516.82 | 1516.8375 |
| GM1 | d18:1, C16:0-OH | II3Neu5Ac-Gg4Cer | 1532.84 | 1532.8324 |
| GM1 | d18:1, C18:0 | II3Neu5Ac-Gg4Cer | 1544.87 | 1544.8688 |
| GM1 | d18:1, C20:0 | II3Neu5Ac-Gg4Cer | 1572.90 | 1572.9001 |
| Band 2 3 | ||||
| GM1 | d18:1, C16:0 | II3Neu5Ac-Gg4Cer | 1516.84 | 1516.8375 |
| GM1 | d18:1, C18:0 | II3Neu5Ac-Gg4Cer | 1544.87 | 1544.8688 |
| GM1 | d18:1, C20:0 | II3Neu5Ac-Gg4Cer | 1572.90 | 1572.9001 |
| GM1 | d18:1, C22:0 | II3Neu5Ac-Gg4Cer | 1600.91 | 1600.9314 |
| GD1a | d18:1, C16:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1829.92 | 1829.9149 |
| GD1a | d18:1, C18:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1857.93 | 1857.9462 |
| GD1a | d18:1, C20:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1885.96 | 1885.9775 |
| GD1a | d18:1, C22:0 | IV3Neu5Ac, II3Neu5Ac-Gg4Cer | 1913.97 | 1914.0088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detzner, J.; Püttmann, C.; Pohlentz, G.; Müthing, J. Ingenious Action of Vibrio cholerae Neuraminidase Recruiting Additional GM1 Cholera Toxin Receptors for Primary Human Colon Epithelial Cells. Microorganisms 2022, 10, 1255. https://doi.org/10.3390/microorganisms10061255
Detzner J, Püttmann C, Pohlentz G, Müthing J. Ingenious Action of Vibrio cholerae Neuraminidase Recruiting Additional GM1 Cholera Toxin Receptors for Primary Human Colon Epithelial Cells. Microorganisms. 2022; 10(6):1255. https://doi.org/10.3390/microorganisms10061255
Chicago/Turabian StyleDetzner, Johanna, Charlotte Püttmann, Gottfried Pohlentz, and Johannes Müthing. 2022. "Ingenious Action of Vibrio cholerae Neuraminidase Recruiting Additional GM1 Cholera Toxin Receptors for Primary Human Colon Epithelial Cells" Microorganisms 10, no. 6: 1255. https://doi.org/10.3390/microorganisms10061255






