Bacterial Prevalence in Skin, Urine, Diarrheal Stool, and Respiratory Samples from Dogs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Prevalence Pattern among Different Age Groups
3.2. Prevalence of Bacteria in Diarrheal Stool Samples
3.3. Prevalence of Bacteria in Skin Samples
3.4. Prevalence of Bacteria in Urine Samples
3.5. Prevalence of Bacteria from Respiratory Tract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Chun, B.C. Association between companion animal ownership and overall life satisfaction in Seoul, Korea. PLoS ONE 2021, 16, e0258034. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Steneroden, K.K.; Hill, A.E.; Salman, M.D. Zoonotic disease awareness in animal shelter workers and volunteers and the effect of training. Zoonoses Public Health 2011, 58, 449–453. [Google Scholar] [CrossRef] [PubMed]
- So, J.H.; Kim, J.; Bae, I.K.; Jeong, S.H.; Kim, S.H.; Lim, S.K.; Park, Y.H.; Lee, K. Dissemination of multidrug-resistant Escherichia coli in Korean veterinary hospitals. Diagn. Microbiol. Infect. Dis. 2012, 73, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.; Voelter, K.; Hartnack, S.; Spiess, B.M.; Pot, S.A. Septic keratitis in dogs, cats, and horses in Switzerland: Associated bacteria and antibiotic susceptibility. Vet. Ophthalmol. 2018, 21, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Rocha, M.F.; Brito, E.H.; Girao, M.D.; Monteiro, A.J.; Teixeira, M.F.; Sidrim, J.J. Survey of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Fortaleza, Ceara, Brazil. Vet. Ophthalmol. 2005, 8, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tolar, E.L.; Hendrix, D.V.; Rohrbach, B.W.; Plummer, C.E.; Brooks, D.E.; Gelatt, K.N. Evaluation of clinical characteristics and bacterial isolates in dogs with bacterial keratitis: 97 cases (1993–2003). J. Am. Vet. Med. Assoc. 2006, 228, 80–85. [Google Scholar] [CrossRef]
- Auten, C.R.; Urbanz, J.L.; Dees, D.D. Comparison of bacterial culture results collected via direct corneal ulcer vs conjunctival fornix sampling in canine eyes with presumed bacterial ulcerative keratitis. Vet. Ophthalmol. 2020, 23, 135–140. [Google Scholar] [CrossRef]
- McDonald, P.J.; Watson, D.J. Microbial flora of normal canine conjunctivae. J. Small Anim. Pract. 1976, 17, 809–812. [Google Scholar] [CrossRef]
- Hindley, K.E.; Groth, A.D.; King, M.; Graham, K.; Billson, F.M. Bacterial isolates, antimicrobial susceptibility, and clinical characteristics of bacterial keratitis in dogs presenting to referral practice in Australia. Vet. Ophthalmol. 2016, 19, 418–426. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Q.; Zhang, L.; Xue, Q.; Cui, J.; Qi, C. Investigation of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Beijing, China. Vet. Ophthalmol. 2008, 11, 145–149. [Google Scholar] [CrossRef]
- Lin, C.T.; Petersen-Jones, S.M. Antibiotic susceptibility of bacterial isolates from corneal ulcers of dogs in Taiwan. J. Small Anim. Pract. 2007, 48, 271–274. [Google Scholar] [CrossRef]
- Ekapopphan, D.; Srisutthakarn, A.; Moonarmart, W.; Buddhirongawatr, R.; Bangphoomi, N. Identification and antimicrobial susceptibility of microorganisms isolated from severe corneal ulcers of dogs in Thailand. J. Vet. Med. Sci. 2018, 80, 1259–1265. [Google Scholar] [CrossRef]
- Gomez-Beltran, D.A.; Villar, D.; Lopez-Osorio, S.; Ferguson, D.; Monsalve, L.K.; Chaparro-Gutierrez, J.J. Prevalence of Antimicrobial Resistance in Bacterial Isolates from Dogs and Cats in a Veterinary Diagnostic Laboratory in Colombia from 2016–2019. Vet. Sci. 2020, 7, 173. [Google Scholar] [CrossRef]
- Kumwenda, P.; Adukwu, E.C.; Tabe, E.S.; Ujor, V.C.; Kamudumuli, P.S.; Ngwira, M.; Wu, J.T.S.; Chisale, M.R.O. Prevalence, distribution and antimicrobial susceptibility pattern of bacterial isolates from a tertiary Hospital in Malawi. BMC Infect. Dis. 2021, 21, 34. [Google Scholar] [CrossRef]
- Nam, H.M.; Lee, H.S.; Byun, J.W.; Yoon, S.S.; Jung, S.C.; Joo, Y.S.; Lim, S.K. Prevalence of antimicrobial resistance in fecal Escherichia coli isolates from stray pet dogs and hospitalized pet dogs in Korea. Microb. Drug Resist. 2010, 16, 75–79. [Google Scholar] [CrossRef]
- Authier, S.; Paquette, D.; Labrecque, O.; Messier, S. Comparison of susceptibility to antimicrobials of bacterial isolates from companion animals in a veterinary diagnostic laboratory in Canada between 2 time points 10 years apart. Can. Vet. J. 2006, 47, 774–778. [Google Scholar]
- Awosile, B.B.; McClure, J.T.; Saab, M.E.; Heider, L.C. Antimicrobial resistance in bacteria isolated from cats and dogs from the Atlantic Provinces, Canada from 1994–2013. Can. Vet. J. 2018, 59, 885–893. [Google Scholar]
- Kang, J.H.; Chung, T.H.; Hwang, C.Y. Clonal distribution of methicillin-resistant Staphylococcus pseudintermedius isolates from skin infection of dogs in Korea. Vet. Microbiol. 2017, 210, 32–37. [Google Scholar] [CrossRef]
- Chung, Y.S.; Hu, Y.S.; Shin, S.; Lim, S.K.; Yang, S.J.; Park, Y.H.; Park, K.T. Mechanisms of quinolone resistance in Escherichia coli isolated from companion animals, pet-owners, and non-pet-owners. J. Vet. Sci. 2017, 18, 449–456. [Google Scholar] [CrossRef]
- Moon, D.C.; Choi, J.H.; Boby, N.; Kim, S.J.; Song, H.J.; Park, H.S.; Gil, M.C.; Yoon, S.S.; Lim, S.K. Prevalence of Bacterial Species in Skin, Urine, Diarrheal Stool, and Respiratory Samples in Cats. Pathogens 2022, 11, 324. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Moon, B.Y.; Youn, J.H.; Shin, S.; Hwang, S.Y.; Park, Y.H. Genetic and phenotypic characterization of methicillin-resistant staphylococci isolated from veterinary hospitals in South Korea. J. Vet. Diagn. Investig. 2012, 24, 489–498. [Google Scholar] [CrossRef]
- Han, J.I.; Yang, C.H.; Park, H.M. Prevalence and risk factors of Staphylococcus spp. carriage among dogs and their owners: A cross-sectional study. Vet. J. 2016, 212, 15–21. [Google Scholar] [CrossRef] [PubMed]
- De Graef, E.M.; Decostere, A.; Devriese, L.A.; Haesebrouck, F. Antibiotic resistance among fecal indicator bacteria from healthy individually owned and kennel dogs. Microb. Drug Resist. 2004, 10, 65–69. [Google Scholar] [CrossRef]
- Adesiyun, A.A.; Campbell, M.; Kaminjolo, J.S. Prevalence of bacterial enteropathogens in pet dogs in Trinidad. Zentralbl. Veterinarmed. B 1997, 44, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Lee, K.J.; Lee, S.Y.; Chae, M.J.; Park, J.K.; Yoo, J.H.; Park, H.M. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J. Microbiol. Biotechnol. 2010, 20, 1764–1768. [Google Scholar] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shah, H.N.; Misra, R.; Chen, J.; Zhang, W.; Liu, Y.; Cutler, R.R.; Mkrtchyan, H.V. The prevalence, antibiotic resistance and mecA characterization of coagulase negative staphylococci recovered from non-healthcare settings in London, UK. Antimicrob. Resist. Infect. Control 2018, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Hommez, J.; Kilpper-Bälz, R.; Schleifer, K.-H. Streptococcus canis sp. nov.: A species of group G streptococci from animals. Int. J. Syst. Evol. Microbiol. 1986, 36, 422–425. [Google Scholar] [CrossRef]
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and antimicrobial susceptibility of bacteria and yeasts isolated from healthy dogs and dogs with otitis externa. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 559–563. [Google Scholar] [CrossRef]
- Timoney, J.F.; Velineni, S.; Ulrich, B.; Blanchard, P. Biotypes and ScM types of isolates of Streptococcus canis from diseased and healthy cats. Vet. Rec. 2017, 180, 358. [Google Scholar] [CrossRef]
- DeWinter, L.M.; Low, D.E.; Prescott, J.F. Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet. Microbiol. 1999, 70, 95–110. [Google Scholar] [CrossRef]
- Morrow, B.L.; McNatt, R.; Joyce, L.; McBride, S.; Morgan, D.; Tressler, C.; Mellits, C. Highly pathogenic beta-hemolytic streptococcal infections in cats from an institutionalized hoarding facility and a multi-species comparison. J. Feline Med. Surg. 2016, 18, 318–327. [Google Scholar] [CrossRef]
- Norris, C.R.; Williams, B.J.; Ling, G.V.; Franti, C.E.; Johnson; Ruby, A.L. Recurrent and persistent urinary tract infections in dogs: 383 cases (1969–1995). J. Am. Anim. Hosp. Assoc. 2000, 36, 484–492. [Google Scholar] [CrossRef]
- Seguin, M.A.; Vaden, S.L.; Altier, C.; Stone, E.; Levine, J.F. Persistent urinary tract infections and reinfections in 100 dogs (1989–1999). J. Vet. Intern. Med. 2003, 17, 622–631. [Google Scholar] [CrossRef]
- Morrissey, I.; Moyaert, H.; de Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of bacterial pathogens isolated from respiratory tract infections in dogs and cats across Europe: ComPath results. Vet. Microbiol. 2016, 191, 44–51. [Google Scholar] [CrossRef]
- Saputra, S.; Jordan, D.; Mitchell, T.; Wong, H.S.; Abraham, R.J.; Kidsley, A.; Turnidge, J.; Trott, D.J.; Abraham, S. Antimicrobial resistance in clinical Escherichia coli isolated from companion animals in Australia. Vet. Microbiol. 2017, 211, 43–50. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Heuer, O.E. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis. 2009, 48, 916–921. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Sabbuba, N.A.; Suller, M.T.; Stickler, D.J.; Feneley, R.C. Genotyping of urinary and fecal Proteus mirabilis isolates from individuals with long-term urinary catheters. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.M.; Stickler, D.; Mobley, H.; Shirtliff, M. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Qekwana, D.N.; Naidoo, V.; Oguttu, J.W.; Odoi, A. Occurrence and Predictors of Bacterial Respiratory Tract Infections and Antimicrobial Resistance among Isolates from Dogs Presented with Lower Respiratory Tract Infections at a Referral Veterinary Hospital in South Africa. Front. Vet. Sci. 2020, 7, 304. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yachi, A.; Ohshima, T.; Ohuchi, A.; Ishida, T. Etiologic study of upper respiratory infections of household dogs. J. Vet. Med. Sci. 2008, 70, 563–569. [Google Scholar] [CrossRef]
- Lavan, R.; Knesl, O. Prevalence of canine infectious respiratory pathogens in asymptomatic dogs presented at US animal shelters. J. Small Anim. Pract. 2015, 56, 572–576. [Google Scholar] [CrossRef]
- Lehner, G.; Linek, M.; Bond, R.; Lloyd, D.H.; Prenger-Berninghoff, E.; Thom, N.; Straube, I.; Verheyen, K.; Loeffler, A. Case-control risk factor study of methicillin-resistant Staphylococcus pseudintermedius (MRSP) infection in dogs and cats in Germany. Vet. Microbiol. 2014, 168, 154–160. [Google Scholar] [CrossRef]
- Haenni, M.; de Moraes, N.A.; Chatre, P.; Medaille, C.; Moodley, A.; Madec, J.Y. Characterisation of clinical canine meticillin-resistant and meticillin-susceptible Staphylococcus pseudintermedius in France. J. Glob. Antimicrob. Resist. 2014, 2, 119–123. [Google Scholar] [CrossRef]
- Bloom, P. Canine superficial bacterial folliculitis: Current understanding of its etiology, diagnosis and treatment. Vet. J. 2014, 199, 217–222. [Google Scholar] [CrossRef]
- Weber, D.J.; Wolfson, J.S.; Swartz, M.N.; Hooper, D.C. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine 1984, 63, 133–154. [Google Scholar] [CrossRef]
- Klein, N.C.; Cunha, B.A. Pasteurella multocida pneumonia. Semin. Respir. Infect. 1997, 12, 54–56. [Google Scholar]
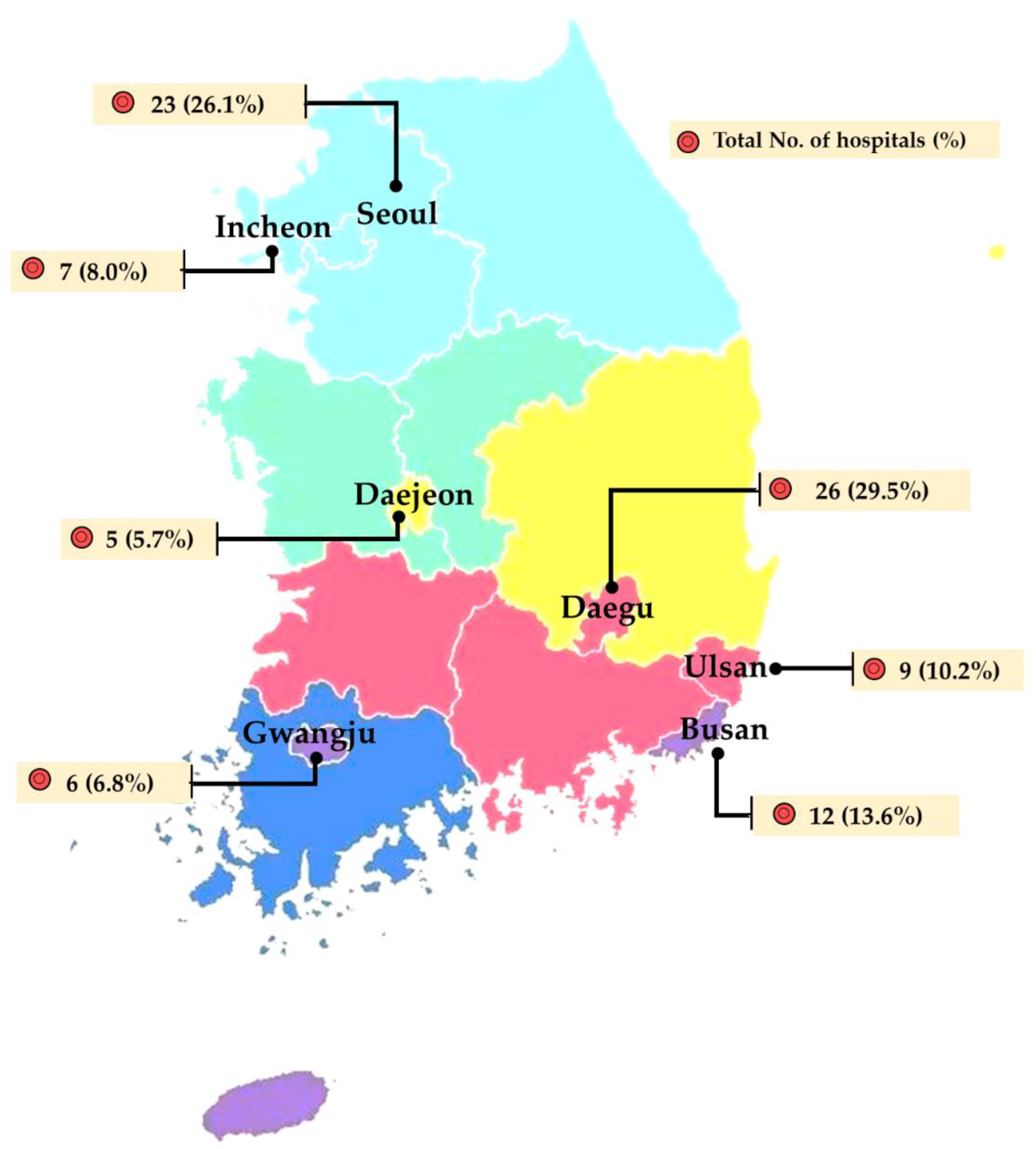
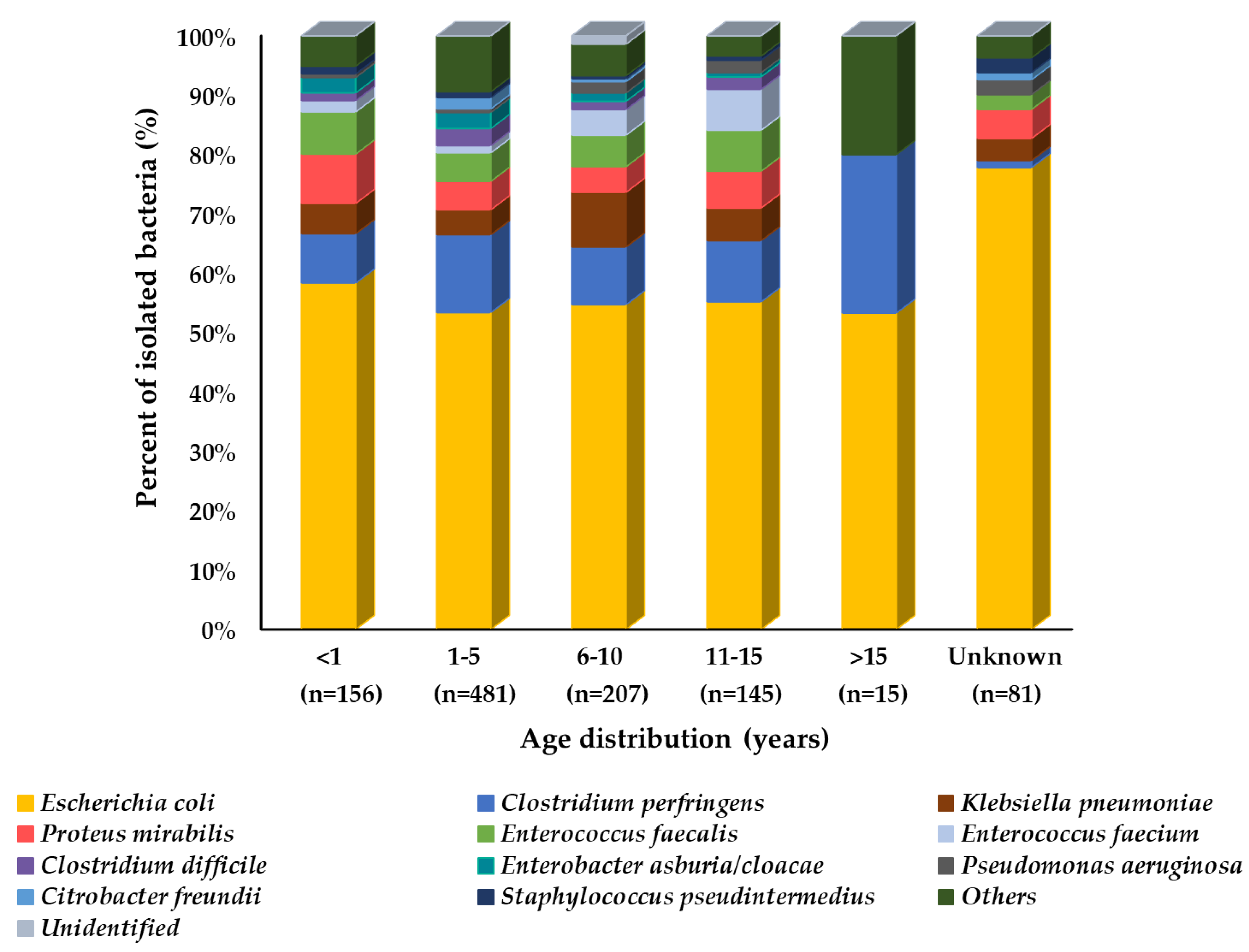

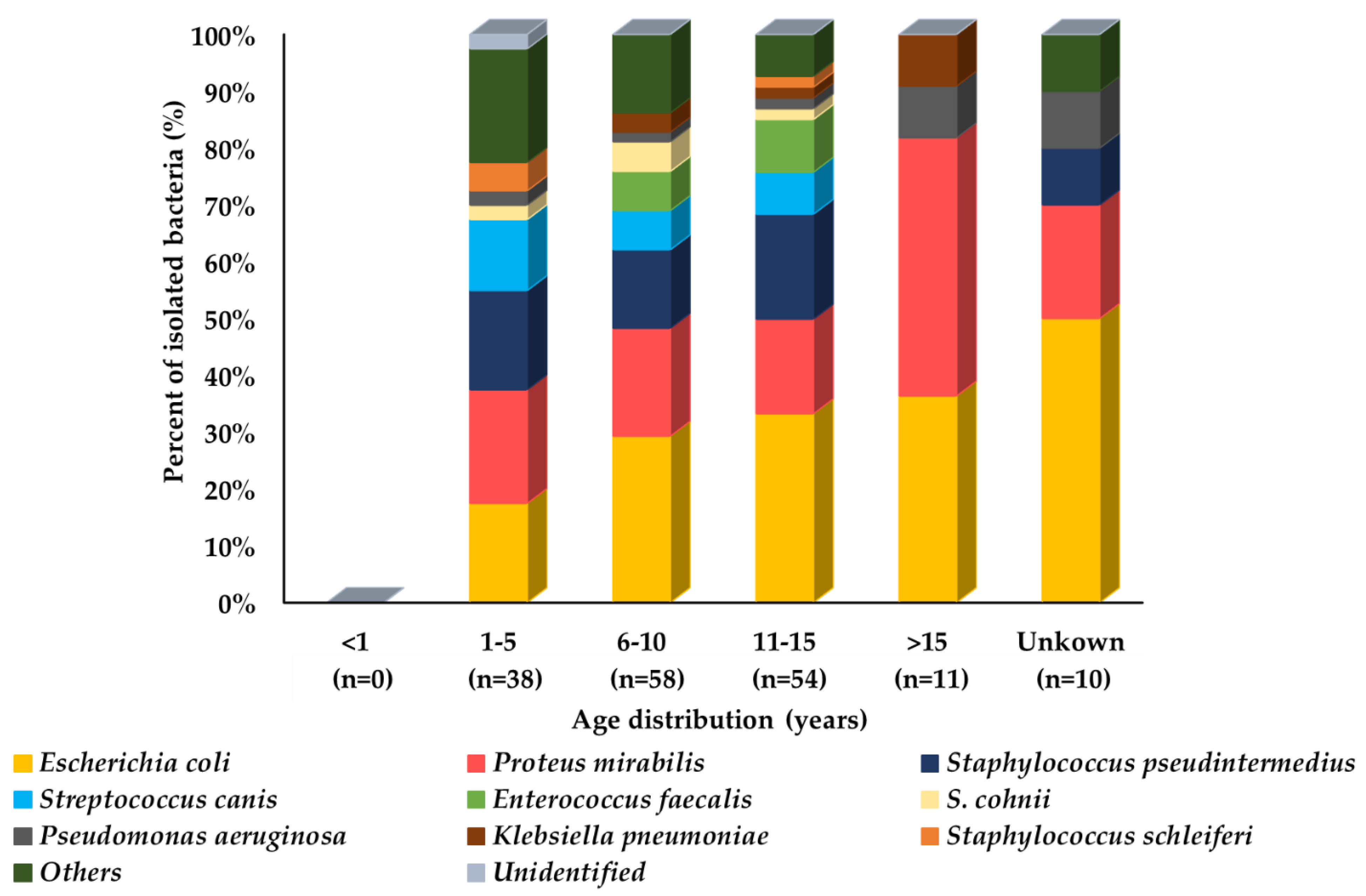

| Sampling Site | No. of Isolates (%) | Total | |||||
|---|---|---|---|---|---|---|---|
| <1 year | 1–5 years | 6–10 years | 11–15 years | >15 years | Unknown | ||
| Diarrhea | 156 (14.4) | 481 (44.3) | 207 (19.1) | 145 (13.4) | 15 (1.4) | 81 (7.5) | 1085 (34.6) |
| Skin | 88 (5.0) | 608 (34.5) | 639 (36.3) | 340 (19.3) | 31 (1.8) | 55 (3.1) | 1761 (56.2) |
| Urine | - | 38 (22.2) | 58 (33.9) | 54 (31.6) | 11 (6.4) | 10 (5.8) | 171 (5.5) |
| Respiratory | 15 (12.7) | 35 (29.7) | 19 (16.1) | 22 (18.6) | 18 (15.3) | 9 (7.6) | 118 (3.8) |
| Total | 259 (8.3) | 1162 (37.1) | 923 (29.4) | 561 (17.9) | 75 (2.4) | 155 (4.9) | 3135 (100) |
| Bacterial Species | No. of Isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seoul (n = 502) | Busan (n = 123) | Daegu (n = 29) | Incheon (n = 55) | Gwangju (n = 41) | Daejeon (n = 258) | Ulsan (n = 77) | Total (n = 1085) | |
| E. coli | 227 (45.2) | 116 (94.3) | 26 (89.7) | 30 (54.5) | 36 (87.8) | 145 (56.2) | 32 (41.6) | 612 (56.4) |
| C. perfringens | 63 (12.5) | 1 (0.8) | 1 (3.4) | - | - | 37 (14.3) | 14 (18.2) | 116 (10.7) |
| K. pneumoniae | 36 (7.2) | 2 (1.6) | - | 6 (10.9) | 1 (2.4) | 8 (3.1) | 5 (6.5) | 58 (5.3) |
| P. mirabilis | 22 (4.4) | - | - | 5 (9.1) | - | 23 (8.9) | 8 (10.4) | 58 (5.3) |
| E. faecalis | 31 (6.2) | - | - | 1 (1.8) | 1 (2.4) | 21 (8.1) | 3 (3.9) | 57 (5.3) |
| E. faecium | 20 (4.0) | - | - | - | - | 6 (2.3) | 2 (2.6) | 28 (2.6) |
| C. difficile | 22 (4.4) | - | - | - | - | - | - | 22 (2.0) |
| E. asburia/cloacae | 15 (3.0) | - | - | - | - | 2 (0.8) | 4 (5.2) | 21 (1.9) |
| P. aeruginosa | 5 (1.0) | 1 (0.8) | - | 7 (12.7) | - | - | - | 13 (1.2) |
| C. freundii | 9 (1.8) | - | - | 1 (1.8) | 1 (2.4) | - | - | 11 (1.0) |
| S. pseudintermedius | 8 (1.6) | - | - | - | 2 (4.9) | 1 (0.4) | - | 11 (1.0) |
| Others * | 41 (8.2) | 3 (2.4) | 2(6.9) | 5 (9.1) | - | 15 (5.8) | 9 (11.7) | 75 (6.9) |
| Unidentified | 3 (0.6) | - | - | - | - | - | - | 3 (0.3) |
| Bacterial Species | No. of Isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seoul (n = 375) | Busan (n = 359) | Daegu (n = 185) | Incheon (n = 141) | Gwangju (n = 100) | Daejeon (n = 457) | Ulsan (n = 144) | Total (n = 1761) | |
| S. pseudintermedius | 170 (45.3) | 166 (46.2) | 93 (50.3) | 40 (28.4) | 67 (67.0) | 252 (55.1) | 76 (52.8) | 864 (49.1) |
| S. schleiferi | 78 (20.8) | 68 (18.9) | 22 (11.9) | 21 (14.9) | 23 (23.0) | 70 (15.3) | 29 (20.1) | 311 (17.7) |
| P. aeruginosa | 20 (5.3) | 23 (6.4) | 25 (13.5) | 20 (14.2) | 2 (2.0) | 22 (4.8) | 6 (4.2) | 118 (6.7) |
| E. coli | 21 (5.6) | 21 (5.8) | 20 (10.8) | 12 (8.5) | 3 (3.0) | 28 (6.1) | 12 (8.3) | 117 (6.6) |
| P. mirabilis | 14 (3.7) | 18 (5.0) | 1 (0.5) | 5 (3.5) | - | 34 (7.4) | 3 (2.1) | 75 (4.3) |
| E. faecalis | 5 (1.3) | 1 (0.3) | - | 24 (17.0) | - | 5 (1.1) | 1 (0.7) | 36 (2.0) |
| S. epidermidis | 6 (1.6) | 5 (1.4) | 3 (1.6) | - | - | 4 (0.9) | 2 (1.4) | 20 (1.1) |
| C. auriscanis | 14 (3.7) | 1 (0.3) | - | - | - | 1 (0.2) | 2 (1.4) | 18 (1.0) |
| S. haemolyticus | 1 (0.3) | 5 (1.4) | 1 (0.5) | 1 (0.7) | 1 (1.0) | 5 (1.1) | 2 (1.4) | 16 (0.9) |
| S. canis | 5 (1.3) | 6 (1.7) | 3 (1.6) | 1 (0.7) | - | 1 (0.2) | - | 16 (0.9) |
| Others * | 39 (10.4) | 44 (12.3) | 15 (8.1) | 17 (12.1) | 4 (4.0) | 33 (7.2) | 11 (7.6) | 163 (9.3) |
| Unidentified | 2 (0.5) | 1 (0.3) | 2 (1.1) | - | - | 2 (0.4) | - | 7 (0.4) |
| Bacterial Species | No. of Isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seoul (n = 35) | Busan (n = 51) | Daegu (n = 16) | Incheon (n = 18) | Gwangju (n = 3) | Daejeon (n = 38) | Ulsan (n = 11) | Total (n = 171) | |
| E. coli | 16 (46.0) | 6 (11.8) | 3 (18.8) | 4 (22.2) | 2 (66.7) | 15 (39.5) | 5 (45.5) | 51 (29.7) |
| P. mirabilis | 7 (20.0) | 4 (7.8) | 2 (12.5) | 12 (66.7) | - | 10 (26.3) | - | 35 (20.3) |
| S. pseudintermedius | 2 (5.7) | 10 (19.6) | 3 (18.8) | 1 (5.6) | - | 10 (26.3) | - | 26 (15.1) |
| S. canis | - | 13 (25.5) | - | - | - | - | - | 13 (7.6) |
| E. faecalis | 1 (2.9) | 1 (2.0) | 5 (31.3) | - | - | - | 2 (18.2) | 9 (5.2) |
| S. cohnii | 2 (5.7) | 3 (5.9) | - | - | - | - | - | 5 (2.9) |
| P. aeruginosa | - | 2 (3.9) | 1 (6.3) | - | 1 (33.3) | - | 1 (9.1) | 5 (2.9) |
| K. pneumoniae | 3 (8.6) | 1 (2.0) | - | - | - | - | - | 4 (2.3) |
| S. schleiferi | - | 2 (3.9) | 1 (6.3) | - | - | - | - | 3 (1.7) |
| Others * | 4 (11.4) | 9 (17.6) | 1 (6.3) | 1 (5.6) | - | 3 (7.9) | 3 (27.3) | 21 (12.2) |
| Unidentified | - | - | - | - | - | 1 (2.6) | - | 1 (0.6) |
| Bacterial Species | No. of Isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seoul (n = 45) | Busan (n = 7) | Daegu (n = 13) | Incheon (n = 38) | Gwangju (n = 7) | Daejeon (n = 4) | Ulsan (n = 6) | Total (n = 118) | |
| S. pseudintermedius | 12 (26.7) | 2 (28.6) | 5 (38.5) | 20 (52.6) | 1 (14.3) | - | - | 40 (33.3) |
| E. coli | 2 (4.4) | 1 (14.3) | 3 (23.1) | - | 2 (28.6) | 1 (25.0) | 1 (16.7) | 10 (8.3) |
| E. faecium | 3 (6.7) | - | - | 1 (2.6) | 1 (14.3) | - | - | 5 (4.2) |
| P. aeruginosa | 1 (2.2) | - | 1 (7.7) | 3 (7.9) | - | - | - | 5 (4.2) |
| S. schleiferi | 1 (2.2) | 1 (14.3) | - | 1 (2.6) | - | - | 1 (16.7) | 4 (3.3) |
| K. pneumoniae | 3 (6.7) | - | - | - | - | 1 (25.0) | - | 4 (3.3) |
| R. nasimurium | 4 (8.9) | - | - | - | - | - | - | 4 (3.3) |
| S. epidermidis | 2 (4.4) | - | 1 (7.7) | - | - | - | - | 3 (2.5) |
| E. aerogenes | - | - | - | 3 (7.9) | - | - | - | 3 (2.5) |
| P. canis | 2 (4.4) | - | - | - | 1 (14.3) | - | - | 3 (2.5) |
| P. mirabilis | 1 (2.2) | - | 1 (7.7) | 1 (2.6) | - | - | - | 3 (2.5) |
| Others * | 14 (31.1) | 3 (42.9) | 2 (15.4) | 9 (23.7) | 2 (28.6) | 2 (50.0) | 4 (66.7) | 36 (30.0) |
| Unidentified | - | - | - | 1 (2.6) | - | - | 1 (16.7) | 2 (1.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.-C.; Choi, J.-H.; Boby, N.; Kang, H.-Y.; Kim, S.-J.; Song, H.-J.; Park, H.-S.; Gil, M.-C.; Yoon, S.-S.; Lim, S.-K. Bacterial Prevalence in Skin, Urine, Diarrheal Stool, and Respiratory Samples from Dogs. Microorganisms 2022, 10, 1668. https://doi.org/10.3390/microorganisms10081668
Moon D-C, Choi J-H, Boby N, Kang H-Y, Kim S-J, Song H-J, Park H-S, Gil M-C, Yoon S-S, Lim S-K. Bacterial Prevalence in Skin, Urine, Diarrheal Stool, and Respiratory Samples from Dogs. Microorganisms. 2022; 10(8):1668. https://doi.org/10.3390/microorganisms10081668
Chicago/Turabian StyleMoon, Dong-Chan, Ji-Hyun Choi, Naila Boby, Hee-Young Kang, Su-Jeong Kim, Hyun-Ju Song, Ho-Sung Park, Min-Chan Gil, Soon-Seek Yoon, and Suk-Kyung Lim. 2022. "Bacterial Prevalence in Skin, Urine, Diarrheal Stool, and Respiratory Samples from Dogs" Microorganisms 10, no. 8: 1668. https://doi.org/10.3390/microorganisms10081668






