Secondary Metabolites from the Endoparasitic Nematophagous Fungus Harposporium anguillulae YMF 1.01751
Abstract
:1. Introduction
2. Materials and Methods
2.1. Normal Materials
2.2. General Experimental Instruments
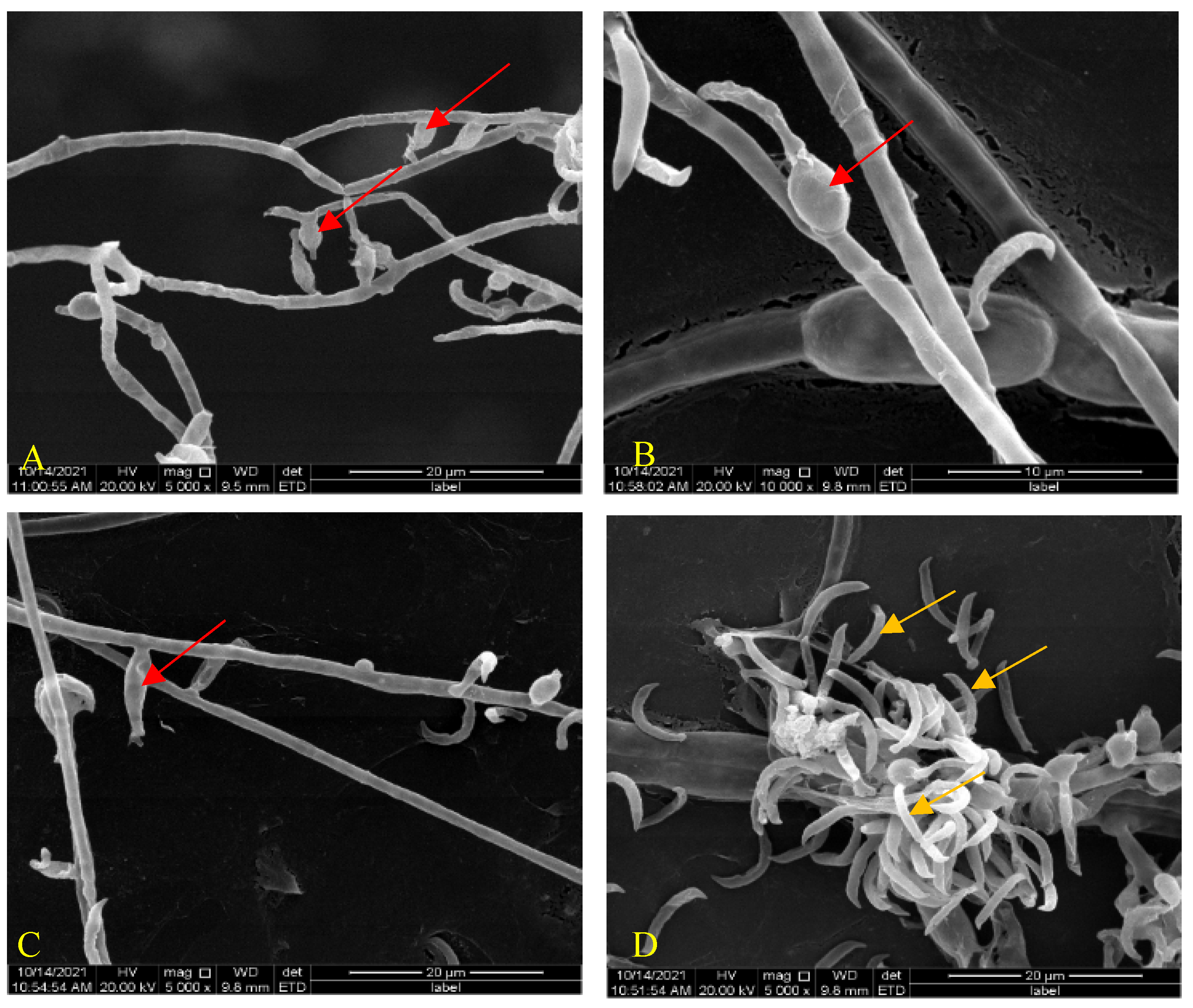
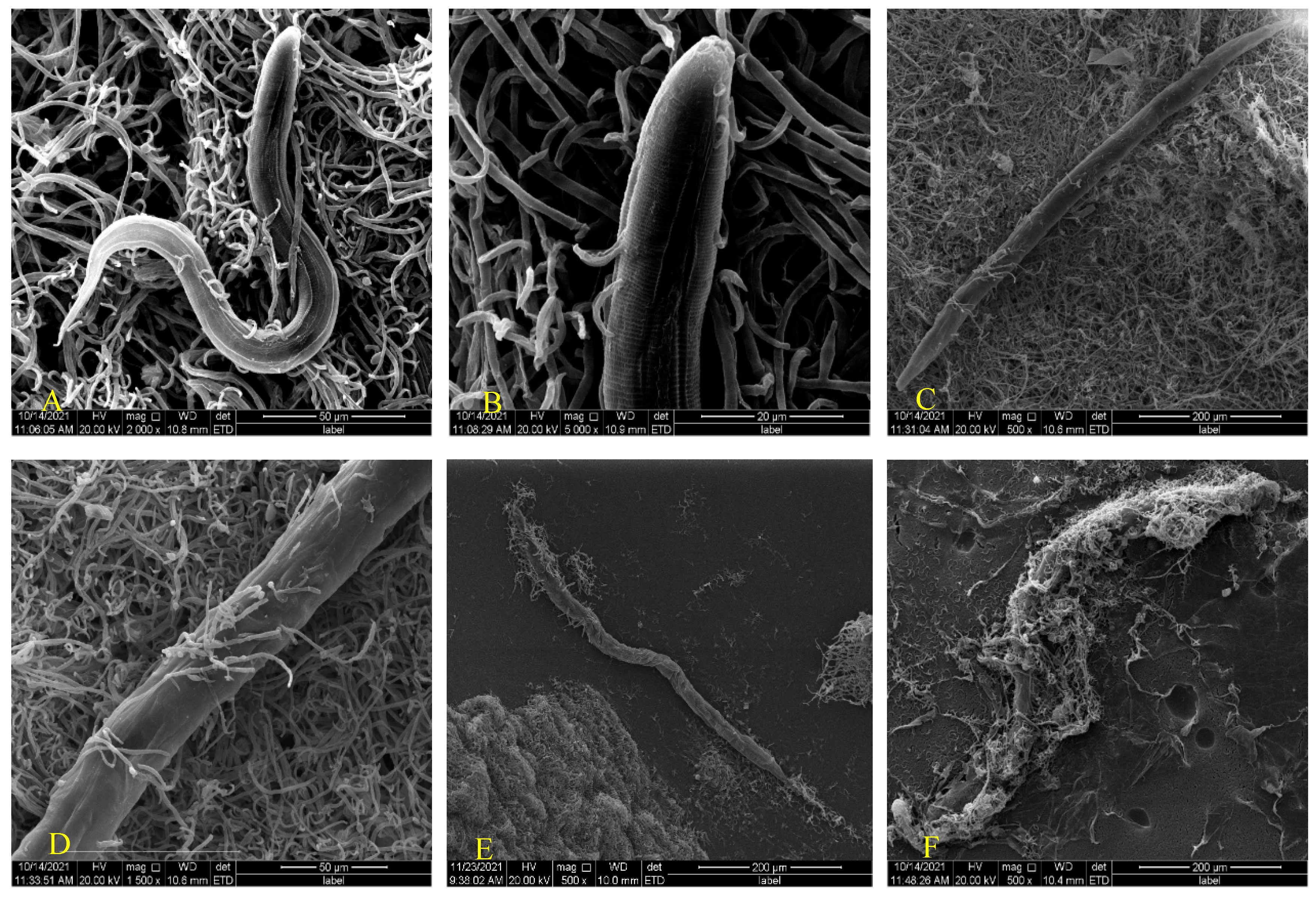
2.3. The Morphology and Pathogenicity against Nematodes of H. anguillulae YMF 1.01751 Observed by Scanning Electron Microscopy
2.4. Extraction and Isolation of Metabolites from H. anguillulae YMF 1.01751
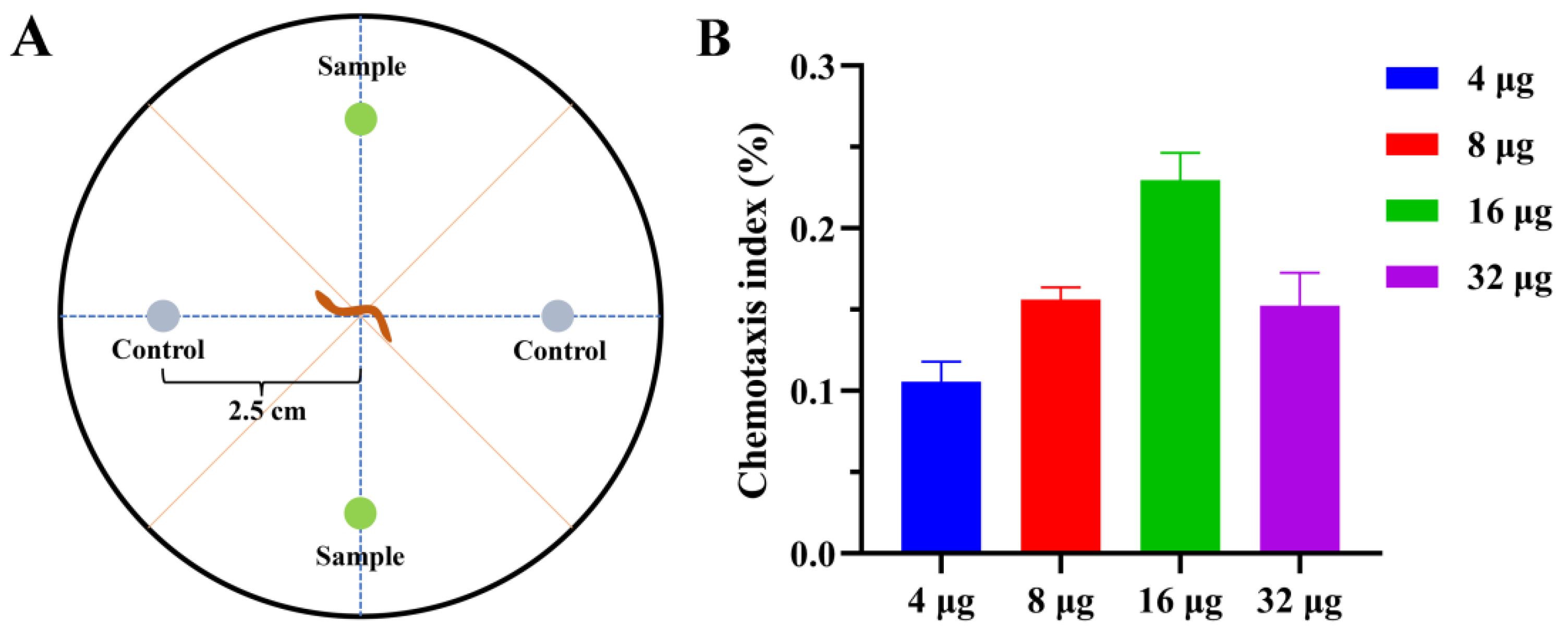
2.5. Nematicidal and Chemotaxis Activity of Metabolites
3. Results
3.1. Scanning Electron Microscopy Observation of Interactions between H. anguillulae YMF 1.01751 and P. redivivus
3.2. Structure Identification
3.3. The Nematicidal and Chemotaxis Activity of Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranier, M.-S.; Pognant-Gros, J.; Quiroz, R.D.; Gonzalez, C.N.A.; Mateille, T.; Roussos, S. Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Braz. Arch. Biol. Technol. 2014, 57, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Nordbring-Hertz, B.; Jansson, H.B.; Tunlid, A. Nematophagous Fungi. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Shimazu, M.; Glockling, S.L. A new species of Harposporium with two spore types isolated from the larva of a cerambycid beetle. Mycol. Res. 1997, 101, 1371–1376. [Google Scholar] [CrossRef]
- Lohde, G. Ueber einige neue parasitische Pilze. Tagebl. Versamml. Dtsch. Nat. Aerzte 1874, 47, 203–206. [Google Scholar]
- Liu, X.Z.; Zhang, K.Q.; Gao, R.H. Nematophagous fungi: Harposporium species from China. Mycosystema 1997, 03, 189–191. [Google Scholar]
- Tian, C.J.; Chen, J.K.; Zhong, Y. Phylogenetic diversity of microbes and its perspectives in conservation biology. Chin. J. Appl. Ecol. 2003, 14, 609–612. [Google Scholar]
- Liu, Z.Q.; Wan, Y.L.; Hao, Y.E. Research advances on resource and biological control of endoparasitic fungi. Chin. J. Appl. Ecol. 2019, 30, 2129–2136. [Google Scholar]
- Li, X.; Luo, H.; Zhang, K.Q. A new species of Harposporium parasitic on nematodes. Can. J. Bot. 2005, 83, 558–562. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Souvanhnachit, S.; Wang, X.; Li, G.H. A new furan derivative from Harposporium sp. YMF 1.01735. Chem. Nat. Compd. 2020, 56, 845–847. [Google Scholar] [CrossRef]
- Lohmann, U.; Sikora, R.A.; HÖfer, M. Influence of phospholipids on growth, sporulation and virulence of the endoparasitic fungi Drechmeria coniospora, Verticillium balanoides and Harposporium anguillulae in liquid culture. J. Phytopathol. 1989, 125, 139–147. [Google Scholar] [CrossRef]
- Charles, T.P.; Roque, M.V.C.; Santos, C.D.P. Reduction of Haemonchus contortus infective larvae by Harposporium anguillulae in sheep faecal cultures. Int. J. Parasitol. 1996, 26, 509–510. [Google Scholar] [CrossRef]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent nematicidal activity of maleimide derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]
- Dyer, H.C.; Boddy, L.; Preston-Meek, C.M. Effect of the nematode Panagrellus redivivus on growth and enzyme production by Phanerochaete velutina and Stereum hirsutum. Mycol. Res. 1992, 96, 1019–1028. [Google Scholar] [CrossRef]
- Wan, J.; Dai, Z.B.; Zhang, K.Q.; Li, G.H.; Zhao, P.J. Pathogenicity and metabolites of endoparasitic nematophagous fungus Drechmeria coniospora YMF 1.01759 against nematodes. Microorganisms 2021, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Jansson, H.B. Adhesion to nematodes of conidia from the nematophagous fungus Drechmeria coniospora. J. Gen. Microbiol. 1993, 139, 1899–1906. [Google Scholar] [CrossRef] [Green Version]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mangoni, A.; Pansini, M. Incisterols, a new class of highly degraded sterols from the marine sponge Dictyonella incisa. Cheminform 1990, 21, 3505–3509. [Google Scholar] [CrossRef]
- Wu, F.E.; Koike, K.; Nikaido, T.; Ishii, K.; Ohmoto, T.; Ikeda, K. Terpenoids and flavonoids from Arenaria kansuensis. Chem. Pharm. Bull. 1990, 38, 2281–2282. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.M.; Chen, S.N.; Lin, Z.W. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 51, 801–806. [Google Scholar] [CrossRef]
- Huang, X.H.; Nishida, H.; Tomoda, H.; Tabata, N.; Shiomi, K.; Yang, D.J.; Takayanagi, H.; Omura, S. Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. II. Structure elucidation of terpendoles A, B, C and D. J. Antibiot. 1995, 48, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.P.; Xu, J.; Yue, J.M. Sterols from the fungus Catathelasma imperiale. Chin. J. Chem. 2003, 21, 1390–1394. [Google Scholar] [CrossRef]
- Kwon, H.C.; Sang, D.Z.; Cho, S.Y.; Sang, U.C.; Kang, R.L. Cytotoxic ergosterols from Paecilomyces sp. J300. Arch. Pharm. Res. 2002, 25, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Meyer, S.L.F.; Chitwood, D.J.; Chauhan, K.R.; Dong, D.; Zhang, T.T.; Li, J.; Liu, W.C. New nematotoxic indoloditerpenoid produced by Gymnoascus reessii za-130. J. Agric. Food Chem. 2017, 65, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Glockling, S.L. Eelworm-eaters the Harposporium and the host. Mycologist 1993, 7, 139–142. [Google Scholar] [CrossRef]
- Niu, Q.H.; Huang, X.W.; Zhang, L.; Xu, J.P.; Yang, D.M.; Wei, K.B.; Niu, X.M.; An, Z.Q.; Bennett, J.W.; Zou, C.G.; et al. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 16631–16636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Chai, H.; Jia, L.; Shao, L.; Liu, X.; Chen, Y. Antitumor secondary metabolites of an endophytic fungus Aspergillus terreus TZS-201607 from Pseudostellaria heterophylla. Nat. Prod. Res. Dev. 2021, 33, 1156–1164. [Google Scholar]
- Gaulin, E.; Bottin, A.; Dumas, B. Sterol biosynthesis in oomycete pathogens. Plant Signal. Behav. 2010, 5, 258–260. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-S.; Bae, K.; Yoo, J.K.; Ryoo, I.-J.; Kim, B.Y.; Ahn, J.S.; Yoo, I.-D. Inhibition of human neutrophil elastase by ergosterol derivatives from the mycelium of Phellinus linteus. J. Antibiot. 2012, 65, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Kobori, H.; Kawaide, M.; Suzuki, T.; Choi, J.-H.; Yasuda, N.; Noguchi, K.; Matsumoto, T.; Hirai, H.; Kawagishi, H. Isolation of bioactive steroids from the Stropharia rugosoannulata mushroom and absolute configuration of strophasterol B. Biosci. Biotechnol. Biochem. 2013, 77, 1779–1781. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.H.; Tomoda, H.; Nishida, H.; Masuma, R.; Omura, S. Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. I. Production, isolation and biological properties. J. Antibiot. 1995, 48, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Kim, Y.P.; Namatame, I.; Tomoda, H.; Omura, S. Sespendole, a new inhibitor of lipid droplet synthesis in macrophages, produced by Pseudobotrytis terrestris FKA-25. J. Antibiot. 2006, 59, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Punyain, W.; Saortep, W.; Choowong, W.; Rachtawee, P.; Pittayakhajonwut, P. Chromanones and aryl glucoside analogs from the entomopathogenic fungus Aschersonia confluens BCC53152. Fitoterapia 2020, 144, 104606. [Google Scholar] [CrossRef] [PubMed]

| Position | 1H | 13C | HMBC |
|---|---|---|---|
| 1 | - | 168.2, s | - |
| 2 | - | 146.3, s | - |
| 3 | 7.59 (1H, s) | 138.3, d | 148.7, 168.2, 146.3 |
| 4 | - | 148.7, s | - |
| 5 | - | 141.9, s | - |
| 6 | 2.36 (3H, s) | 14.6, q | 141.9, 148.7 |
| 7 | 3.79 (2H, s) | 57.0, t | 146.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Gan, Y.; Zhao, P.; Li, G. Secondary Metabolites from the Endoparasitic Nematophagous Fungus Harposporium anguillulae YMF 1.01751. Microorganisms 2022, 10, 1553. https://doi.org/10.3390/microorganisms10081553
Dai Z, Gan Y, Zhao P, Li G. Secondary Metabolites from the Endoparasitic Nematophagous Fungus Harposporium anguillulae YMF 1.01751. Microorganisms. 2022; 10(8):1553. https://doi.org/10.3390/microorganisms10081553
Chicago/Turabian StyleDai, Zebao, Yang Gan, Peiji Zhao, and Guohong Li. 2022. "Secondary Metabolites from the Endoparasitic Nematophagous Fungus Harposporium anguillulae YMF 1.01751" Microorganisms 10, no. 8: 1553. https://doi.org/10.3390/microorganisms10081553





