All Kinds of Sunny Colors Synthesized from Methane: Genome-Encoded Carotenoid Production by Methylomonas Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation Procedures
2.2. Morphological Characterization and Growth Tests
2.3. Identification of Carotenoids
2.4. DNA Extraction
2.5. Genome Sequencing and Annotation
2.6. Phylogenomic Analysis
2.7. Identification of Carotenoid Biosynthesis Genes
2.8. Sequence Accession Numbers
3. Results
3.1. Identification and Characterization of Novel Isolates
3.2. Carotenoid Profiles
3.3. Genome Sequencing and Assembly
3.4. Genome-Based Phylogeny and Genome-to-Genome Comparison
3.5. Analysis of Carotenoid Biosynthesis Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polyakov, N.E.; Focsan, A.L.; Gao, Y.; Kispert, L.D. The Endless World of Carotenoids—Structural, Chemical and Biological Aspects of Some Rare Carotenoids. Int. J. Mol. Sci. 2023, 24, 9885. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Dewanjee, S.; Riaz, M. Carotenoids: Structure and Function in the Human Body; Springer International Publishing: New York, NY, USA, 2021; ISBN 3-030-46458-8. [Google Scholar]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cámara, S.; Ibañez, A.; Rubio, S.; Barreiro, C.; Barredo, J.-L. Main Carotenoids Produced by Microorganisms. Encyclopedia 2021, 1, 1223–1245. [Google Scholar] [CrossRef]
- Siziya, I.N.; Hwang, C.Y.; Seo, M.J. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants 2022, 11, 1963. [Google Scholar] [CrossRef]
- López, G.D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Non-Mevalonate Isoprenoid Biosynthesis: Enzymes, Genes and Inhibitors. Biochem. Soc. Trans. 2000, 28, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Sangari, F.J.; Pérez-Gil, J.; Carretero-Paulet, L.; García-Lobo, J.M.; Rodríguez-Concepción, M. A New Family of Enzymes Catalyzing the First Committed Step of the Methylerythritol 4-Phosphate (MEP) Pathway for Isoprenoid Biosynthesis in Bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 14081–14086. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.; Olmos-Soto, J.; Ruiz, M.A. Pathways of Carotenoid Biosynthesis in Bacteria and Microalgae. Methods Mol. Biol. 2012, 892, 1–12. [Google Scholar] [CrossRef]
- Lieberman, R.L.; Rosenzweig, A.C. Crystal Structure of a Membrane-Bound Metalloenzyme That Catalyses the Biological Oxidation of Methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hakemian, A.S.; Rosenzweig, A.C. The Biochemistry of Methane Oxidation. Annu. Rev. Biochem. 2007, 76, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C. Biochemistry: Breaking Methane. Nature 2015, 518, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Knief, C. Diversity and Phylogeny of Described Aerobic Methanotrophs. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M., Xing, X., Eds.; Springer: Cham, Switzerland, 2018; pp. 17–42. ISBN 9783319748665. [Google Scholar]
- Bowman, J.P. Methylomonas. Bergey’s Man. Syst. Archaea Bact. 2016, 8, 748. [Google Scholar] [CrossRef]
- Suleimanov, R.Z.; 3Tikhonova, E.N.; Oshkin, I.Y.; Danilova, O.V.; Dedysh, S.N. Methylomonas montana sp. nov., the First Nonpigmented Methanotroph of the Genus Methylomonas, Isolated from Mountain River Sediments. Microbiology 2023, 92, 766–774. [Google Scholar]
- Yazdian, F.; Hajizadeh, S.; Shojaosadati, S.A.; Jahanshahi, M.; Nosrati, M. Production of Single Cell Protein from Natural Gas: Parameter Optimization and RNA Evaluation. Iran. J. Biotechnol. 2005, 3, 235–242. [Google Scholar]
- Skrede, A.; Berge, G.M.; Storebakken, T.; Herstad, O.; Aarstad, K.G.; Sundstøl, F. Digestibility of Bacterial Protein Grown on Natural Gas in Mink, Pigs, Chicken and Atlantic Salmon. Anim. Feed Sci. Technol. 1998, 76, 103–116. [Google Scholar] [CrossRef]
- Bjorck, C.E.; Dobson, P.D.; Pandhal, J. Biotechnological Conversion of Methane to Methanol: Evaluation of Progress and Potential. AIMS Bioeng. 2018, 5, 1–38. [Google Scholar] [CrossRef]
- Safaeian, P.; Yazdian, F.; Khosravi-Darani, K.; Rashedi, H.; Lackner, M. P3HB from CH4 Using Methanotrophs: Aspects of Bioreactor, Fermentation Process and Modelling for Cost-Effective Biopolymer Production. Front. Bioeng. Biotechnol. 2023, 11, 1137749. [Google Scholar] [CrossRef]
- Fei, Q.; Puri, A.W.; Smith, H.; Dowe, N.; Pienkos, P.T. Enhanced Biological Fixation of Methane for Microbial Lipid Production by Recombinant Methylomicrobium buryatense. Biotechnol. Biofuels 2018, 11, 129. [Google Scholar] [CrossRef]
- Henard, C.A.; Smith, H.; Dowe, N.; Kalyuzhnaya, M.G.; Pienkos, P.T.; Guarnieri, M.T. Bioconversion of Methane to Lactate by an Obligate Methanotrophic Bacterium. Sci. Rep. 2016, 6, 21585. [Google Scholar] [CrossRef]
- Oshkin, I.Y.; Danilova, O.V.; But, S.Y.; Miroshnikov, K.K.; Suleimanov, R.Z.; Belova, S.E.; Tikhonova, E.N.; Kuznetsov, N.N.; Khmelenina, V.N.; Pimenov, N.V.; et al. Expanding Characterized Diversity and the Pool of Complete Genome Sequences of Methylococcus Species, the Bacteria of High Environmental and Biotechnological Relevance. Front. Microbiol. 2021, 12, 756830. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; He, R.; Wu, M.; Chen, W.; Gao, F.; Zhang, Z.; Yao, Y.; Yu, L.; Chen, S. Synthesizing Value-Added Products from Methane by a New Methylomonas. J. Appl. Microbiol. 2017, 123, 1214–1227. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Kim, D.; Lee, E.Y. A Comparative Transcriptome Analysis of the Novel Obligate Methanotroph methylomonas sp. DH-1 Reveals Key Differences in Transcriptional Responses in C1 and Secondary Metabolite Pathways during Growth on Methane and Methanol. BMC Genom. 2019, 20, 130. [Google Scholar] [CrossRef]
- Bothe, H.; Møller Jensen, K.; Mergel, A.; Larsen, J.; Jørgensen, C.; Bothe, H.; Jørgensen, L. Heterotrophic Bacteria Growing in Association with Methylococcus Capsulatus (Bath) in a Single Cell Protein Production Process. Appl. Microbiol. Biotechnol. 2002, 59, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Koffas, M.; Odom, J.; Schenzle, A. High Growth Methanotrophic Bacterial Strain. EP1320579A2, 25 June 2003. [Google Scholar]
- Gayazov, R.R.; Shishkina, V.N.; Mshenskii, Y.N.; Trotsenko, Y.A.; Ivanov, M.V. Effect of Temperature on Growth and Metabolism of Methylomonas methanica. Dokl. Acad. Sci. 1985, 284, 746–748. [Google Scholar]
- Tikhonova, E.N.; Suleimanov, R.Z.; Miroshnikov, K.K.; Oshkin, I.Y.; Belova, S.E.; Danilova, O.V.; Ashikhmin, A.A.; Konopkin, A.A.; But, S.Y.; Khmelenina, V.N.; et al. Methylomonas rapida sp. nov., a Novel Species of Fast-Growing, Carotenoid-Producing Obligate Methanotrophs with High Biotechnological Potential. Syst. Appl. Microbiol. 2023, 46, 126398. [Google Scholar] [CrossRef]
- Ye, R.W.; Yao, H.; Stead, K.; Wang, T.; Tao, L.; Cheng, Q.; Sharpe, P.L.; Suh, W.; Nagel, E.; Arcilla, D.; et al. Construction of the Astaxanthin Biosynthetic Pathway in a Methanotrophic Bacterium Methylomonas sp. Strain 16a. J. Ind. Microbiol. Biotechnol. 2007, 289–299. [Google Scholar] [CrossRef]
- Sharpe, P.L.; Dicosimo, D.; Bosak, M.D.; Knoke, K.; Tao, L.; Cheng, Q.; Ye, R.W. Use of Transposon Promoter-Probe Vectors in the Metabolic Engineering of the Obligate Methanotroph Methylomonas sp. Strain 16a for Enhanced C40 Carotenoid Synthesis. Appl. Environ. Microbiol. 2007, 73, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Schenzle, A.; Odom, J.M.; Cheng, Q. Novel Carotenoid Oxidase Involved in Biosynthesis of 4,4′- Diapolycopene Dialdehyde. Appl. Environ. Microbiol. 2005, 71, 3294–3301. [Google Scholar] [CrossRef]
- Kleinig, H.; Schmitt, R. On the Biosynthesis of C30 Carotenoic Acid Glucosyl Esters in Pseudomonas rhodos. Analysis of Car-Mutants. Z. Naturforsch.-Sect. C J. Biosci. 1982, 37, 758–760. [Google Scholar] [CrossRef]
- Takaichi, S.; Inoue, K.; Akaike, M.; Kobayashi, M.; Oh-oka, H.; Madigan, M.T. The Major Carotenoid in All Known Species of Heliobacteria Is the C30 Carotenoid 4,4′-Diaponeurosporene, Not Neurosporene. Arch. Microbiol. 1997, 168, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Oh-oka, H.; Maoka, T.; Jung, D.O.; Madigan, M.T. Novel Carotenoid Glucoside Esters from Alkaliphilic Heliobacteria. Arch. Microbiol. 2003, 179, 95–100. [Google Scholar] [CrossRef]
- Marshall, J.H.; Wilmoth, G.J. Proposed Pathway of Triterpenoid Carotenoid Biosynthesis in Staphylococcus aureus: Evidence from a Study of Mutants. J. Bacteriol. 1981, 147, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.H.; Wilmoth, G.J. Pigments of Staphylococcus aureus, a Series of Triterpenoid Carotenoids. J. Bacteriol. 1981, 147, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Ashikhmin, A.; Makhneva, Z.; Bolshakov, M.; Moskalenko, A. Incorporation of Spheroidene and Spheroidenone into Light-Harvesting Complexes from Purple Sulfur Bacteria. J. Photochem. Photobiol. B. 2017, 170, 99–107. [Google Scholar] [CrossRef]
- Umeno, D.; Tobias, A.V.; Arnold, F.H. Evolution of the C30 Carotenoid Synthase CrtM for Function in a C40 Pathway. J. Bacteriol. 2002, 184, 6690–6699. [Google Scholar] [CrossRef]
- Steiger, S.; Takaichi, S.; Sandmann, G. Heterologous Production of Two Unusual Acyclic Carotenoids, 1,1-Dihydroxy-3,4-Didehydrolycopene and 1-Hydroxy-3,4,3,4-Tetradehydrolycopene by Combination of the CrtC and CrtD Genes from Rhodobacter and Rubrivivax. J. Biotechnol. 2002, 97, 51–58. [Google Scholar] [CrossRef]
- Enzell, C.R.; Francis, G.W.; Liaaen-Jensen, S. Mass Spectrometric Studies of Carotenoids. 2. A Survey of Fragmentation Reactions. Acta Chem. Scand. 1969, 23, 727–750. [Google Scholar] [CrossRef]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids, V. 1B., Spectroscopy; Britton, G., Liaaen-Jensen, S.H.P., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995. [Google Scholar]
- Wilson, K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2–4. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and Taxonomy in Diagnostics for Food Security: Soft-Rotting Enterobacterial Plant Pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M. Data, Information, Knowledge and Principle: Back to Metabolism in KEGG. Nucleic Acids Res. 2014, 42, 199–205. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome Analysis of Multiple Pathogenic Isolates of Streptococcus agalactiae: Implications for the Microbial “Pan-Genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER Web Server: 2018 Update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Tiedje, J.M. Genomic Insights That Advance the Species Definition for Prokaryotes Konstantinos. Proc. Natl. Acad. Sci. USA 2005, 102, 2567–2572. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA Hybridization Values and Their Relationship to Whole-Genome Sequence Similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Thulasiram, H.V.; Poulter, C.D. Farnesyl Diphosphate Synthase: The Art of Compromise between Substrate Selectivity and Stereoselectivity. J. Am. Chem. Soc. 2006, 128, 15819–15823. [Google Scholar] [CrossRef]
- Wieland, B.; Feil, C.; Gloria-Maercker, E.; Thumm, G.; Lechner, M.; Bravo, J.M.; Poralla, K.; Gotz, F. Genetic and Biochemical Analyses of the Biosynthesis of the Yellow Carotenoid 4,4′-Diaponeurosporene of Staphylococcus aureus. J. Bacteriol. 1994, 176, 7719–7726. [Google Scholar] [CrossRef]
- Pelz, A.; Wieland, K.P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and Biosynthesis of Staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef]
- Santana-Molina, C.; Henriques, V.; Hornero-Méndez, D.; Devos, D.P.; Rivas-Marin, E. The Squalene Route to C30 Carotenoid Biosynthesis and the Origins of Carotenoid Biosynthetic Pathways. Proc. Natl. Acad. Sci. USA 2022, 119, e2210081119. [Google Scholar] [CrossRef]
- Pan, J.J.; Solbiati, J.O.; Ramamoorthy, G.; Hillerich, B.S.; Seidel, R.D.; Cronan, J.E.; Almo, S.C.; Poulter, C.D. Biosynthesis of Squalene from Farnesyl Diphosphate in Bacteria: Three Steps Catalyzed by Three Enzymes. ACS Cent. Sci. 2015, 1, 77–82. [Google Scholar] [CrossRef]
- Furubayashi, M.; Umeno, D. Directed Evolution of Carotenoid Synthases for the Production of Unnatural Carotenoids; Humana Press: Totowa, NJ, USA, 2012; Volume 892, ISBN 9781617798788. [Google Scholar]
- Bussmann, I.; Horn, F.; Hoppert, M.; Klings, K.W.; Saborowski, A.; Warnstedt, J.; Liebner, S. Methylomonas albis sp. nov. and Methylomonas fluvii sp. nov.: Two Cold-Adapted Methanotrophs from the River Elbe and Emended Description of the Species Methylovulum psychrotolerans. Syst. Appl. Microbiol. 2021, 44, 126248. [Google Scholar] [CrossRef]
- Maeda, I. Genetic Modification in Bacillus subtilis for Production of C30 Carotenoids. Methods Mol. Biol. 2012, 892, 197–205. [Google Scholar] [CrossRef]
- Summers, C.; Karst, F.; Charles, A.D. Cloning, Expression and Characterisation of the CDNA Encoding Human Hepatic Squalene Synthase, and Its Relationship to Phytoene Synthase. Gene 1993, 136, 185–192. [Google Scholar] [CrossRef]
- Robinson, G.W.; Tsay, Y.H.; Kienzle, B.K.; Smith-Monroy, C.A.; Bishop, R.W. Conservation between Human and Fungal Squalene Synthetases: Similarities in Structure, Function, and Regulation. Mol. Cell. Biol. 1993, 13, 2706–2717. [Google Scholar] [CrossRef]
- Nakano, T.; Wiegertjes, G. Properties of Carotenoids in Fish Fitness: A Review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- de Carvalho, C.C.; Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4, 93. [Google Scholar] [CrossRef]

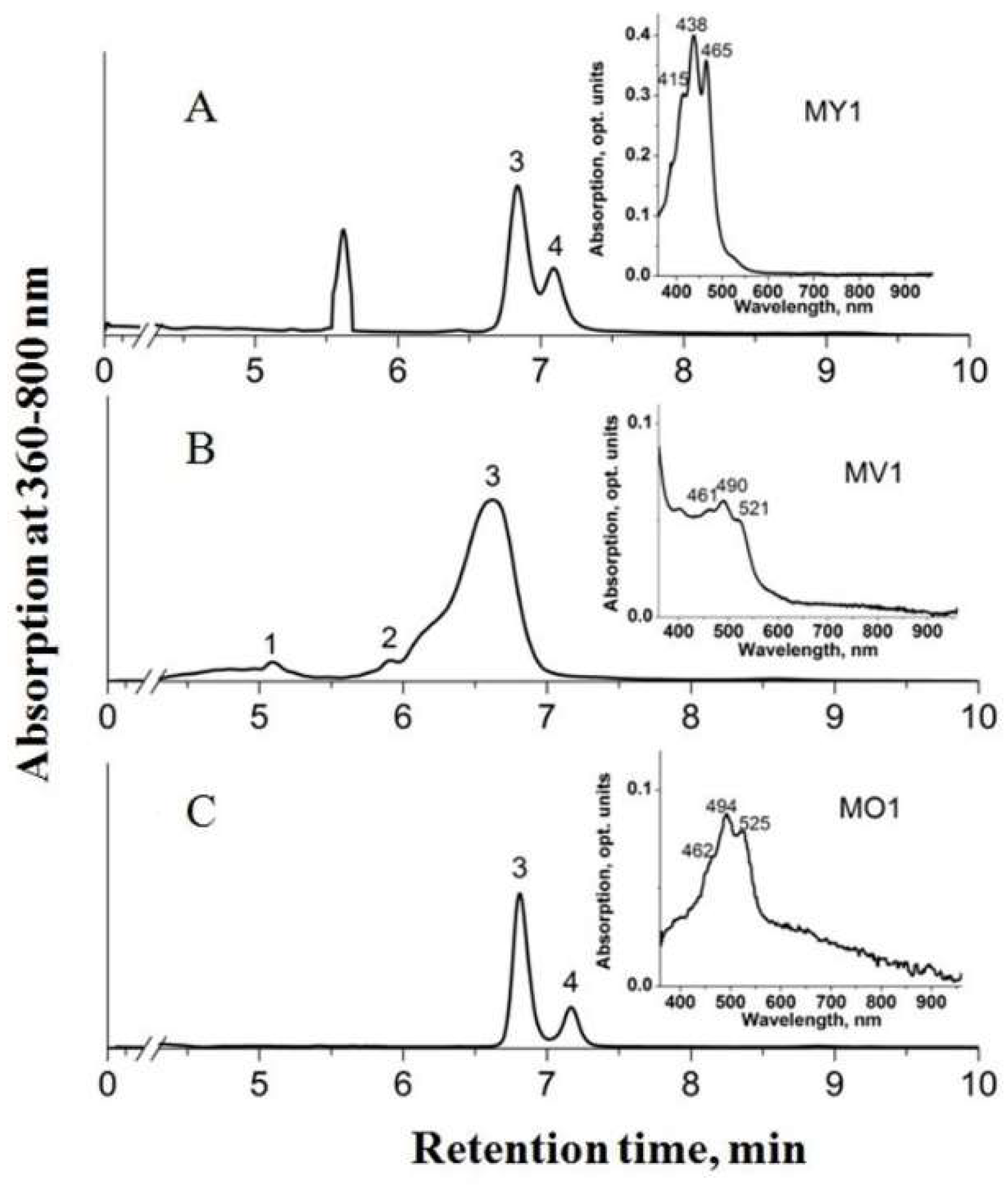
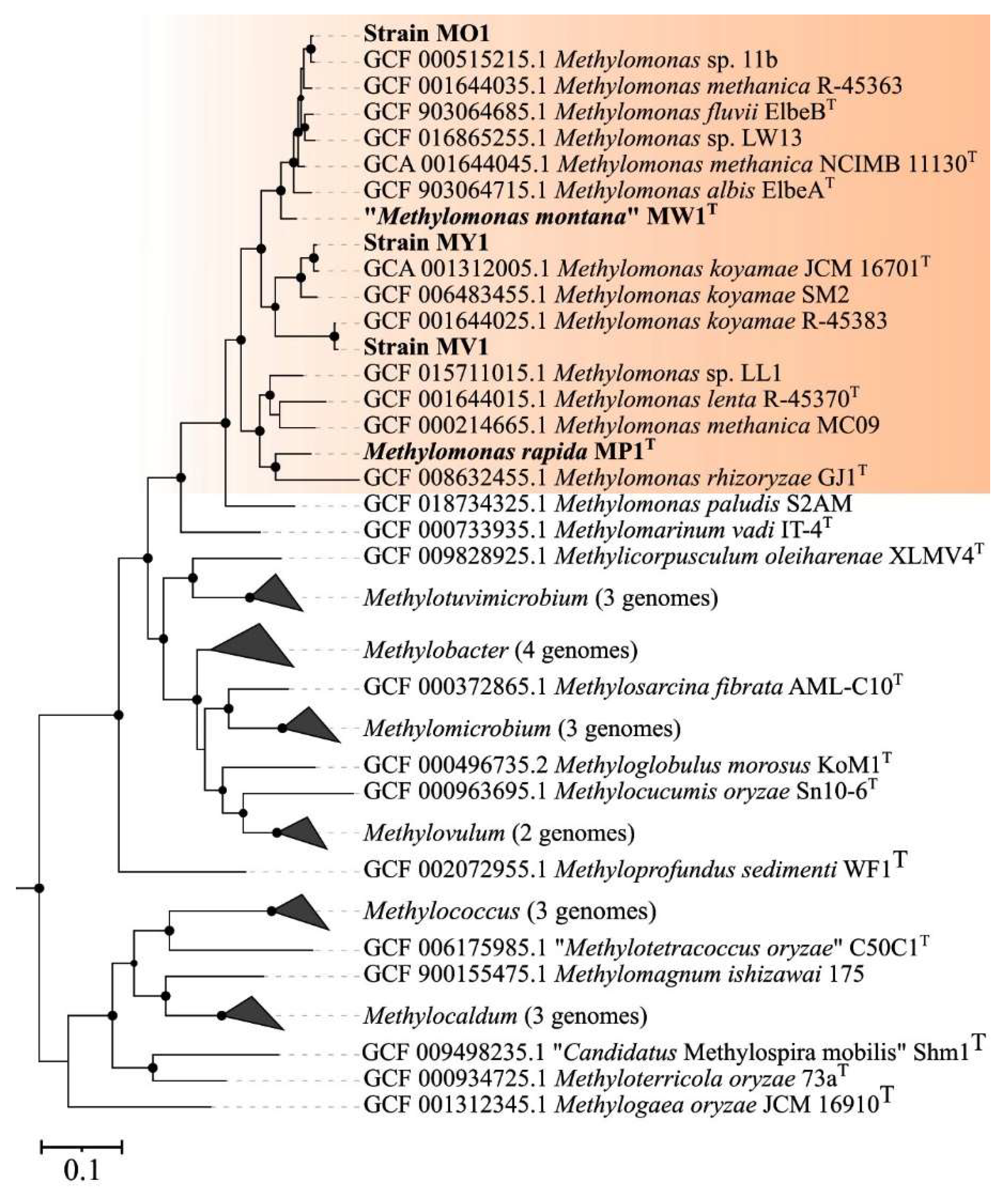
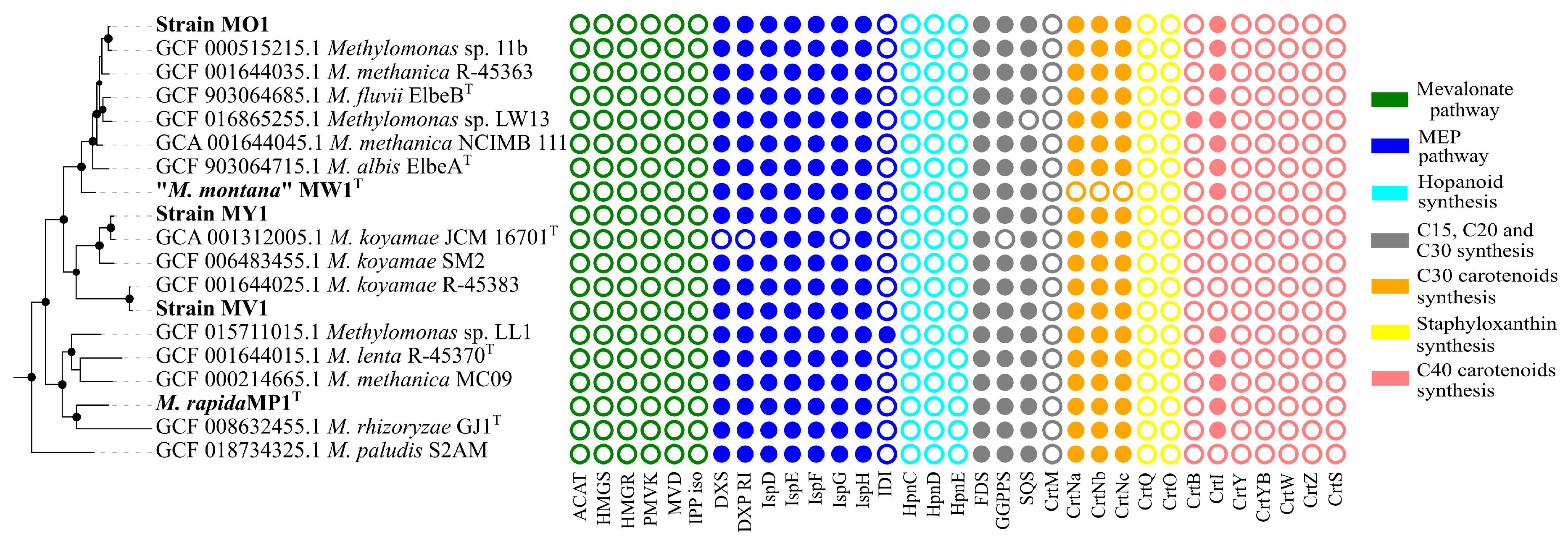
| Strain | GenBank 16S rRNA | Sampling Site | Closest Relative (% Similarity to 16S rRNA) | Color | Cell Size, Wide/Long, μm | Growth Temperature Range (Optimum), °C | Growth Rate in Batch Culture, h−1 |
|---|---|---|---|---|---|---|---|
| MP1 | ON819564 | Unnamed lake, Krasnodar region, Russia (N 44.42°; E 39.18°) | M. rapida (100) | pink to red | 1.10 ± 0.03/2.10 ± 0.08 | 8–45 (35) | 0.33 |
| MY1 | OR234855 | M. koyamae Fw12E-Y (100) | yellow | 1.5 ± 0.07/1.9 ± 0.1 | 8–37 (30) | 0.29 | |
| MO1 | OR234854 | Meshchersky pond, Moscow, Russia (N 55.67°; E 37.40°) | M. denitrificans FJG1 (99.65) | orange | 0.9 ± 0.04/1.3 ± 0.07 | 5–37 (32) | 0.22 |
| MV1 | OR234856 | Unnamed pond, Krasnodar region, Russia (N 44.42°; E 39.18°) | M. koyamae Fw12E-Y (97.56) | pink | 0.7 ± 0.03/2.2 ± 0.09 | 4–38 (30) | 0.25 |
| MW1 | OR237191 | Khosta river, Krasnodar region, Russia (N 43.53°; E 39.97°) | M. methanica S1 (97.29) | white | 0.9 ± 0.04/1.5 ± 0.2 | 10–35 (30) | 0.13 |
| Retention Time, min | Peak Absorption Maxima, nm | Identity | Formula | Strains | ||||
|---|---|---|---|---|---|---|---|---|
| MY1 | MO1 | MP1 | MW1 | MV1 | ||||
| 5.7 | 467/489/519 | 1,2-Dihydro-3,4-dehydrolycopene | C40H56 | – | – | – | – | 5.0 |
| 6.5 | 469/498/527 | 4’-Apo-3,4-didehydrolycopene | C35H46 | – | – | 2.9 | – | 4.8 |
| 7.0 | 465/489/521 | 4,4’-Diaplycopene-4,4’-dioic acid | C30H36O4 | 68.7 | 78.3 | 67.9 | – | 90.2 |
| 7.3 | 456/484/513 | 1,1’-Dihydroxy-3,4-didehydrolycopene | C40H58O2 | 31.3 | 21.7 | 18.0 | – | – |
| 7.8 | 470/508/540 | Tetradehydrolycopene | C40H52 | – | – | 1.8 | – | – |
| 9.0 | 451/484/510 | 4,4’-Diaplycopenoic acid | C30H38O2 | – | – | 9.3 | – | – |
| Characteristics | Strain MO1 | Strain MV1 | Strain MY1 | “M. montana” MW1T | M. rapida MP1T | |
|---|---|---|---|---|---|---|
| Illumina | Number of reads | 2,022,040 | 3,782,980 | 3,724,476 | 3,643,858 | 1,304,732 |
| Total bases, Gb | 0.6 | 1.1 | 1.1 | 1.1 | 0.4 | |
| Mean read length, bp | 301 | 301 | 301 | 301 | 301 | |
| Nanopore | Number of reads | 258,525 | 216,073 | 211,318 | 266,732 | 150,333 |
| Total bases, Gb | 1.2 | 1.4 | 1.4 | 1.6 | 0.9 | |
| Mean read length, bp | 4497.9 | 6464.9 | 6411 | 6126.4 | 6263.5 | |
| Total coverage | 352 | 468 | 500 | 589 | 290 | |
| Strain MO1 | Strain MV1 | Strain MY1 | “M. montana” MW1T | M. rapida MP1T | |
|---|---|---|---|---|---|
| Genome size (Mb) | 5.03 | 5.42 | 4.95 | 4.63 | 4.60 |
| Contigs | 2 | 10 | 1 | 1 | 1 |
| G + C content (mol %) | 51 | 55.5 | 56 | 52 | 52.5 |
| CDS | 4556 | 4820 | 4469 | 4296 | 4343 |
| Repeat region | 0 | 2 | 5 | 2 | 3 |
| tRNA | 46 | 49 | 52 | 47 | 51 |
| 5S, 16S, 23S | 3, 3, 3 | 3, 3, 3 | 3, 3, 3 | 3, 3, 3 | 4, 4, 4 |
| pMMO operon | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshkin, I.Y.; Tikhonova, E.N.; Suleimanov, R.Z.; Ashikhmin, A.A.; Ivanova, A.A.; Pimenov, N.V.; Dedysh, S.N. All Kinds of Sunny Colors Synthesized from Methane: Genome-Encoded Carotenoid Production by Methylomonas Species. Microorganisms 2023, 11, 2865. https://doi.org/10.3390/microorganisms11122865
Oshkin IY, Tikhonova EN, Suleimanov RZ, Ashikhmin AA, Ivanova AA, Pimenov NV, Dedysh SN. All Kinds of Sunny Colors Synthesized from Methane: Genome-Encoded Carotenoid Production by Methylomonas Species. Microorganisms. 2023; 11(12):2865. https://doi.org/10.3390/microorganisms11122865
Chicago/Turabian StyleOshkin, Igor Y., Ekaterina N. Tikhonova, Ruslan Z. Suleimanov, Aleksandr A. Ashikhmin, Anastasia A. Ivanova, Nikolai V. Pimenov, and Svetlana N. Dedysh. 2023. "All Kinds of Sunny Colors Synthesized from Methane: Genome-Encoded Carotenoid Production by Methylomonas Species" Microorganisms 11, no. 12: 2865. https://doi.org/10.3390/microorganisms11122865






