Lipid Production of Schizochytrium sp. HBW10 Isolated from Coastal Waters of Northern China Cultivated in Food Waste Hydrolysate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Identification
2.2. Growth Curve and Lipid Production of S. sp. HBW10
2.3. Lipid Production with Varied Carbon and Nitrogen Substrates
2.4. Batch Axenic Culture with Food Waste Hydrolysate (FWH)
2.4.1. Preparation of Food Waste Hydrolysate (FWH)
2.4.2. Lipid Production with Different Content Levels of FWH
2.4.3. Influence of Initial pH on Lipid Production
2.5. Analytical Methods
2.5.1. Biomass Analysis
2.5.2. Lipid extraction and analysis
3. Results
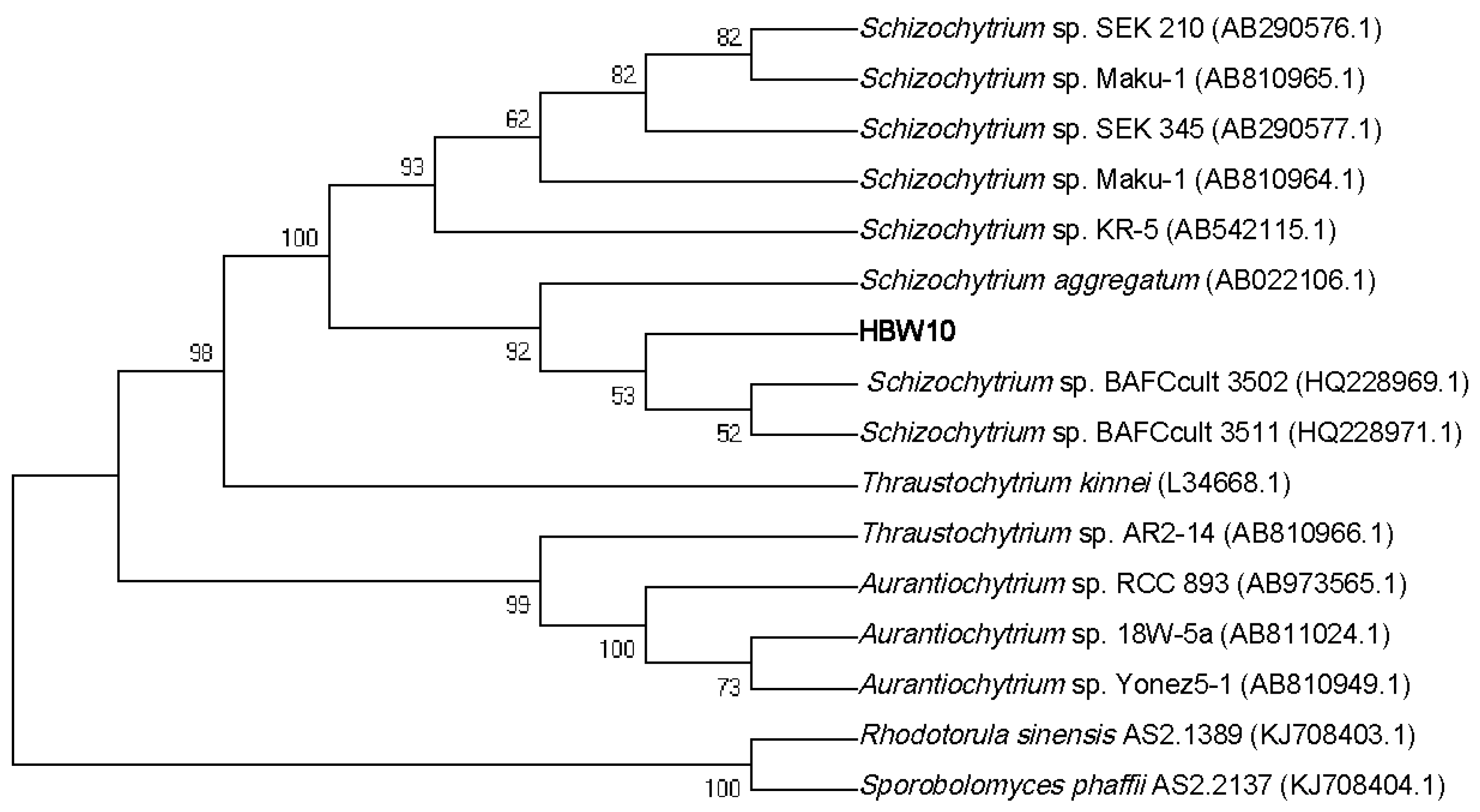
3.1. Strain Isolation and Phylogenetic Analysis
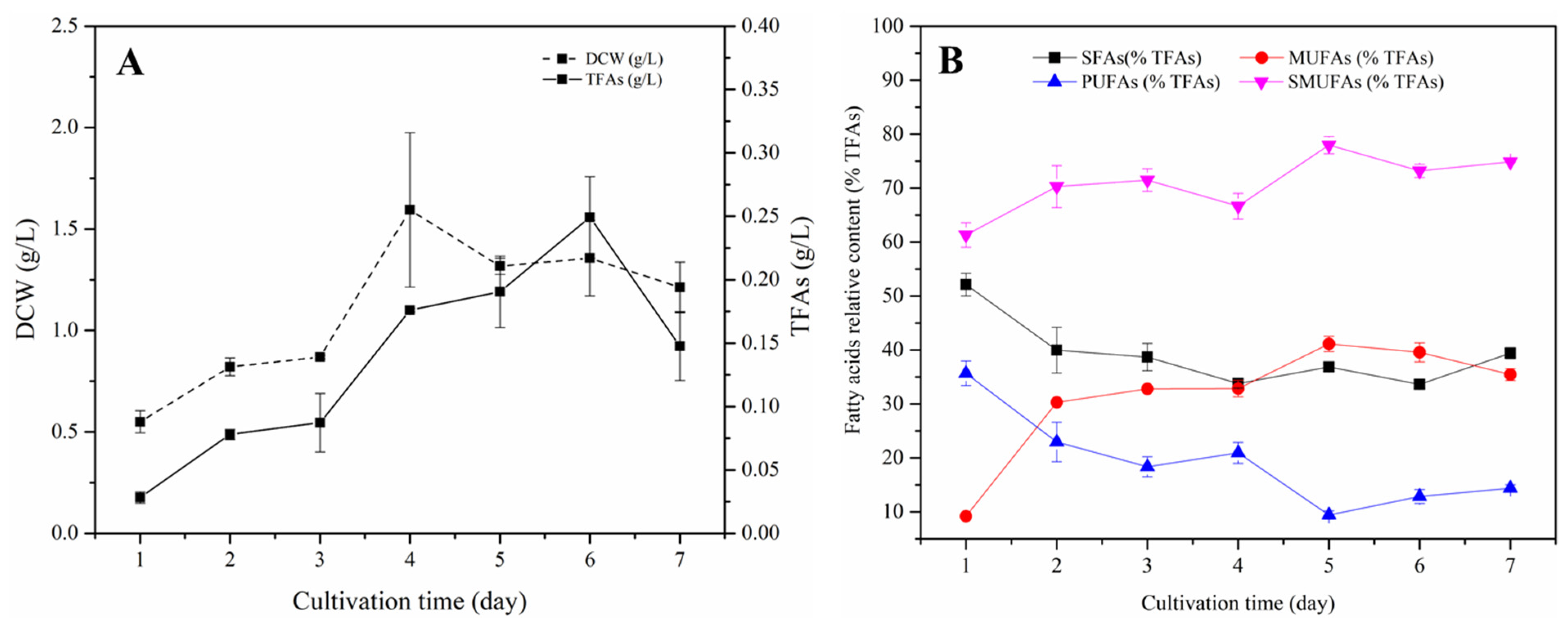
3.2. Characteristics of S. sp. HBW10 for Biomass and Lipid Accumulation
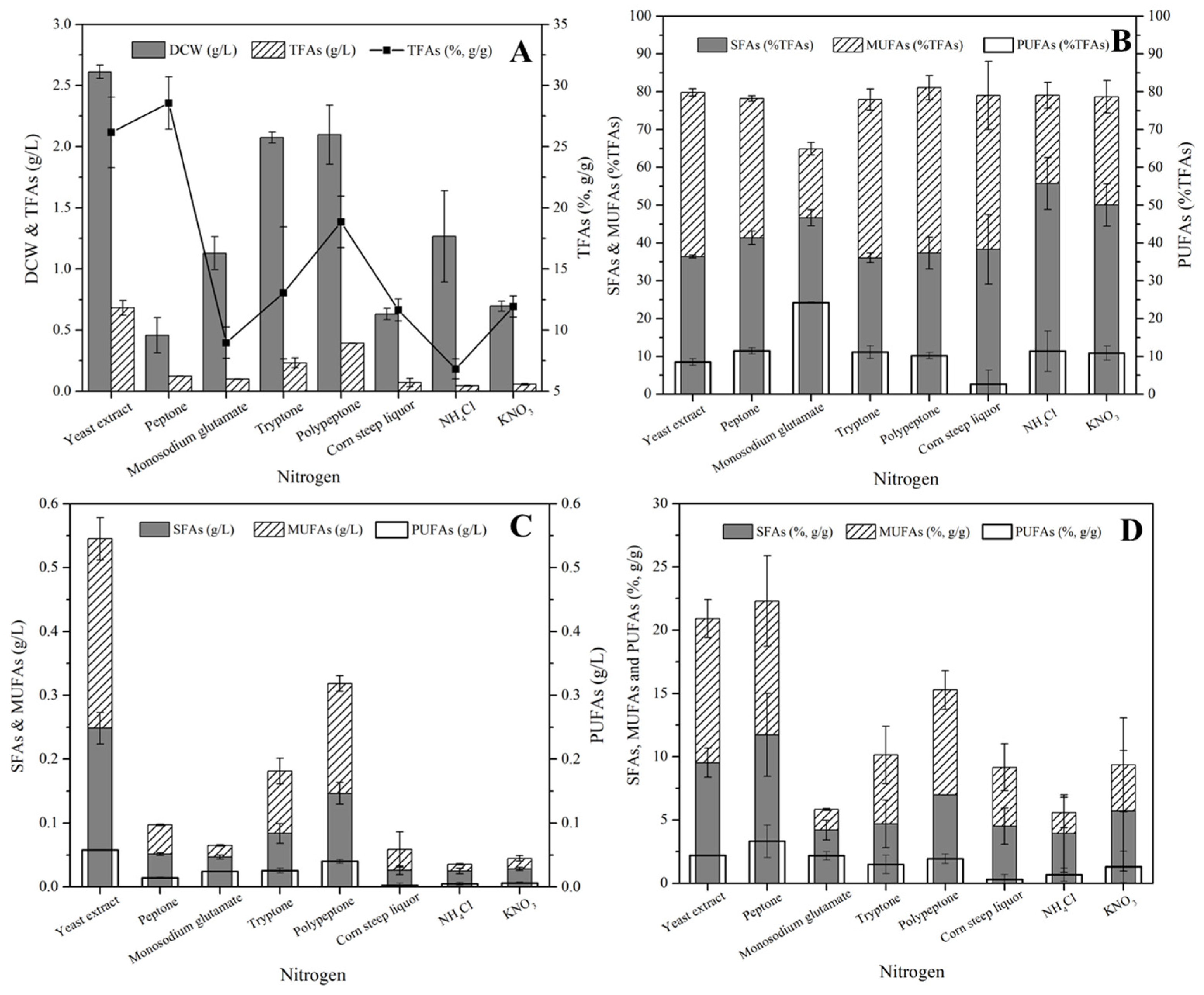
3.3. Lipid Production of S. sp. HBW10 under Varied Carbon and Nitrogen Substrates
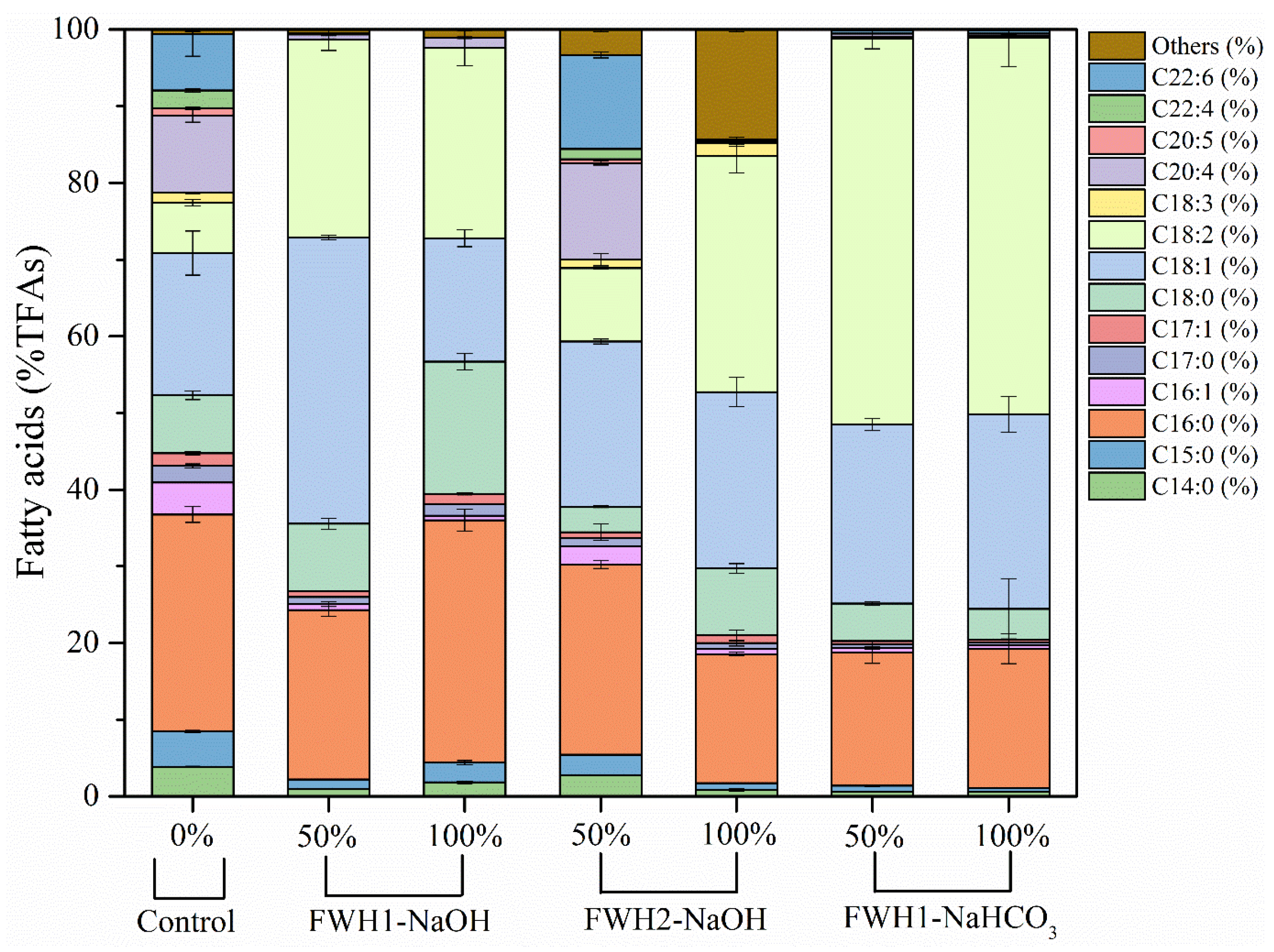
3.4. Utilization of FWH as Feedstock for Lipid Production of S. sp. HBW10
3.5. Influence of the Initial pH on the Biomass and Lipid Production of S. sp. HBW10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, Y.; Li, S.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Effect of lipase addition on hydrolysis and biomethane production of Chinese food waste. Bioresour. Technol. 2015, 179, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Algapani, E.D.; Wang, J.; Qiao, W.; Su, M.; Goglio, A.; Wandera, S.M.; Jiang, M.; Pan, X.; Adani, F.; Dong, R. Improving methane production and anaerobic digestion stability of food waste by extracting lipids and mixing it with sewage sludge. Bioresour. Technol. 2017, 244 Pt 1, 996–1005. [Google Scholar] [PubMed]
- Kaur, G.; Wang, H.; To, M.H.; Roelants, S.L.K.W.; Soetaert, W.; Lin, C.S.K. Efficient sophorolipids production using food waste. J. Clean. Prod. 2019, 232, 1–11. [Google Scholar]
- Rafieenia, R.; Pivato, A.; Lavagnolo, M.C. Optimization of hydrogen production from food waste using anaerobic mixed cultures pretreated with waste frying oil. Renew. Energy 2019, 139, 1077–1085. [Google Scholar]
- Huang, J.; Feng, H.; Huang, L.; Ying, X.; Shen, D.; Chen, T.; Shen, X.; Zhou, Y.; Xu, Y. Continuous hydrogen production from food waste by anaerobic digestion (AD) coupled single-chamber microbial electrolysis cell (MEC) under negative pressure. Waste Manag. 2020, 103, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, X.; Zhu, X.; Wang, Z.; Li, H.; Wang, Z. Transcriptome mechanism of utilizing corn steep liquor as the sole nitrogen resource for lipid and DHA biosynthesis in marine oleaginous protist Aurantiochytrium sp. Biomolecules 2019, 9, 695. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci. Total Environ. 2020, 736, 139691. [Google Scholar] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low-cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Pleissner, D.; Lam, W.C.; Han, W.; Lau, K.Y.; Cheung, L.C.; Lee, M.W.; Lei, H.M.; Lo, K.Y.; Ng, W.Y.; Sun, Z.; et al. Fermentative polyhydroxybutyrate production from a novel feedstock derived from bakery waste. BioMed Res. Int. 2014, 2014, 819474. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Pleissner, D.; Lau, K.Y.; Zhang, C.; Lin, C.S. Plasticizer and surfactant formation from food-waste- and algal biomass-derived lipids. ChemSusChem 2015, 8, 1686–1691. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Cheok, W.L.; de Toledo, R.A.; Shim, H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 2014, 173, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xie, T.; Li, P.; Jian, B.; Li, X.; Xie, Y.; Zhang, Y. Enhanced lipid production and nutrient utilization of food waste hydrolysate by mixed culture of oleaginous yeast Rhodosporidium toruloides and oleaginous microalgae Chlorella vulgaris. Renew. Energy 2018, 126, 915–923. [Google Scholar] [CrossRef]
- Qin, L.; Liu, L.; Wang, Z.; Chen, W.; Wei, D. Efficient resource recycling from liquid digestate by microalgae-yeast mixed culture and the assessment of key gene transcription related to nitrogen assimilation in microalgae. Bioresour. Technol. 2018, 264, 90–97. [Google Scholar] [CrossRef]
- Singh, N.; Choudhury, B. Valorization of food-waste hydrolysate by Lentibacillus salarius NS12IITR for the production of branched chain fatty acid enriched lipid with potential application as a feedstock for improved biodiesel. Waste Manag. 2019, 94, 1–9. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Gong, G.; Zhang, X.; Tan, T. Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour. Technol. 2014, 164, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Ling, T.C.; Arya, S.S.; Chang, J.S. Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresour. Technol. 2018, 267, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, Z.; Gao, M.; Wu, C.; Wang, Q. Microbial lipid production from food waste saccharified liquid under two-stage process. Bioresour. Technol. 2019, 289, 121626. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cabello, M.; García, I.L.; Papadaki, A.; Tsouko, E.; Koutinas, A.; Dorado, M.P. Biodiesel production using microbial lipids derived from food waste discarded by catering services. Bioresour. Technol. 2020, 323, 124597. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Lam, W.C.; Sun, Z.; Lin, C.S. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 2013, 137, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Bian, D.; Xie, Y.; Jiang, X.; Li, X.; Li, P.; Zhang, Y.; Xie, T. Utilization of food waste hydrolysate for microbial lipid and protein production by Rhodosporidium toruloides Y2. J. Chem. Technol. Biotechnol. 2017, 92, 666–673. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Gao, M.; Ma, Y.; Ma, H.; Zhang, M.; Liu, Y.; Wang, Q. Microbial lipid production from food waste saccharified liquid and the effects of compositions. Energy Convers. Manag. 2018, 172, 306–315. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.M.; Sun, Z.; Liu, S.F.; Qin, Z.H.; Mou, J.H.; Zhou, Z.G.; Lin, C.S.K. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef] [PubMed]
- Heggeset, T.M.B.; Ertesvag, H.; Liu, B.; Ellingsen, T.E.; Vadstein, O.; Aasen, I.M. Lipid and DHA-production in Aurantiochytrium sp.—Responses to nitrogen starvation and oxygen limitation revealed by analyses of production kinetics and global transcriptomes. Sci. Rep. 2019, 9, 19470. [Google Scholar] [CrossRef]
- Leong, H.Y.; Su, C.A.; Lee, B.S.; Lan, J.C.; Law, C.L.; Chang, J.S.; Show, P.L. Development of Aurantiochytrium limacinum SR21 cultivation using salt-rich waste feedstock for docosahexaenoic acid production and application of natural colourant in food product. Bioresour. Technol. 2019, 271, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, H.; Xie, Y.; He, Y.; Sen, B.; Wang, G. Culturable diversity and lipid production profile of Labyrinthulomycete protists isolated from coastal mangrove habitats of China. Mar. Drugs 2019, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, M.; He, M.; Ye, Y.; Huang, J. Illustrating and enhancing the biosynthesis of astaxanthin and docosahexaenoic acid in Aurantiochytrium sp. SK4. Mar. Drugs 2019, 17, 45. [Google Scholar] [CrossRef]
- Chen, W.; Ma, L.; Zhou, P.; Zhu, Y.; Wang, X.; Luo, X.; Bao, Z.; Yu, L. A novel feedstock for biodiesel production: The application of palmitic acid from Schizochytrium. Energy 2015, 86, 128–138. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.W.; Oh, B.R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef]
- Wang, Q.; Sen, B.; Liu, X.; He, Y.; Xie, Y.; Wang, G. Enhanced saturated fatty acids accumulation in cultures of newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. for large-scale biodiesel production. Sci. Total Environ. 2018, 631–632, 994–1004. [Google Scholar]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Lee Chang, K.J.; Paul, H.; Nichols, P.D.; Koutoulis, A.; Blackburn, S.I. Australian thraustochytrids: Potential production of dietary long-chain omega-3 oils using crude glycerol. J. Funct. Foods 2015, 19, 810–820. [Google Scholar] [CrossRef]
- Jung, I.S.; Lovitt, R.W. Intgrated production of long chain polyunsaturated fatty acids (PUFA)-rich Schizochytrium biomass using a nutrient supplemented marine aquaculture wastewater. Aquacult. Eng. 2010, 43, 51–61. [Google Scholar] [CrossRef]
- Yin, F.W.; Zhu, S.Y.; Guo, D.S.; Ren, L.J.; Ji, X.J.; Huang, H.; Gao, Z. Development of a strategy for the production of docosahexaenoic acid by Schizochytrium sp. from cane molasses and algae-residue. Bioresour. Technol. 2019, 271, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.C.K.W.; Jones, E.B.G.; Vrijmoed, L.L.P. Utilization of food processing waste by Thraustochytrids. Fungal Divers. 2000, 5, 185–194. [Google Scholar]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, H.; Sen, B.; Xie, Y.; He, Y.; Park, S.; Wang, G. Improved production of docosahexaenoic acid in batch fermentation by newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. through bioprocess optimization. Syn. Syst. Biotechnol. 2018, 3, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Song, X.; Feng, Y.; Li, W.; Cui, Q. Isolation and characterization of Aurantiochytrium species: High docosahexaenoic acid (DHA) production by the newly isolated microalga, Aurantiochytrium sp. SD116. J. Oleo Sci. 2013, 62, 143–151. [Google Scholar] [CrossRef]
- Zhang, A.; Xie, Y.; He, Y.; Wang, W.; Sen, B.; Wang, G. Bio-based squalene production by Aurantiochytrium sp. through optimization of culture conditions, and elucidation of the putative biosynthetic pathway genes. Bioresour. Technol. 2019, 287, 121415. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, E.; Loperena, L.; Hinzpeter, I.; Pradel, P.; Gordillo, F.; Corsini, G.; Tello, M.; Lavin, P.; Gonzalez, A.R. Isolation and molecular characterization of Thraustochytrium strain isolated from Antarctic Peninsula and its biotechnological potential in the production of fatty acids. Braz. J. Microbiol. 2017, 48, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, D.; Byreddy, A.R.; Thyagarajan, T.; Sonkar, S.P.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J.; Puri, M. Exploring omega-3 fatty acids, enzymes and biodiesel producing thraustochytrids from Australian and Indian marine biodiversity. Biotechnol. J. 2016, 11, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Marchan, L.F.; Lee Chang, K.J.; Nichols, P.D.; Polglase, J.L.; Mitchell, W.J.; Gutierrez, T. Screening of new British thraustochytrids isolates for docosahexaenoic acid (DHA) production. J. Appl. Phycol. 2017, 29, 2831–2843. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.J.; Ji, X.J.; Huang, H.; Qu, L.; Feng, Y.; Tong, Q.Q.; Ouyang, P.K. Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl. Microbiol. Biot. 2010, 87, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ji, X.J.; Ren, L.J.; Nie, Z.K.; Feng, Y.; Wu, W.J.; Ouyang, P.K.; Huang, H. Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two-stage oxygen supply control strategy based on oxygen transfer coefficient. Lett. Appl. Microbiol. 2011, 52, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ji, X.J.; Lian, M.; Ren, L.J.; Jin, L.J.; Ouyang, P.K.; Huang, H. Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308. Appl. Biochem. Biotechnol. 2011, 164, 249–255. [Google Scholar] [PubMed]
- Huang, T.Y.; Lu, W.C.; Chu, I.M. A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Balamurugan, S.; Liu, S.F.; Zhang, M.M.; Yang, W.D.; Liu, J.S.; Li, H.Y.; Sze Ki Lin, C. Enhanced polyunsaturated fatty acid production using food wastes and biofuels byproducts by an evolved strain of Phaeodactylum tricornutum. Bioresour. Technol. 2020, 296, 122351. [Google Scholar] [CrossRef] [PubMed]

| Cultivation Time (day) | FWH (%, v/v) | TFAs (g/L) | SFAs (%TFAs) | MUFAs (%TFAs) | PUFAs (%TFAs) |
|---|---|---|---|---|---|
| Initial pH adjustment of FWH1 medium by NaOH (FWH1-NaOH) | |||||
| 1 | 0 | 0.11 ± 0.02 | 57.36 ± 1.39 | 11.24 ± 1.05 | 20.01 ± 1.68 |
| 1 | 50 | 0.06 ± 0.01 | 45.90 ± 5.69 | 17.06 ± 3.18 | 8.32 ± 1.16 |
| 1 | 100 | 0.04 ± 0.00 | 55.26 ± 3.83 | 17.6 ± 1.09 | 3.43 ± 0.64 |
| 3 | 0 | 0.16 ± 0.04 | 47.28 ± 0.08 | 25.93 ± 0.85 | 19.61 ± 0.55 |
| 3 | 50 | 0.06 ± 0.02 | 43.68 ± 3.32 | 13.8 ± 0.29 | 5.20 ± 0.91 |
| 3 | 100 | 0.05 ± 0.00 | 45.91 ± 5.85 | 15.36 ± 5.51 | 7.65 ± 1.87 |
| 5 | 0 | 0.17 ± 0.01 | 46.5 ± 1.39 | 24.35 ± 3.04 | 22.02 ± 4.14 |
| 5 | 50 | 0.10 ± 0.00 | 34.07 ± 1.56 | 38.82 ± 0.16 | 0.85 ± 0.17 |
| 5 | 100 | 0.04 ± 0.00 | 54.80 ± 2.73 | 17.97 ± 1.10 | 1.30 ± 0.11 |
| Initial pH adjustment of FWH2 medium by NaOH (FWH2-NaOH) | |||||
| 5 | 0 | 0.17 ± 0.01 | 46.50 ± 1.39 | 24.35 ± 3.04 | 22.02 ± 4.14 |
| 5 | 50 | 0.34 ± 0.03 | 34.61 ± 0.67 | 24.72 ± 0.92 | 27.81 ± 0.08 |
| 5 | 100 | 0.08 ± 0.01 | 28.06 ± 1.43 | 24.69 ± 1.28 | 0.92 ± 0.07 |
| Initial pH adjustment of FWH1 medium by NaHCO3 (FWH1-NaHCO3) | |||||
| 5 | 0 | 0.17 ± 0.01 | 46.50 ± 1.39 | 24.35 ± 3.04 | 22.02 ± 4.14 |
| 5 | 50 | 0.20 ± 0.02 | 24.14 ± 2.64 | 24.38 ± 1.05 | 0.91 ± 0.26 |
| 5 | 100 | 0.11 ± 0.01 | 23.7 ± 1.80 | 26.15 ± 2.30 | 0.91 ± 0.25 |
| Food Waste Hydrolysate (%, v/v) | pH | Biomass (g/L) | TFAs (g/L) | SFAs (%TFAs) | MUFAs (%TFAs) | PUFAs (%TFAs) |
|---|---|---|---|---|---|---|
| 0 | 6.2 | 4.06 ± 0.27 | 0.17 ± 0.01 | 46.50 ± 1.39 | 24.35 ± 3.04 | 22.02 ± 4.14 |
| 0 | 7 | 4.07 ± 0.28 | 0.10 ± 0.01 | 65.23 ± 5.34 | 5.30 ± 0.83 | 29.48 ± 5.46 |
| 0 | 8 | 4.09 ± 0.09 | 0.17 ± 0.01 | 72.07 ± 4.02 | 4.55 ± 0.16 | 23.39 ± 3.95 |
| 50 | 6.2 | 0.77 ± 0.14 | 0.10 ± 0.02 | 34.07 ± 0.31 | 38.82 ± 1.41 | 0.85 ± 0.02 |
| 50 | 7 | 3.45 ± 0.0.54 | 0.11 ± 0.01 | 88.25 ± 3.14 | 11.75 ± 3.14 | 0.00 ± 0.00 |
| 50 | 8 | 3.80 ± 0.48 | 0.12 ± 0.01 | 89.84 ± 3.60 | 9.31 ± 2.23 | 0.85 ± 0.00 |
| 100 | 6.4 | 1.48 ± 0.59 | 0.04 ± 0.00 | 54.80 ± 0.40 | 17.97 ± 0.40 | 1.30 ± 0.00 |
| 100 | 7 | 1.92 ± 0.22 | 0.04 ± 0.00 | 97.70 ± 2.07 | 0.00 ± 0.00 | 2.30 ± 0.07 |
| 100 | 8 | 2.50 ± 0.18 | 0.05 ± 0.01 | 98.61 ± 1.96 | 0.00 ± 0.00 | 1.39 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yu, X.; Liu, Q.; Zhang, Y.; Wang, Q. Lipid Production of Schizochytrium sp. HBW10 Isolated from Coastal Waters of Northern China Cultivated in Food Waste Hydrolysate. Microorganisms 2023, 11, 2714. https://doi.org/10.3390/microorganisms11112714
Li X, Yu X, Liu Q, Zhang Y, Wang Q. Lipid Production of Schizochytrium sp. HBW10 Isolated from Coastal Waters of Northern China Cultivated in Food Waste Hydrolysate. Microorganisms. 2023; 11(11):2714. https://doi.org/10.3390/microorganisms11112714
Chicago/Turabian StyleLi, Xiaofang, Xinping Yu, Qian Liu, Yong Zhang, and Qiuzhen Wang. 2023. "Lipid Production of Schizochytrium sp. HBW10 Isolated from Coastal Waters of Northern China Cultivated in Food Waste Hydrolysate" Microorganisms 11, no. 11: 2714. https://doi.org/10.3390/microorganisms11112714





