The Infection Rate of Bird-Feeding Ixodes ricinus Ticks with Borrelia garinii and B. valaisiana Varies with Host Haemosporidian Infection Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Field Methods and Study Species
2.2. DNA Extraction
2.3. Borrelia Molecular Analyses
2.4. Avian Haemosporidian Molecular Analyses
2.5. Statistical Analysis
3. Results
3.1. General Tick Infestation and Borrelia Infection Patterns
3.2. General Haemosporidian Infection Patterns
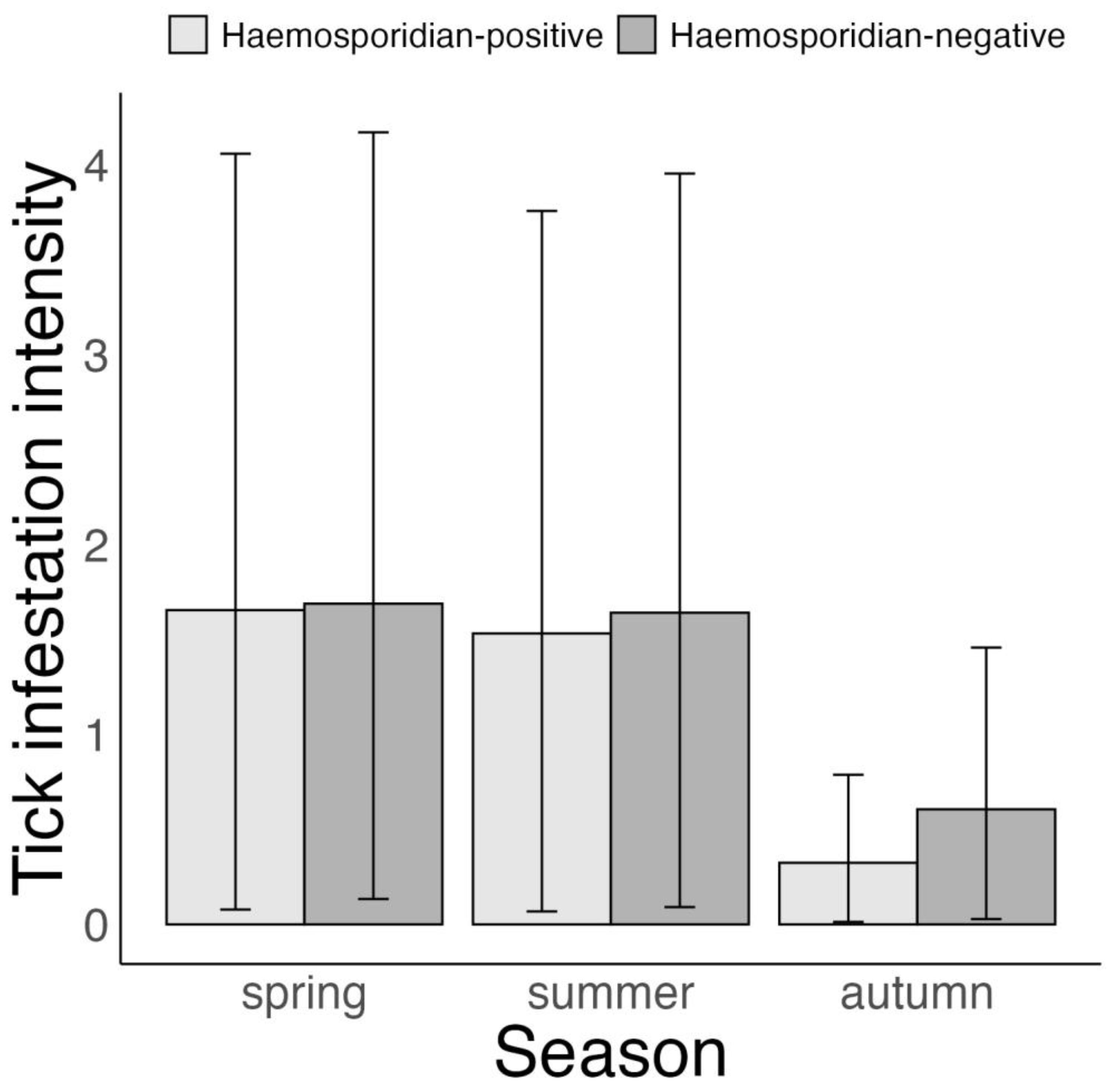
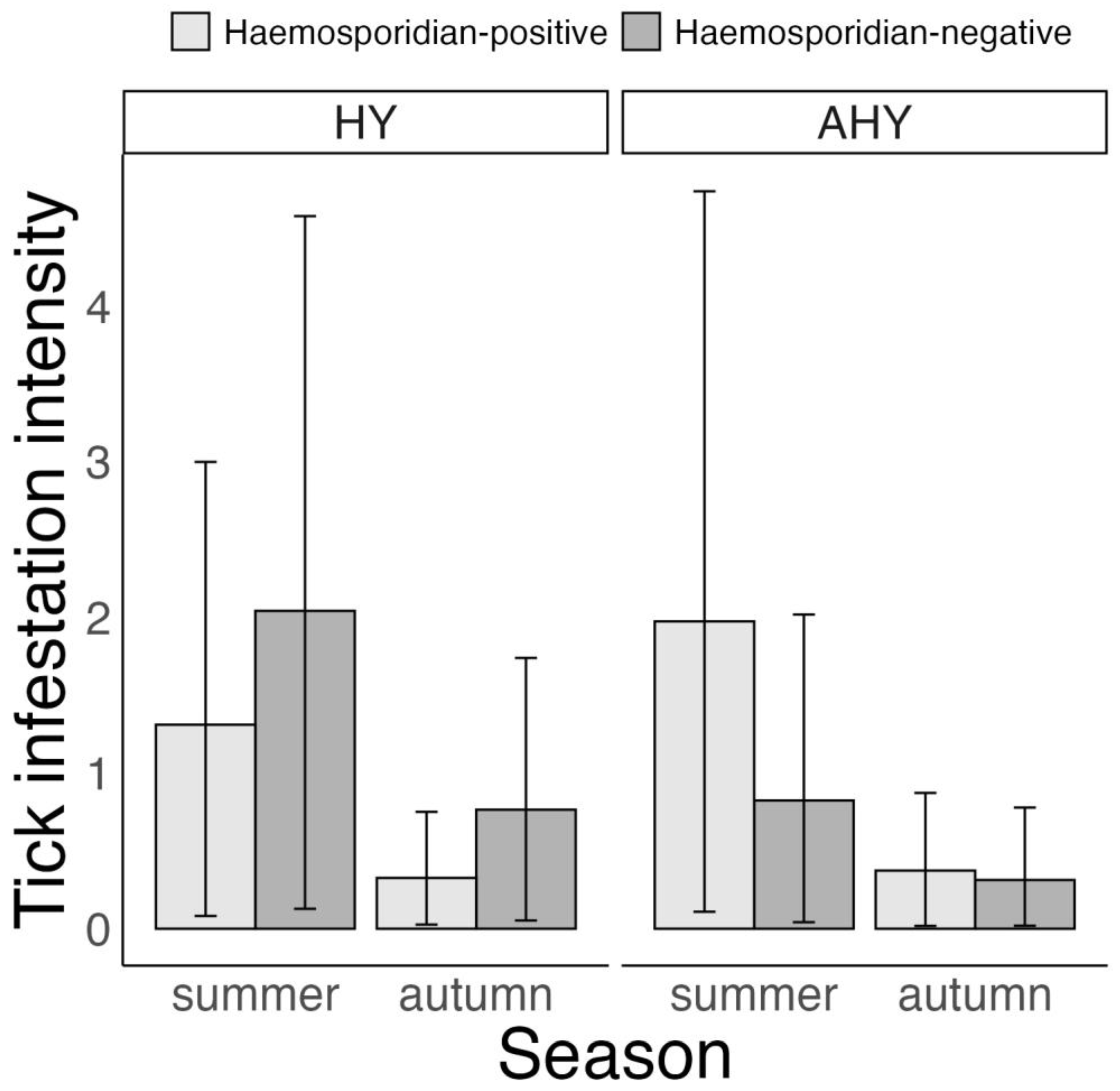
3.3. Association between Tick Infestation and Host Haemosporidian Infection Status
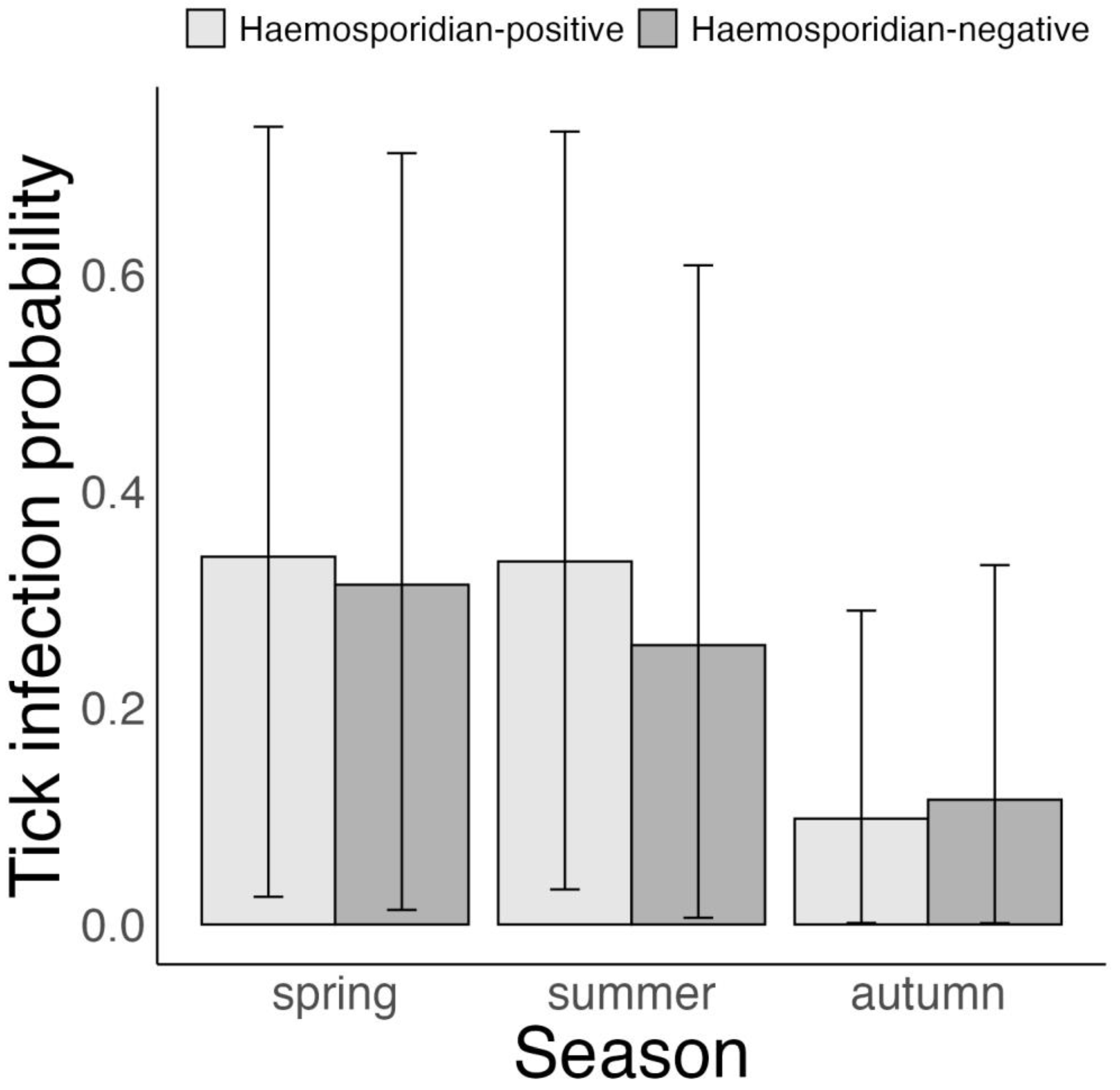
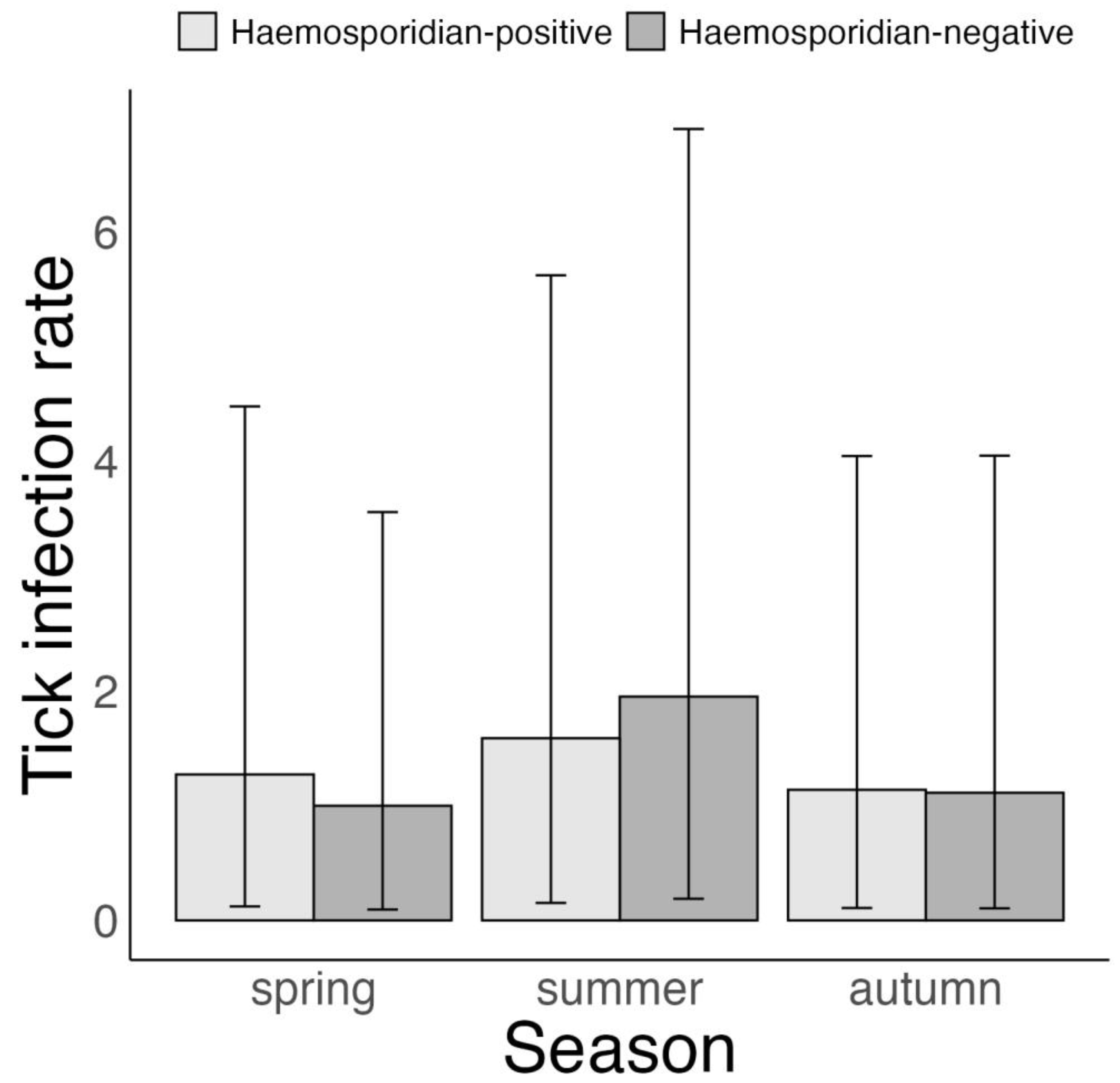
3.4. Association between Tick Borrelia Infection and Host Haemosporidian Infection Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme Borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, Migration and Emerging Zoonoses: West Nile Virus, Lyme Disease, Influenza A and Enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humair, P.-F. Birds and Borrelia. Int. J. Med. Microbiol. 2002, 291, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Humair, P.-F.; Postic, D.; Wallich, R.; Gern, L. An Avian Reservoir (Turdus Merula) of the Lyme Borreliosis Spirochetes. Zent. Bakteriol. 1998, 287, 521–538. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef] [Green Version]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Biard, C.; Monceau, K.; Motreuil, S.; Moreau, J. Interpreting Immunological Indices: The Importance of Taking Parasite Community into Account. An Example in Blackbirds Turdus Merula. Methods Ecol. Evol. 2015, 6, 960–972. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Lambert, S.; Paton, D.C. Ticks (Ixodes Sp.) and Blood Parasites (Haemoproteus Spp.) in New Holland Honeyeaters (Phylidonyris Novaehollandiae): Evidence for Site Specificity and Fitness Costs. Emu 2006, 106, 113–118. [Google Scholar] [CrossRef]
- Norte, A.; Araújo, P.; Augusto, L.; Guímaro, H.; Santos, S.; Lopes, R.; Núncio, M.; Ramos, J.; Lopes de Carvalho, I. Effects of Stress Exposure in Captivity on Physiology and Infection in Avian Hosts: No Evidence of Increased Borrelia Burgdorferi Sl Infectivity to Vector Ticks. Microb. Ecol. 2022, 83, 202–215. [Google Scholar] [CrossRef]
- Norte, A.C.; Lobato, D.N.C.; Braga, E.M.; Antonini, Y.; Lacorte, G.; Gonçalves, M.; Lopes De Carvalho, I.; Gern, L.; Núncio, M.S.; Ramos, J.A. Do Ticks and Borrelia Burgdorferi s.l. Constitute a Burden to Birds? Parasitol. Res. 2013, 112, 1903–1912. [Google Scholar] [CrossRef]
- Newman, E.A.; Eisen, L.; Eisen, R.J.; Fedorova, N.; Hasty, J.M.; Vaughn, C.; Lane, R.S. Borrelia Burgdorferi Sensu Lato Spirochetes in Wild Birds in Northwestern California: Associations with Ecological Factors, Bird Behavior and Tick Infestation. PLoS ONE 2015, 10, e0118146. [Google Scholar] [CrossRef]
- Piesman, J.; Dolan, M.C.; Schriefer, M.E.; Burkot, T.R. Ability of Experimentally Infected Chickens to Infect Ticks with the Lyme Disease Spirochete, Borrelia Burgdorferi. Am. J. Trop. Med. Hyg. 1996, 54, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Barber, I.; Dingemanse, N.J. Parasitism and the Evolutionary Ecology of Animal Personality. Philos. Trans. R. Soc. B: Biol. Sci. 2010, 365, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Rollins, R.E.; Mouchet, A.; Margos, G.; Chitimia-Dobler, L.; Fingerle, V.; Becker, N.S.; Dingemanse, N.J. Repeatable Differences in Exploratory Behaviour Predict Tick Infestation Probability in Wild Great Tits. Behav. Ecol. Sociobiol. 2021, 75, 1–10. [Google Scholar] [CrossRef]
- Sih, A.; Spiegel, O.; Godfrey, S.; Leu, S.; Bull, C.M. Integrating Social Networks, Animal Personalities, Movement Ecology and Parasites: A Framework with Examples from a Lizard. Anim. Behav. 2018, 136, 195–205. [Google Scholar] [CrossRef]
- Heylen, D.J.A.; Matthysen, E. Experimental Evidence for Host Preference in a Tick Parasitizing Songbird Nestlings. Oikos 2011, 120, 1209–1216. [Google Scholar] [CrossRef]
- Christe, P.; Giorgi, M.S.; Vogel, P.; Arlettaz, R. Differential Species-specific Ectoparasitic Mite Intensities in Two Intimately Coexisting Sibling Bat Species: Resource-mediated Host Attractiveness or Parasite Specialization? J. Anim. Ecol. 2003, 72, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Christe, P.; Møller, A.P.; de Lope, F. Immunocompetence and Nestling Survival in the House Martin: The Tasty Chick Hypothesis. Oikos 1998, 83, 175–179. [Google Scholar] [CrossRef]
- Václav, R.; Valera, F. Host Preference of a Haematophagous Avian Ectoparasite: A Micronutrient Supplementation Experiment to Test an Evolutionary Trade-Off. Biol. J. Linn. Soc. 2018, 125, 171–183. [Google Scholar] [CrossRef]
- Benelli, G. Pathogens Manipulating Tick Behavior—Through a Glass, Darkly. Pathogens 2020, 9, 664. [Google Scholar] [CrossRef]
- Faulde, M.K.; Robbins, R.G. Tick Infestation Risk and Borrelia Burgdorferi s.l. Infection-Induced Increase in Host-Finding Efficacy of Female Ixodes Ricinus under Natural Conditions. Exp. Appl. Acarol. 2008, 44, 137–145. [Google Scholar] [CrossRef]
- Herrmann, C.; Gern, L. Search for Blood or Water Is Influenced by Borrelia Burgdorferi in Ixodes Ricinus. Parasites Vectors 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, C.; Gern, L. Do the Level of Energy Reserves, Hydration Status and Borrelia Infection Influence Walking by Ixodes Ricinus (Acari: Ixodidae) Ticks? Parasitology 2012, 139, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia Burgdorferi Promotes the Establishment of Babesia Microti in the Northeastern United States. PLoS ONE 2015, 9, e115494. [Google Scholar] [CrossRef] [PubMed]
- Chvostáč, M.; Špitalská, E.; Václav, R.; Vaculová, T.; Minichová, L.; Derdáková, M. Seasonal Patterns in the Prevalence and Diversity of Tick-Borne Borrelia Burgdorferi Sensu Lato, Anaplasma Phagocytophilum and Rickettsia Spp. in an Urban Temperate Forest in South Western Slovakia. Int. J. Environ. Res. Public Health 2018, 15, 994. [Google Scholar] [CrossRef] [Green Version]
- Mtierová, Z.; Derdáková, M.; Chvostáč, M.; Didyk, Y.M.; Mangová, B.; Rusňáková Tarageľová, V.; Selyemová, D.; Šujanová, A.; Václav, R. Local Population Structure and Seasonal Variability of Borrelia Garinii Genotypes in Ixodes Ricinus Ticks, Slovakia. Int. J. Environ. Res. Public Health 2020, 17, 3607. [Google Scholar] [CrossRef]
- Šujanová, A.; Špitalská, E.; Václav, R. Seasonal Dynamics and Diversity of Haemosporidians in a Natural Woodland Bird Community in Slovakia. Diversity 2021, 13, 439. [Google Scholar] [CrossRef]
- Berndtson, K. Review of Evidence for Immune Evasion and Persistent Infection in Lyme Disease. Int. J. Gen. Med. 2013, 6, 291–306. [Google Scholar] [CrossRef] [Green Version]
- Brissette, C.; Kraiczy, P. Pathogenesis and Immune Defense. In Lyme Borreliosis; Hunfeld, K.-P., Gray, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–75. ISBN 978–3-030–93680–8. [Google Scholar]
- Duc, M.; Ilgūnas, M.; Valkiūnas, G. Patterns of Haemoproteus Majoris (Haemosporida, Haemoproteidae) Megalomeront Development. Acta Trop. 2020, 212, 105706. [Google Scholar] [CrossRef]
- Grillon, A.; Westermann, B.; Cantero, P.; Jaulhac, B.; Voordouw, M.J.; Kapps, D.; Collin, E.; Barthel, C.; Ehret-Sabatier, L.; Boulanger, N. Identification of Borrelia Protein Candidates in Mouse Skin for Potential Diagnosis of Disseminated Lyme Borreliosis. Sci. Rep. 2017, 7, 16719. [Google Scholar] [CrossRef] [Green Version]
- Gylfe, Å.; Bergström, S.; Lundstróm, J.; Olsen, B. Reactivation of Borrelia Infection in Birds. Nature 2000, 403, 724–725. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Bairlein, F.; Iezhova, T.A.; Dolnik, O.V. Factors Affecting the Relapse of Haemoproteus Belopolskyi Infections and the Parasitaemia of Trypanosoma Spp. in a Naturally Infected European Songbird, the Blackcap, Sylvia Atricapilla. Parasitol. Res. 2004, 93, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Reinoso-Pérez, M.T.; Dhondt, K.V.; Sydenstricker, A.V.; Heylen, D.; Dhondt, A.A. Complex Interactions between Bacteria and Haemosporidia in Coinfected Hosts: An Experiment. Ecol. Evol. 2020, 10, 5801–5814. [Google Scholar] [CrossRef] [PubMed]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-Infection of Blacklegged Ticks with Babesia Microti and Borrelia Burgdorferi Is Higher than Expected and Acquired from Small Mammal Hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Krause, P.J. Concurrent Lyme Disease and Babesiosis: Evidence for Increased Severity and Duration of Illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Dietz, K.; Lackner, P.; Pasche, B.; Fendel, R.; Mordmüller, B.; Ben-Smith, A.; Hoffmann, W.H. Schistosoma Co-Infection Protects against Brain Pathology but Does Not Prevent Severe Disease and Death in a Murine Model of Cerebral Malaria. Int. J. Parasitol. 2011, 41, 21–31. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Hayward, A.; Lemon, H.; Sweeny, A.R.; Bal, X.; Kenyon, F.; Pilkington, J.G.; Pemberton, J.M.; Nussey, D.H.; McNeilly, T.N. Functionally Distinct T-Helper Cell Phenotypes Predict Resistance to Different Types of Parasites in a Wild Mammal. Sci. Rep. 2022, 12, 3197. [Google Scholar] [CrossRef]
- Hartmann, W.; Haben, I.; Fleischer, B.; Breloer, M. Pathogenic Nematodes Suppress Humoral Responses to Third-Party Antigens In Vivo by IL-10–Mediated Interference with Th Cell Function. J.I. 2011, 187, 4088–4099. [Google Scholar] [CrossRef] [Green Version]
- Nacher, M.; Singhasivanon, P.; Yimsamran, S.; Manibunyong, W.; Thanyavanich, N.; Wuthisen, P.; Looareesuwan, S. Intestinal Helminth Infections Are Associated with Increased Incidence of Plasmodium Falciparum Malaria in Thailand. J. Parasitol. 2002, 88, 55–58. [Google Scholar] [CrossRef]
- Václav, R.; Blažeková, J. The Effect of Anthelmintic Treatment on Coccidia Oocyst Shedding in a Wild Mammal Host with Intermittent Cestode Infection. Sci. World J. 2014, 2014, e302903. [Google Scholar] [CrossRef] [Green Version]
- Knowles, S.C.L.; Nakagawa, S.; Sheldon, B.C. Elevated Reproductive Effort Increases Blood Parasitaemia and Decreases Immune Function in Birds: A Meta-Regression Approach. Funct. Ecol. 2009, 23, 405–415. [Google Scholar] [CrossRef]
- Degen, W.G.J.; van Daal, N.; Rothwell, L.; Kaiser, P.; Schijns, V.E.J.C. Th1/Th2 Polarization by Viral and Helminth Infection in Birds. Vet. Microbiol. 2005, 105, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by the Tick-Borne Pathogens Babesia Microti and Borrelia Burgdorferi: Ecological, Epidemiological and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šujanová, A.; Václav, R. Phylogeographic Patterns of Haemoproteid Assemblages of Selected Avian Hosts: Ecological and Evolutionary Implications. Microorganisms 2022, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Slovák, M. Obrázkový kľúč lariev a nýmf kliešťov (Acari: Ixodida) fauny Slovenska. Entomofauna Carpathica 2014, 26, 12–18. [Google Scholar]
- Slovák, M. Obrázkový Kľúč Dospelých Kliešťov (Acari: Ixodida) Fauny Slovenska. Entomofauna Carpathica 2010, 22, 8–13. [Google Scholar]
- Eisen, L. Vector Competence Studies with Hard Ticks and Borrelia Burgdorferi Sensu Lato Spirochetes: A Review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
- Guy, E.C.; Stanek, G. Detection of Borrelia Burgdorferi in Patients with Lyme Disease by the Polymerase Chain Reaction. J. Clin. Pathol. 1991, 44, 610. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, S.A.; Bormane, A.; Dinnis, R.E.; Seelig, F.; Dobson, A.D.M.; Aanensen, D.M.; James, M.C.; Donaghy, M.; Randolph, S.E.; Feil, E.J.; et al. Host Migration Impacts on the Phylogeography of Lyme Borreliosis Spirochaete Species in Europe. Environ. Microbiol. 2011, 13, 184–192. [Google Scholar] [CrossRef]
- Black, W.C., 4th; Roehrdanz, R.L. Mitochondrial Gene Order Is Not Conserved in Arthropods: Prostriate and Metastriate Tick Mitochondrial Genomes. Mol. Biol. Evol. 1998, 15, 1772–1785. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Springs Harbor Laboratory Press: Cold Springs Harbor, NY, USA, 1989. [Google Scholar]
- Derdáková, M.; Beati, L.; Pet’ko, B.; Stanko, M.; Fish, D. Genetic Variability within Borrelia Burgdorferi Sensu Lato Genospecies Established by PCR-Single-Strand Conformation Polymorphism Analysis of the RrfA-RrlB Intergenic Spacer in Ixodes Ricinus Ticks from the Czech Republic. Appl. Environ. Microbiol. 2003, 69, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.A.; Weckstein, J.D.; Fecchio, A.; Tkach, V.V. A New Real-Time PCR Protocol for Detection of Avian Haemosporidians. Parasites Vectors 2015, 8, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Jönsson, J.; Bensch, S. Persistence of Avian Haemosporidians in the Wild: A Case Study to Illustrate Seasonal Infection Patterns in Relation to Host Life Stages. Int. J. Parasitol. 2020, 50, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Bürkner, P.-C. Advanced Bayesian Multilevel Modeling with the R Package Brms. R J. 2018, 10, 395–411. [Google Scholar] [CrossRef]
- Bürkner, P.-C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Stan Development Team Stan User’s Guide. Available online: https://mc-stan.org/docs/stan-users-guide/index.html (accessed on 16 September 2022).
- Kruschke, J.K. Bayesian Analysis Reporting Guidelines. Nat. Hum. Behav. 2021, 5, 1282–1291. [Google Scholar] [CrossRef]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical Bayesian Model Evaluation Using Leave-One-out Cross-Validation and WAIC. Stat. Comput. 2017, 27, 1413–1432. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. RStudio Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Available online: https://ggplot2.tidyverse.org (accessed on 4 November 2022).
- Kay, M.; Mastny, T. Tidybayes: Tidy Data and “Geoms” for Bayesian Models. Available online: https://mjskay.github.io/tidybayes (accessed on 14 October 2022).
- Chepkwony, R.; van Bommel, S.; van Langevelde, F. Interactive Effects of Biological, Human and Environmental Factors on Tick Loads in Boran Cattle in Tropical Drylands. Parasites Vectors 2021, 14, 188. [Google Scholar] [CrossRef]
- Møller, A.P. Parasitism and the Evolution of Host Life History. In Host-Parasite Evolution. General Principles and Avian Models; Clayton, D.H., Moore, J., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 105–127. [Google Scholar]
- Waite, J.L.; Henry, A.R.; Owen, J.P.; Clayton, D.H. An Experimental Test of the Effects of Behavioral and Immunological Defenses against Vectors: Do They Interact to Protect Birds from Blood Parasites? Parasites Vectors 2014, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Taragel’ová, V.; Koči, J.; Hanincová, K.; Kurtenbach, K.; Derdáková, M.; Ogden, N.H.; Literák, I.; Kocianová, E.; Labuda, M. Blackbirds and Song Thrushes Constitute a Key Reservoir of Borrelia Garinii, the Causative Agent of Borreliosis in Central Europe. Appl. Environ. Microbiol. 2008, 74, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.T.; van Riper, C., III. Pathogenicity and Epizootiology of Avian Haematozoa: Plasmodium, Haemoproteus, and Leucocytozoon. In Bird-Parasite Interactions: Ecology, Evolution, and Behavior; Loye, J.E., Zuk, M., Eds.; Oxford University Press: London, UK, 1991; pp. 19–48. [Google Scholar]
- Olsen, B. Borrelia. In Infectious Diseases of Wild Birds; Thomas, N.J., Hunter, D.B., Atkinson, C.T., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 341–351. [Google Scholar]
- Norte, A.C.; Costantini, D.; Araújo, P.M.; Eens, M.; Ramos, J.A.; Heylen, D. Experimental Infection by Microparasites Affects the Oxidative Balance in Their Avian Reservoir Host the Blackbird Turdus Merula. Ticks Tick Borne Dis. 2018, 9, 720–729. [Google Scholar] [CrossRef]
- Sürth, V.; Lopes de Carvalho, I.; Núncio, M.S.; Norte, A.C.; Kraiczy, P. Bactericidal Activity of Avian Complement: A Contribution to Understand Avian-Host Tropism of Lyme Borreliae. Parasites Vectors 2021, 14, 451. [Google Scholar] [CrossRef]
- Heylen, D.; Bisaglia, B.; Fracasso, G.; Prinsen, E.; Müller, W.; Matthysen, E. Ineffective Humoral Anti-Tick IgY-Response in Birds: Reaction against Pathogen Constituents. Open Res. Eur. 2021, 1, 8. [Google Scholar] [CrossRef]
- Heylen, D.J.A.; Müller, W.; Vermeulen, A.; Sprong, H.; Matthysen, E. Virulence of Recurrent Infestations with Borrelia-Infected Ticks in a Borrelia-Amplifying Bird. Sci. Rep. 2015, 5, 16150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heylen, D.J.A.; Madder, M.; Matthysen, E. Lack of Resistance against the Tick Ixodes Ricinus in Two Related Passerine Bird Species. Int. J. Parasitol. 2010, 40, 183–191. [Google Scholar] [CrossRef]
- Radolf, J.D.; Caimano, M.J.; Stevenson, B.; Hu, L.T. Of Ticks, Mice and Men: Understanding the Dual-Host Lifestyle of Lyme Disease Spirochaetes. Nat. Rev. Microbiol. 2012, 10, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, P.A.; Labuda, M. Tick-Host Interactions: Saliva-Activated Transmission. Parasitology 2004, 129, S177–S189. [Google Scholar] [CrossRef]
- Zeidner, N.; Mbow, M.L.; Dolan, M.; Massung, R.; Baca, E.; Piesman, J. Effects of Ixodes Scapularis and Borrelia Burgdorferi on Modulation of the Host Immune Response: Induction of a TH2 Cytokine Response in Lyme Disease-Susceptible (C3H/HeJ) Mice but Not in Disease-Resistant (BALB/c) Mice. Infect. Immun. 1997, 65, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Hartgers, F.; Yazdanbakhsh, M. Co-infection of Helminths and Malaria: Modulation of the Immune Responses to Malaria. Parasite Immunol. 2006, 28, 497–506. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Schäfer, S.M.; Sewell, H.-S.; Peacey, M.; Hoodless, A.; Nuttall, P.A.; Randolph, S.E. Differential Survival of Lyme Borreliosis Spirochetes in Ticks That Feed on Birds. Infect. Immun. 2002, 70, 5893–5895. [Google Scholar] [CrossRef] [Green Version]
- Vannier, E.; Krause, P.J. Human Babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.L.; Gaston, K.J.; Sharp, S.P.; McGowan, A.; Simeoni, M.; Hatchwell, B.J. Effects of Urbanisation on Disease Prevalence and Age Structure in Blackbird Turdus Merula Populations. Oikos 2009, 118, 774–782. [Google Scholar] [CrossRef]
- Gregoire, A.; Faivre, B.; Heeb, P.; Cezilly, F. A Comparison of Infestation Patterns by Ixodes Ticks in Urban and Rural Populations of the Common Blackbird Turdus Merula. Ibis 2002, 144, 640–645. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Brunton, D.; Stidworthy, M.F.; Elsheikha, H.M.; Pennycott, T.; Schulze, C.; Braun, M.; Wink, M.; Gerlach, H.; Pendl, H.; et al. Haemoproteus Minutus Is Highly Virulent for Australasian and South American Parrots. Parasites Vectors 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; Iezhova, T.A.; Krizanauskiene, A.; Markovets, M.Y.; Bensch, S.; Valkiūnas, G. Molecular Characterization and Distribution of Haemoproteus Minutus (Haemosporida, Haemoproteidae): A Pathogenic Avian Parasite. Parasitol. Int. 2013, 62, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Telfer, S.; Lambin, X.; Birtles, R.; Beldomenico, P.; Burthe, S.; Paterson, S.; Begon, M. Species Interactions in a Parasite Community Drive Infection Risk in a Wildlife Population. Science 2010, 330, 243–246. [Google Scholar] [CrossRef]


| Bird Species | No. Birds Sampled | No. Birds Infested with Ticks | No. Birds with Infected Ticks | No. Ticks | No. B. burgdorferi s.l. Infected Ticks | No. B. garinii Infected Ticks (L/N) | No. B. valaisiana Infected Ticks (L/N) | No. B. garinii + B. valaisiana Infected Ticks (L/N) | No. B. afzelii Infected Ticks (L/N) | No. B. lusitaniae Infected Ticks (L/N) | No. B. burgdorferi s.s Infected Ticks (L/N) | No. B. spielmanii Infected Ticks (L/N) | No. B. afzelii + B. garinii Infected Ticks (L/N) | No. B. garinii + B. lusitaniae Infected Ticks (L/N) | No. B. afzelii + B. valaisiana Infected Ticks (L/N) | No. B. b. s.s. + B. lusitaniae Infected Ticks (L/N) | No. B. garinii + ? Infected Ticks (L/N) | No. B. valaisiana + ? Infected Ticks (L/N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. coccothraustes | 44 | 19 | 5 | 60 | 7 | 1 (0/1) | 3 (0/3) | 1 (0/1) | 2 (1/1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyanistes caeruleus | 121 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Emberiza schoeniclus | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erithacus rubecula | 355 | 120 | 21 | 338 | 26 | 12 (8/4) | 7 (1/6) | 0 | 6 (0/6) | 0 | 0 | 1 (1/0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fringilla coelebs | 31 | 4 | 3 | 16 | 5 | 2 (0/2) | 0 | 0 | 3 (0/3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Garrulus glandarius | 11 | 5 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chloris chloris | 12 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 1 (0/1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luscinia megarhynchos | 17 | 5 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parus major | 175 | 35 | 8 | 67 | 8 | 4 (3/1) | 2 (1/1) | 0 | 2 (0/2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Passer montanus | 5 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phylloscopus collybita | 74 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phylloscopus trochilus | 27 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poecile palustris | 23 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prunella modularis | 75 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 (0/1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyrrhula pyrrhula | 39 | 5 | 1 | 9 | 1 | 0 | 0 | 0 | 1 (0/1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sylvia atricapilla | 372 | 17 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sylvia communis | 12 | 3 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T. troglodytes | 29 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Turdus merula | 96 | 86 | 76 | 909 | 610 | 322 (204/118) | 179 (57/122) | 81 (29/52) | 11 (0/11) | 4 (0/4) | 3 (0/3) | 0 | 3 (0/3) | 1 (0/1) | 1 (0/1) | 1 (0/1) | 1 (0/1) | 3 (1/2) |
| Turdus philomelos | 40 | 27 | 19 | 169 | 105 | 86 (63/23) | 6 (0/6) | 10 (5/5) | 2 (0/2) | 0 | 0 | 0 | 0 | 1 (0/1) | 0 | 0 | 0 | 0 |
| Total | 1561 | 340 | 135 | 1653 | 764 | 427 (278/148) | 197 (59/138) | 92 (34/58) | 29 (1/28) | 4 (0/4) | 3 (0/3) | 1 (1/0) | 3 (0/3) | 2 (0/2) | 1 (0/1) | 1 (0/1) | 1 (0/1) | 3 (1/2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šujanová, A.; Čužiová, Z.; Václav, R. The Infection Rate of Bird-Feeding Ixodes ricinus Ticks with Borrelia garinii and B. valaisiana Varies with Host Haemosporidian Infection Status. Microorganisms 2023, 11, 60. https://doi.org/10.3390/microorganisms11010060
Šujanová A, Čužiová Z, Václav R. The Infection Rate of Bird-Feeding Ixodes ricinus Ticks with Borrelia garinii and B. valaisiana Varies with Host Haemosporidian Infection Status. Microorganisms. 2023; 11(1):60. https://doi.org/10.3390/microorganisms11010060
Chicago/Turabian StyleŠujanová, Alžbeta, Zuzana Čužiová, and Radovan Václav. 2023. "The Infection Rate of Bird-Feeding Ixodes ricinus Ticks with Borrelia garinii and B. valaisiana Varies with Host Haemosporidian Infection Status" Microorganisms 11, no. 1: 60. https://doi.org/10.3390/microorganisms11010060





