Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects
Abstract
:1. Introduction
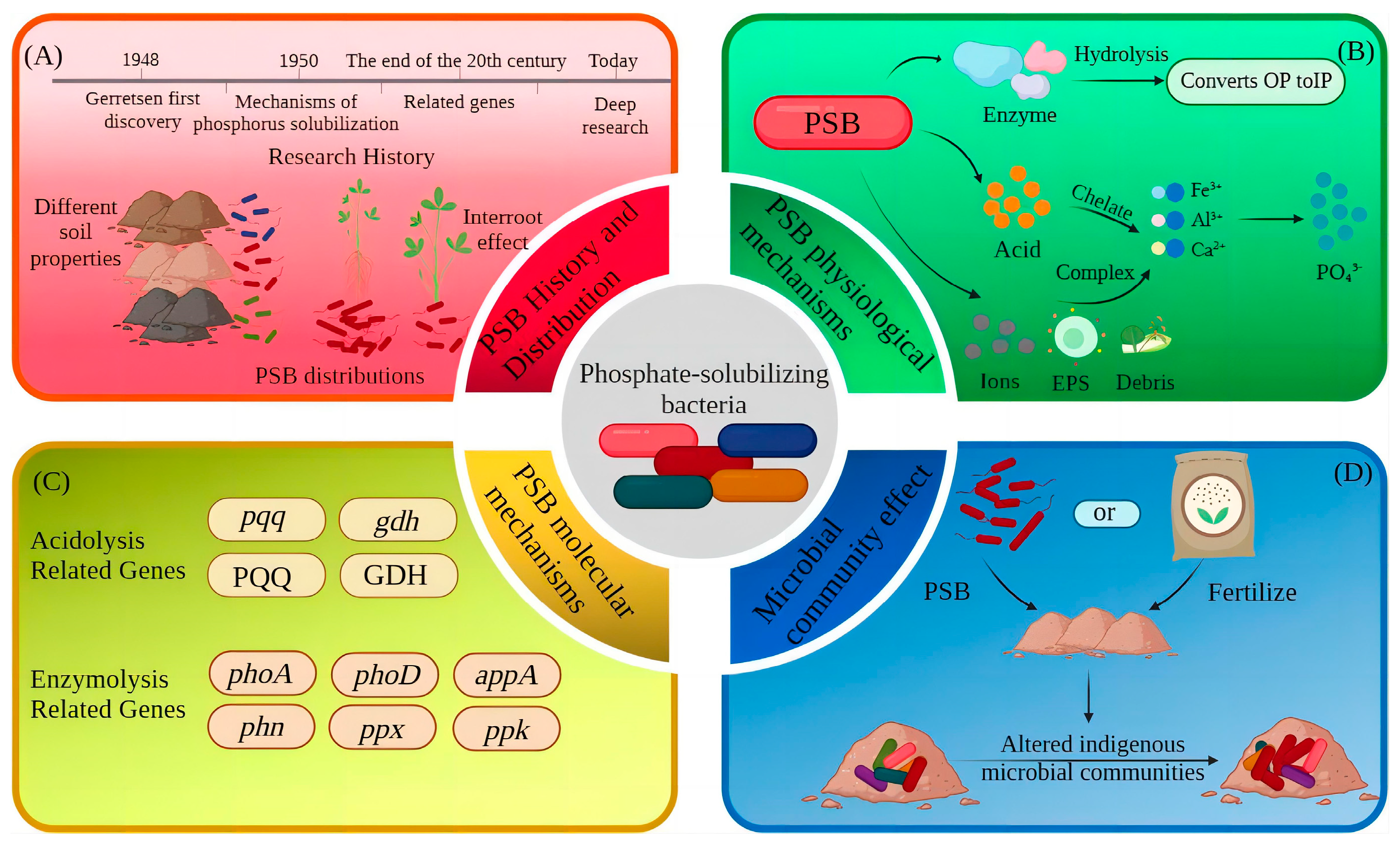
2. Overview of PSB
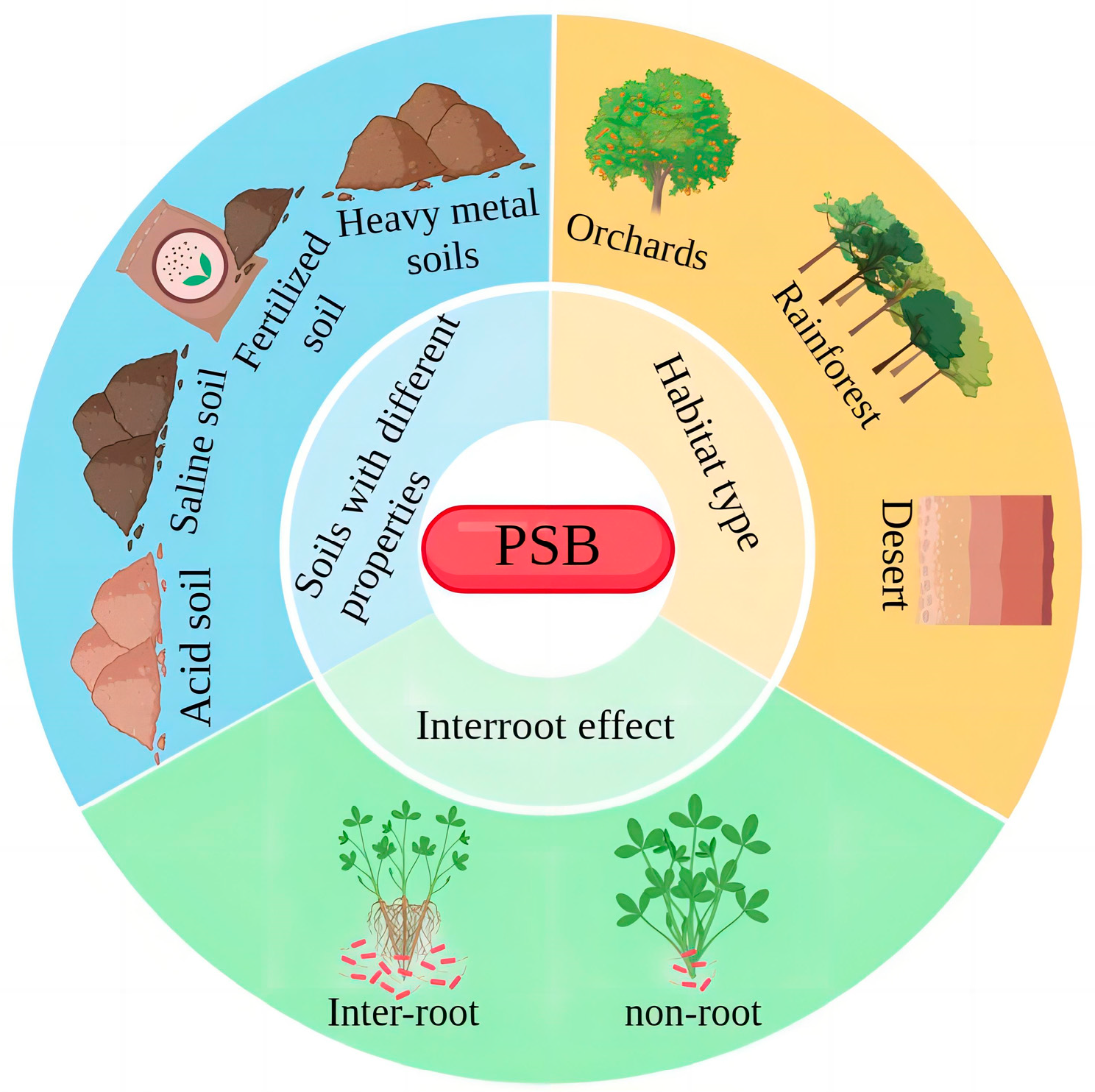
Distribution and Species of PSB
| PSB | Gram Stain | Glucose Hydrolysis | Starch Hydrolysis | Gelatin Liquefaction | Citrate Utilization | Hydrogen Sulfide Generation | V-P | Methyl Red | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pantoea roadsii | - | + | + | + | - | - | + | [45] | |
| Pseudomonas donghuensis | - | + | + | + | - | - | + | [45] | |
| Ochrobactrum pseudogrignonense | - | + | - | - | - | - | + | [45] | |
| Pseudomonas moraviensis | - | + | + | + | + | + | [46] | ||
| Bacillus safensis | + | + | + | + | + | + | [46] | ||
| Falsibacillus pallidus | - | + | - | - | - | - | [46] | ||
| Pseudomonas sp. | - | - | - | - | [47] | ||||
| Acinetobacter calcoaceticus | - | - | - | - | [47] | ||||
| Antagonistic Bacillus | + | + | + | + | + | + | - | [48] |
| Crop Species | Distribution | PSB | Impact on Crop Performance | Reference |
|---|---|---|---|---|
| Cereals | Maize rhizosphere soil | Pseudomonas fluorescens, Pseudomonas poae, Bacillus subtilis | Increase in dry weight | [49] |
| Wheat rhizosphere soil | Bacillus safensis, Falsibacillus pallidus | Root length, root surface area, root volume and number of root tips increased significantly | [46] | |
| Maize rhizosphere soil | S. marcensens, P. brenneri | Substantial increase in maize production | [50] | |
| Pulses | Legumes in Fes-Meknes region rhizosphere soil | PSB WJEF38 | Agronomic traits of peas and broad beans were improved | [51] |
| Phaseolus vulgaris L. rhizosphere soil | Pseudomonas kribbensis | Biomass increased dramatically | [13] | |
| Peanut rhizosphere soil | Bacillus amyloliquefaciens | Significant increase in aboveground dry and fresh weights | [48] | |
| Horticultural crops | Walnut, feijoa, jujube, apple rhizosphere soil | Pantoea gavini, Acinetobacter sp. | Increase in root length | [52] |
| Chenopodium quinoa rhizosphere soil | Licheniformis, Enterobacter, Asburiae, | Increase in fresh weight and root length | [53] | |
| Tomato rhizosphere soil | Acinetobacter, Stenotrophomonas maltophilia | Tomato plant height and leaf area were increased | [54] | |
| Cash crops (economics) | Cotton rhizosphere soil | Bacillus halotolerans | Increased cotton yield | [55] |
| Tobacco rhizosphere soil | Burkholderia cenocepacia | Significant increase in plant height | [56] |
| Source | Source Location | PSB General Category | Screening Method | Reference |
|---|---|---|---|---|
| Soil | Arid land | Pseudomonas azotoformans, Acinetobacter baumannii, Bacillus paramycoides | Flatbed screening | [57] |
| Cinnamomum camphora soil | Bacteroidetes, Proteobacteria, Chloroflexi, and Gemmatimonadetes | High-throughput sequencing | [45] | |
| Saline soil | Bacillus amyloliquefaciens | Flatbed screening | [36] | |
| Brazilian cerrado soil | Pseudomonas aeruginosa, Bacillus cereus | Flatbed screening | [58] | |
| Rhizosphere | Rhizosphere of Taxus chinensis var. mairei | Pseudomonas fluorescens, Bacillus cereus, Sinorhizobium meliloti, Bacillus licheniformis | Flatbed screening | [59] |
| Rice rhizosphere | Pseudomonas aeruginosa, Bacillus subtilis strain | Flatbed screening | [41] | |
| Blueberry plant rhizosphere | Buttiauxella sp. | High-throughput sequencing | [60] | |
| Characteristics of rhizosphere | Ascomycetes, Acidobacteria, | High-throughput sequencing | [61] | |
| Sediment | Reservoir sediment | Micromonospora sp., Aminobacter sp. | High-throughput sequencing | [62] |
| Surface sediment in the Changjiang or Yangtze River estuary | Firmicutes, Proteobacteria, Actinobacteria | Flatbed screening | [63] | |
| Lake Taihu sediment | Burkholderia sp. | Flatbed screening | [64] | |
| Plant parts | Roots, stems and leaves of moso bamboo | Alkaloid-producing bacilli of the genus Bacillus, Enterobacter spp., Bacillus spp. | Flatbed screening | [65] |
| Stems of Oryza officinalis | Acinetobacter, Cutibacterium, Dechloromonas | Flatbed screening | [66] | |
| Corn roots | Burkholderia spp. | Flatbed screening | [67] |
3. Mechanisms of Phosphorus Solubilization by PSB
3.1. Physiological Mechanisms
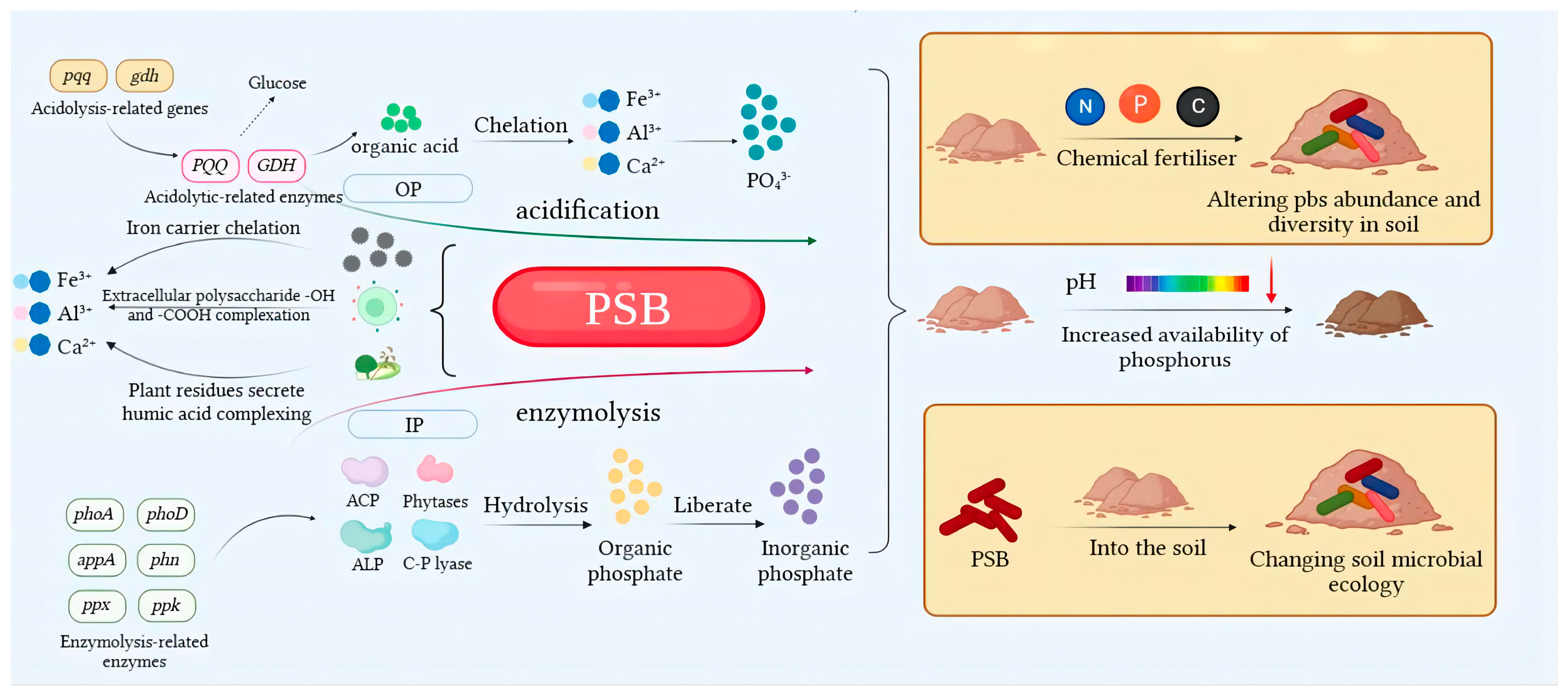
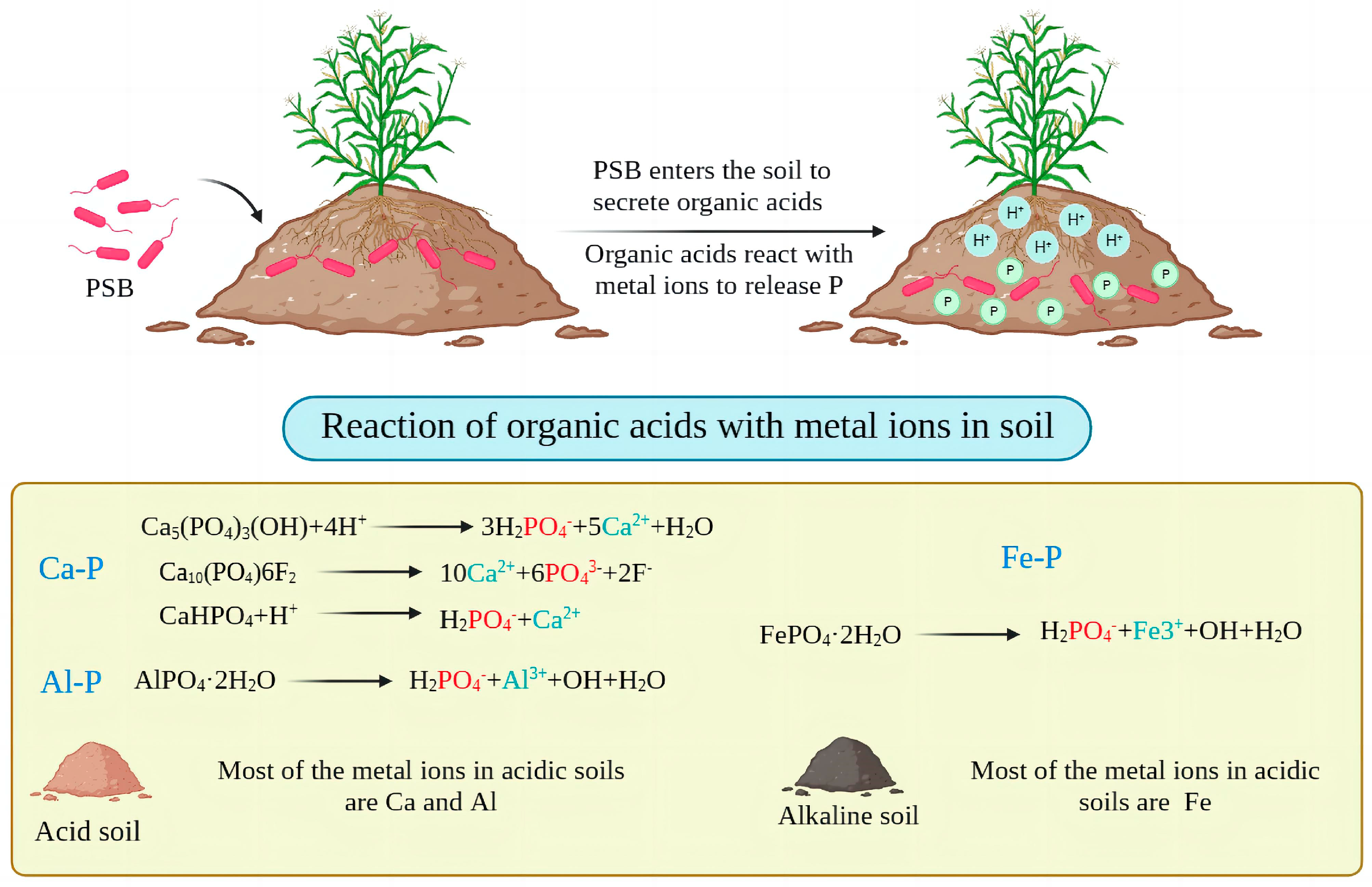
3.1.1. Solubilizing Action of Acids
| Phylum | PSB Species | PSB Source | Secreted Organic Acids | References |
|---|---|---|---|---|
| Proteobacteria | Enterobacter aerogenes | Mangrove rhizosphere soil | Lactic, succinic, isovaleric, isobutyric and acetic acids | [84] |
| Pantoea dispersa | Corn rhizosphere soil | Citric, malic, succinic and acetic acids | [85] | |
| Pantoea sp. | Nectarine rhizosphere soil | Oxalic, formic, acetic and citric acids | [86] | |
| Firmicutes | Bacillus | Rice paddy soil | Gluco-oxalic acid, citric acid, tartaric acid, succinic acid, formic acid and acetic acid | [87] |
| Bacillus safensis | Turmeric rhizosphere soil | Gluconic acid, alpha-ketogluconic acid, succinic acid, oxalic acid and tartaric acid | [69] | |
| Bacillus siamensis | Wheat rhizosphere soil | Glycolic acid | [88] | |
| Bacillus amyloliquefaciens | Saline soil | Lactic acid, maleic acid and oxalic acid | [36] | |
| Actinobacteria | Tsukamurellatrosinosolvens | Tea tree rhizosphere soil | Lactic acid, maleic acid and oxalic acid | [89] |
3.1.2. Mineralization Action of Enzymes
3.1.3. Chelation and Complexation
3.2. Molecular Mechanisms
3.2.1. Functional Genes Related to the Regulation of Acidolysis
3.2.2. Functional Genes Related to the Regulation of Enzymolysis
| PSB Species | PSB Source | Related Genes | Functions | Reference |
|---|---|---|---|---|
| Pseudomonas putida | Laboratory storage | gcd | Encode glucose dehydrogenase, which promotes the | [116] |
| solubilization of inorganic P | ||||
| Pseudomonas sp. | Wheat rhizosphere soil | gcd | Encode glucose dehydrogenase, which promotes the solubilization of inorganic P | [105] |
| Acinetobacter | Soils in rocky desertification areas | gcd | Mediate the production of gluconic acid | [35] |
| Acinetobacter pittii gp-1 | Laboratory storage | gcd | Promotion of the solubilization of inorganic and organic phosphorus | [94] |
| Ochrobactrum haematophilum | Sweet potato rhizosphere soil | CS, ACO, ODGH, SFD, FH, MDA | Tricarboxylic acid cycle-related genes | [111] |
| Ochrobactrum haematophilum | Sweet potato rhizosphere soil | POX, LDH | Acetic acid and lactic acid regulatory genes | [111] |
| Acinetobacter spp., Pseudomonas spp. | Rice rhizosphere soil | pqqC, pqqE | Regulation of gluconic acid production | [107] |
| PSB Species | PSB Source | Related Genes | Functions | Reference |
|---|---|---|---|---|
| Ochrobactrum sp | Wheat rhizosphere soil | pho | Promotion of the solubilization of inorganic and organic phosphorus | [117] |
| Pantoea agglomerans | Wheat rhizosphere soil | phy | Participate in the dissolution of phytic acid | [117] |
| Acinetobacter pittii gp-1 | Laboratory storage | phoD, bpp, | Promotion of the solubilization of inorganic and organic phosphorus | [94] |
| Arthrobacter | Corn rhizosphere soil | Ppx, ppk | Promotes the synthesis of exonuclease polyphosphatase and polyphosphate kinase | [94] |
| aryabhattai | Laboratory storage | Phn, pho, | Promotion of phosphorus metabolic pathway activity | [95] |
| Pseudomonas | Corn rhizosphere soil | bpp | Phytase-encoding genes | [118] |
| Pantoea brenneri | Soil samples of the Republic of Tatarstan | phnK | C-P lyase regulatory genes | [119] |
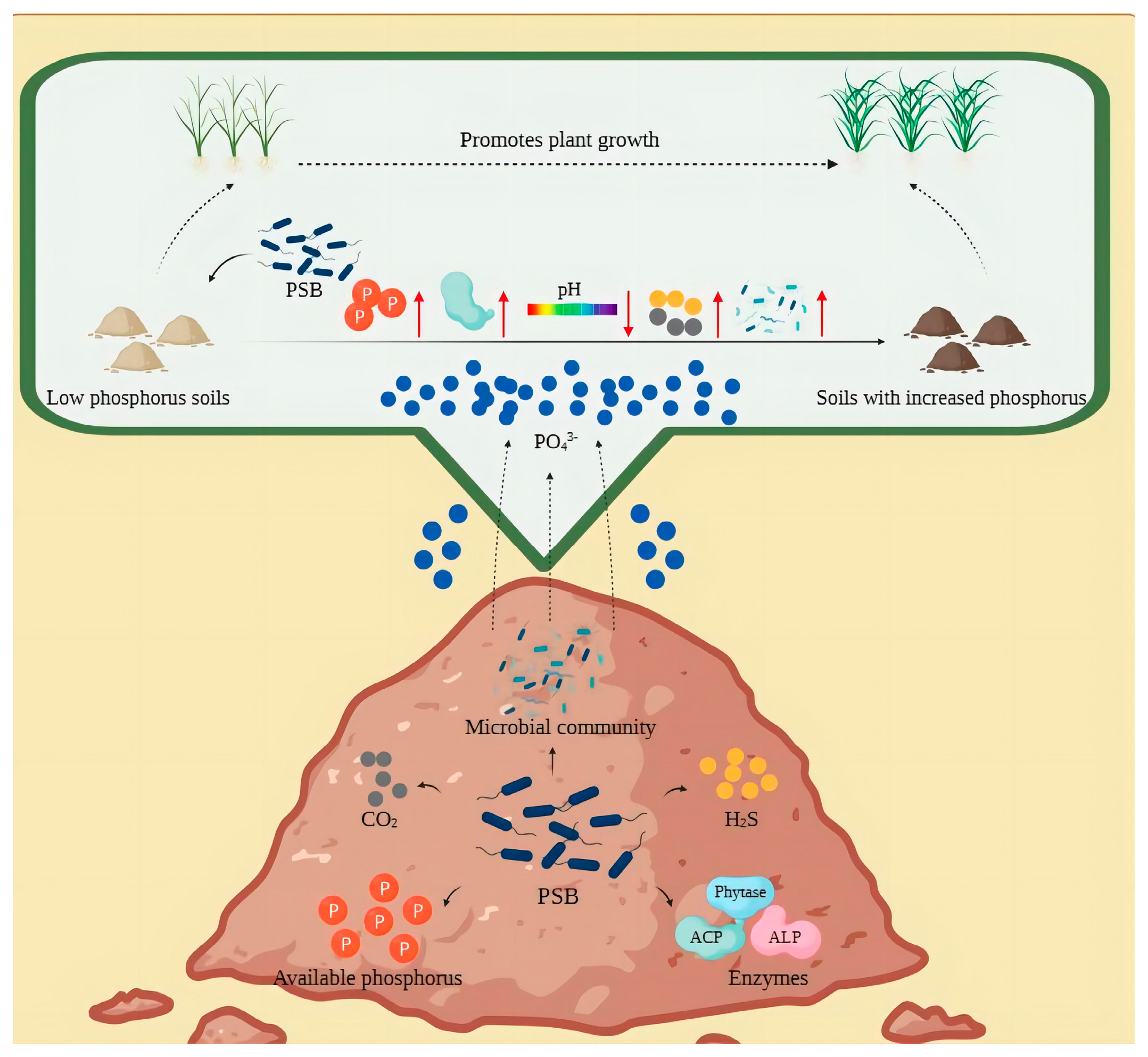
3.3. Mechanisms of Microbial Community Effects
3.3.1. Effects of Soil Nutrient Changes on the Abundance of PSB Communities
3.3.2. Effects of PSB on Soil Microcosm Systems
3.4. PSB Regulates Plant Root Transporter Proteins
4. Problems and Future Outlook
4.1. Multi-omic Synergy in the In-Depth Mining of Functional Genes of PSB
4.2. PSB Regulatory Genes in Soils with Different Phosphorus Levels
4.3. Use of PSB for Making Microbial Preparations in Agriculture
Author Contributions
Funding
Conflicts of Interest
References
- Siedliska, A.; Piotr, B.; Joanna, P.; Monika, Z.; Jaromir, K. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Chouyia, F.; Ventorino, V.; Pepe, O. Diversity, mechanisms and beneficial features of phosphate-solubilizing Streptomyces in sustainable agriculture: A review. Front. Plant Sci. 2022, 13, 1035358. [Google Scholar] [CrossRef]
- Alori, E.; Glick, B.; Babalola, O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Suleimanova, A.D.; Beinhauer, A.; Valeeva, L.R.; Chastukhina, I.B.; Balaban, N.P.; Shakiro, E.V.; Greiner, R.; Sharipova, M.R. Novel glucose-1-phosphatase with high phytase activity and unusual metal ion activation from soil bacterium pantoea sp. strain 3.5.1. Appl. Environ. Microbiol. 2015, 81, 6790–6799. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.; Silva, G.; Carnietto, M.; Oliveira, L.; Nogueira, C.; Silva, M. Bacillus velezensis associated with organomineral fertilizer and reduced phosphate doses improves soil microbial—Chemical properties and biomass of sugarcane. Agronomy 2022, 12, 2701. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Singh, R.; Kantwa, S.; Jha, P.; Ahamad, S.; Singh, A.G.; Prasad, M.; Singh, S.; Singh, S.; et al. Understanding soil carbon and phosphorus dynamics under grass-legume intercropping in a semi-arid region. Agronomy 2023, 13, 1692. [Google Scholar] [CrossRef]
- Cordell, D.; Schmid-Neset, T.; White, S.; Drangert, J.O. Preferred future phosphorus scenarios: A framework for meeting long-term phosphorus needs for global food demand. In Proceedings of the 2009 International Conference on Nutrient Recovery from Wastewater Streams, Vancouver, BC, Canada, 10–13 May 2009. [Google Scholar]
- Gross, A.; Lin, Y.; Weber, P.K.; Pett-Ridge, J.; Silver, W.L. The role of soil redox conditions in microbial phosphorus cycling in humid tropical forests. J. Ecol. 2020, 101, e02928. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Neal, A.; Blackwell, M.; Akkari, E.; Guyomar, C.; Clark, I.; Hirsch, P. Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific acid phosphatase gene diversity and abundance. Plant Soil. 2018, 427, 175–189. [Google Scholar] [CrossRef]
- Udaondo, Z.; Duque, E.; Daddaoua, A.; Caselles, C.; Roca, A.; Pizarro-Tobias, P.; Ramos, J. Developing robust protein analysis profiles to identify bacterial acid phosphatases in genomes and metagenomic libraries. Environ. Microbiol. 2020, 22, 3561–3571. [Google Scholar] [CrossRef]
- Kiprotich, K.; Muoma, J.; Omayio, D.; Ndombi, T.; Wekesa, C. Molecular characterization and mineralizing potential of phosphorus solubilizing bacteria colonizing common bean (Phaseolus vulgaris L.) rhizosphere in Western Kenya. Int. J. Microbiol. 2023, 2023, 6668097. [Google Scholar] [CrossRef] [PubMed]
- Massucato, L.; Almeida, S.; Silva, M.; Mosela, M.; Zeffa, D.; Nogueira, A.; Filho, R.; Mian, S.; Higashi, A.; Teixeira, G.; et al. Efficiency of combining strains Ag87 (Bacillus megaterium) and Ag94 (Lysinibacillus sp.) as phosphate solubilizers and growth promoters in maize. Microorganisms 2020, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Stalstrom, V.A. Boitrag zur kennturs, and Ein-wisking. Sterier use in garung bofindlicher oranischer strofe auf dil losichket der phosphorson des tricalclum phosphate. Zel Bakt. 1903, 724–732. [Google Scholar]
- Gerrestsen, F.C. The influence of micro-organism on the phosphate intaken by the plant. Plant Soil 1948, 1, 51–60. [Google Scholar] [CrossRef]
- Chi, J.L.; Hao, M.; Wang, Z.X. Advances in research and application of phosphorus-solubilizing microorganism. J. Microbiol. 2021, 41, 1–7. (In Chinese) [Google Scholar]
- Liu, J.; Liu, X.; Zhang, Q.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express. 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fu, D.; Liu, T.; Guo, G.; Hu, Z. Phosphorus solubilizing and releasing bacteria screening from the rhizosphere in a natural wetland. Water 2018, 10, 195. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Rong, G.Q.; Qin, X.K.; Yao, Q.J.; Zhang, Z.Q.; Hu, Y.B. Research on application status of soil phosphate solubilizing bacteria in modern agriculture. Farm Prod. Process. 2021, 13, 86–89. [Google Scholar]
- Li, M.; Teng, Z.D.; Zhu, J.; Song, M.Y. Research advances in heavy metal contaminated soil remediation by phosphate solubilizing microorganisms. Acta Ecol. Sin. 2018, 38, 3393–3402. [Google Scholar]
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population distribution of phosphate-solubilizing microorganisms in agricultural soil. Microbes Environ. 2022, 37, ME21041. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; He, Z.; Zhang, Z.; Xu, Y.; Li, Z.; Peng, Y.; Deng, N.; Chen, Y. Integration and potential application ability of culturable functional microorganism in oil tea camellia. Indian J. Microbiol. 2021, 61, 1–9. [Google Scholar] [CrossRef]
- Li, K.; Zeghbroeck, J.V.; Liu, Q.; Zhang, S. Isolating and characterizing phosphorus solubilizing bacteria from rhizospheres of native plants grown in calcareous soils. Front. Environ. Sci. 2021, 9, 802563. [Google Scholar] [CrossRef]
- Sahu, S.; Rajbonshi, M.; Gujre, N.; Gupta, M.; Shelke, R.; Ghose, A.; Rangan, L.; Pakshirajan, K.; Mitra, S. Bacterial strains found in the soils of a municipal solid waste dumping site facilitated phosphate solubilization along with cadmium remediation. Chemosphere 2020, 287, 132320. [Google Scholar] [CrossRef]
- Bahadur, I.; Maurya, B.; Meena, V.; Saha, M.; Kumar, A.; Aeron, A. Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing rhizobacteria isolated from Indo-Gangetic plain of India. Geomicrobiol. J. 2017, 34, 454–466. [Google Scholar] [CrossRef]
- Kashyap, A.; Manzar, N.; Rajawat, M.; Kesharwani, A.; Singh, R.; Dubey, S.; Abhijeet, S.K.; Pattanayak, D.; Dhar, S.; Lai, S.K.; et al. Screening and biocontrol potential of rhizobacteria native to gangetic plains and hilly regions to induce systemic resistance and promote plant growth in Chilli against Bacterial Wilt Disease. Plants 2021, 10, 2125. [Google Scholar] [CrossRef]
- Zhu, Y.; Ku, Y.; Liu, J.; Le, T.; Zhao, Z. Community characteristics and functions of phosphate-solubilizing bacteria in rhizosphere soil of natural and planted Pinus tabuliformis forests on the Loess Plateau, Northwest China. Ying Yong Sheng Tai Xue Bao 2021, 32, 3097–3106. (In Chinese) [Google Scholar]
- Ghosh, R.; Barman, S.; Mukherjee, R.; Mandal, N. Role of phosphate solubilizing Burkholderia spp. for successful colonization and growth promotion of Lycopodium cernuum L. (Lycopodiaceae) in lateritic belt of Birbhum district of West Bengal, India. Microbiol. Res 2016, 183, 80–91. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.; Majid, N.; Jalloh, M. Potential PGPR properties of cellulolytic, nitrogen-fixing, phosphate-solubilizing bacteria in rehabilitated tropical forest soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.; Kučerik, J.; Mumtaz, M.; Holatko, J.; Munaza, N.; Antonin, K.; Mukkaram, E.; Muhammad, N.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A Look at plant-growth-promoting bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, Y.; Wei, Y.; Huang, Y.; Zhang, H.; He, W.; Sheng, H.; An, L. Screening of plant growth promoting bacteria (PGPB) from rhizosphere and bulk soil of Caragana microphylla in different habitats and their effects on the growth of Arabidopsis seedlings. Biotechnol. Biotechnol. Equip. 2019, 33, 921–930. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Z.; Wang, G.; Xue, W.; Li, C.; Chen, X.; Chen, D. A Bacterium isolated from soil in a karst rocky desertification region has efficient phosphate-solubilizing and plant growth-promoting ability. Front. Microbiol. 2021, 11, 625450. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Dai, Y.; Chen, Y.; Tian, Y.; Huo, Z. Isolation and screening of phosphorus solubilizing bacteria from saline alkali soil and their potential for Pb pollution remediation. Front. Bioeng. Biotechnol. 2023, 11, 1134310. [Google Scholar] [CrossRef]
- Li, H.; Su, J.; Yang, X.; Zhu, Y. Distinct rhizosphere effect on active and total bacterial communities in paddy soils. Sci. Total Environ. 2019, 649, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Ahmad, W.; Latif, F.; Haurat, J.; Bally, R.; Normand, P.; Malik, K. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil 2001, 237, 47–54. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Shen, R. Stronger effects of maize rhizosphere than phosphorus fertilization on phosphatase activity and phosphorus-mineralizing-related bacteria in acidic soils. Rhizosphere 2022, 23, 100555. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Shen, R.F. Efects of calcium carbonate and rhizosphere on abundance of phosphate-solubilizing microorqanisms in acidic red soil. Soils 2022, 52, 704–709. (In Chinese) [Google Scholar]
- Gupta, R.; Kumari, A.; Sharma, S.; Alzahrani, O.; Noureldeen, A.; Darwish, H. Identification, characterization and optimization of phosphate solubilizing rhizobacteria (PSRB) from rice rhizosphere. Saudi J. Biol. Sci. 2022, 29, 35–42. [Google Scholar] [CrossRef]
- Yahya, M.; Islam, E.; Rasul, M.; Farooq, I.; Mahreen, N.; Tawab, A.; Muhammad, I.; Lubna, R.; Imran, A.; Sumera, Y. Differential Root Exudation and Architecture for Improved Growth of Wheat Mediated by Phosphate Solubilizing Bacteria. Front. Microbiol. 2021, 12, 744094. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Ray, P.; Mahato, B.; Paramanik, B.; Choudhury, A.; Karforma, J. Performance of phosphate solubilizing bacteria in tea (Camellia sinensis L.) Rhizosphere. Natl. Acad. Sci. Lett. 2021, 44, 561–564. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Gopi, C. Distribution pattern and screening of phosphate solubilizing bacteria isolated from different food and forage crops. Agron. J. 2006, 5, 600–604. [Google Scholar]
- Chen, D.; Sun, W.; Xiang, S.; Zou, S. High-throughput sequencing analysis of the composition and diversity of the bacterial community in Cinnamomum camphora soil. Microorganisms 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Liu, L.; Li, S.; Xie, J.; Xue, X.; Jiang, Y. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef]
- Huang, C.; Yang, K.Y.; Gao, P.; Liang, Y.P.; Han, L.J.; Zhao, X. Screening, ldentification and characteristics of phosphate-solubilizing microorganisms in Lespedeza daurica. Acta Agrestia Sinica. 2022, 30, 2345–2355. [Google Scholar]
- Li, H.; Li, C.; Song, X.; Li, J.; Zhang, P.; Sun, F.; Geng, Z.; Liu, X. Isolation and identification of antagonistic Bacillus amyloliquefaciens HSE-12 and its effects on peanut growth and rhizosphere microbial community. Front. Microbiol. 2023, 14, 1274346. [Google Scholar] [CrossRef]
- Mei, X.L.; Shan, A.Q.; Jiang, Y.; Wei, Z.; Wang, Y.Y.; Wang, S.M. Screening of phosphorus solubng bacteria adapted to maize and their effect on maize growth. Acta Pedologica Sinica. 2016, 53, 502–509. [Google Scholar]
- Ateş, Ç.; Yalçin, G.R.; Taşpinar, K.; Alveroğlu, V. Isolation and characterization of phosphate solubilizing bacteria and effect of growth and nutrient uptake of maize under pot and field conditions. Commun. Soil Sci. Plant Anal. 2022, 53, 2114–2124. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; Ghadraoui, L.; Errachidi, F. Growth stimulation of two legumes (Vicia faba and Pisum sativum) using phosphate-solubilizing bacteria inoculation. Front. Microbiol. 2023, 14, 1212702. [Google Scholar] [CrossRef]
- Xiao, K.; Cui, Y.; Gao, D.Y.; Wang, Z.G.; Yan, A.H. Screening of rhizosphere phosphorus-dissolving bacteria of walnut and studies on their role in walnut promotion. J. Hebei Agric. Univ. 2018, 41, 49–54. [Google Scholar]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric halotolerant phosphate solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020, 80, 948. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Gao, Y.F.; Wang, Y.; Li, C.X.; Gao, X.Y.; Zhang, Z.H. Study on phosphate-solubilizing strain selection of plant growth promoting rhizobacteria and its effect on tomato growth promotion. Southwest China J. Agric. Sci. 2020, 33, 2891–2896. [Google Scholar]
- Shah, S.H.; Hussain, M.B.; Zahir, Z.A.; Haq, T.U.; Matloob, A. Thermal plasticity and cotton production enhancing attributes of phosphate-solubilizing bacteria from cotton rhizosphere. J. Soil. Sci. Plant Nutr. 2022, 22, 3885–3900. [Google Scholar] [CrossRef]
- Liu, C.J.; Du, C.Y.; Liang, Z.J.; Xia, Z.J.; Zhang, D.Z.; Gao, Y.P. Identification of high-efficiency phosphate—Dissolving strain CT45-1 and its promoting effect on tobacco. Shandong Agric. Sci. 2019, 51, 74–78. (In Chinese) [Google Scholar]
- Susilowati, L.; Kusumo, B.; Arifin, Z. Screening of the drought tolerant phosphate solubilizing bacteria in dissolving P-inorganic. J. Phys. Conf. Ser. 2019, 1402, 55082. [Google Scholar] [CrossRef]
- Soares, A.; Nascimento, V.; De Oliveira, E.; Jumbo, L.; Dos Santos, G.; Queiroz, L.; Silva, R.; Filho, R.; Romero, M.; Aguiar, R. Pseudomonas aeruginosa and Bacillus cereus isolated from Brazilian cerrado soil act as phosphate-solubilizing bacteria. Curr. Microbiol. 2023, 80, 146. [Google Scholar] [CrossRef]
- Ren, J.; Liu, H.; Wu, X.; Wang, Q.; Ren, Y.; Liu, Y. Screening, identification, and promoting effect of phosphate-solubilizing bacteria in rhizosphere of Taxus chinensis var. mairei. Wei Sheng Wu Hsüeh Pao 2012, 52, 295–303. [Google Scholar]
- Wang, M.; Sun, H.; Xu, Z. Analysis of blueberry plant rhizosphere bacterial diversity and selection of plant growth promoting rhizobacteria. Curr. Microbiol. 2022, 79, 331. [Google Scholar] [CrossRef]
- Wei, X.; Fu, T.; He, G.; Zhong, Z.; Yang, M.; Lou, F.; He, T. Characteristics of rhizosphere and bulk soil microbial community of Chinese cabbage (Brassica campestris) grown in Karst area. Front. Microbiol. 2023, 14, 1241436. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Li, H.; Zhou, Z.; Wang, S.; Wang, Z.; Song, C.; Zhou, Y. Distribution of phosphorus-solubilizing bacteria in relation to fractionation and sorption behaviors of phosphorus in sediment of the Three Gorges Reservoir. Environ. Sci. Pollut. Res. 2017, 24, 17679–17687. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Shang, K.; Liu, X.; Wei, Q.S.; Ran, X.B.; Du, G.X.; Zhao, S.J.; Qu, L.Y. Diversity characteristics of organic phosphate-solubilizing bacteria in surface sediments of the Yangtze River Estuary. Adv. Mar. Biol. 2019, 37, 495–507. [Google Scholar]
- Qu, J.H.; Zhang, L.J.; Fu, Y.H.; Li, H.F.; Tian, H.L. Isolation Identification and phosphorus-dissolving capacity of an efficient phosphate-solubilizing bacterium. J. Henan Agric. Sci. 2018, 47, 55–91. (In Chinese) [Google Scholar]
- Yuan, Z.; Liu, F.; Zhang, G. Characteristics and biodiversity of endophytic phosphorus- and potassium-solubilizing bacteria in moso bamboo (Phyllostachys edulis). Acta. Biol. Hung. 2015, 66, 449–459. [Google Scholar] [CrossRef]
- Tian, Q.; Gong, Y.; Liu, S.; Ji, M.; Tang, R.; Kong, D.; Qin, S. Endophytic bacterial communities in wild rice (Oryza officinalis) and their plant growth-promoting effects on perennial rice. Front. Plant Sci. 2023, 14, 1184489. [Google Scholar] [CrossRef] [PubMed]
- Baghel, V.; Thakur, J.K.; Yadav, S.S.; Manna, M.C.; Mandal, A.; Shirale, A.O.; Sharma, P.; Sinha, N.K.; Mohanty, M.; Singh, A.B.; et al. Phosphorus and potassium solubilization from rock minerals by Endophytic burkholderia sp. Strain FDN2-1 in soil and shift in diversity of bacterial endophytes of corn root tissue with crop growth stage. Geomicrobiol. J. 2020, 37, 550–563. [Google Scholar] [CrossRef]
- Tang, D.X.; Gao, Y.Z. Advances on the strategies of soil phosphate solubilizing micioorganisms to promote plan. Sheng Tai Xue Bao 2023, 43, 4390–4399. (In Chinese) [Google Scholar]
- Dinesh, R.; Srinivasan, V.; Praveena, R.; Subila, K.; George, P.; Das, A.; Shajina, O.; Anees, N.K.; Leela, P. Exploring the potential of P solubilizing rhizobacteria for enhanced yield and quality in turmeric (Curcuma longa L.). Ind. Crops Prod. 2022, 189, 115826. [Google Scholar] [CrossRef]
- Patel, D.; Murawala, P.; Archana, G.; Naresh Kumar, G. Repression of mineral phosphate solubilizing phenotype in the presence of weak organic acids in plant growth promoting fluorescent pseudomonads. Bioresour. Technol. 2021, 102, 3055–3061. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.; Glick, B. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.; Munir, A.; Gurmani, A. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Dash, S.; Borah, S.S.; Kalamdhad, A.S. Study of the limnology of wetlands through a one-dimensional model for assessing the eutrophication levels induced by various pollution sources. Ecol. Model. 2019, 416, 108907. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Agron. J. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fang, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, J.; Li, X.; Liu, F. Effect of several organic acids on phosphate adsorption by variable charge soils of central China. Environ Int. 2001, 26, 353–358. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Ye, J.; Yang, H. Characteristics of Organic Acid Secretion Associated with the Interaction between Burkholderia multivorans WS-FJ9 and Poplar Root System. Biomed Res. Int. 2018, 2018, 9619724. [Google Scholar] [CrossRef]
- Yue, L.; Jiao, L.; Tao, M.; Xu, L.; Cao, X.; Chen, F.; Wang, C.; Cheng, B.; Wang, Z. Dynamics of organic acid exudation and rhizobacteria in maize rhizosphere respond to N-CDs. Sci. Total Environ. 2023, 901, 166500. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, D.; Xiong, Z.; Wang, Z.; Gao, M. Changes in rhizosphere phosphorus fractions and phosphate-mineralizing microbial populations in acid soil as influenced by organic acid exudation. Soil Tillage Res. 2023, 225, 105543. [Google Scholar] [CrossRef]
- De Weert, S.; Vermeiren, H.; Mulders, I.; Kuiper, I.; Hendrickx, N.; BloeMberg, G.; Vanderleyden, J.; Mot, R.; Lugtenberg, B. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol. Plant Microbe Interact. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.; Paré, P.; Bais, H. Root-Secreted Malic Acid Recruits Beneficial Soil Bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Zhao, X.R.; Lin, Q.M.; Li, B.G. The Relationship Between Rock Phosphate Solubilization and pH and Organic Acid Production of Microorganisms. J. Microbiol. 2003, 23, 5–7. [Google Scholar]
- Qin, L.J.; Wang, Z.H.; Chen, Q.B.; Bai, C.J. Effect of inoculation with phosphate-solubilizing bacteria and soil acidification on the growth of Stylosanthes guianensis Reyan No. 2. Cao Ye Xue Bao 2008, 17, 20–28. [Google Scholar]
- Vazquez, P.; Holguin, G.; Puente, M.; Lopez-Cortes, A.; Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils. 2000, 30, 460–468. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Cao, X.; Cao, A.; Han, Y.; Zhao, N. Isolation and identification of saline tolerance phosphate-solubilizing bacteria derived from salt-affected soils and their mechanisms of p-solubilizing. ICAB 2014, 2, 1259–1266. [Google Scholar]
- Wang, J.; Yan, A.; Wang, W.; Li, J.; Li, Y. Screening, identification and phosphate-solubilizing characteristics of phosphate-solubilizing bacteria strain D2 (Pantoea sp.) in rhizosphere of Pinus tabuliformis in iron tailings yard. Ying Yong Sheng Tai Xue Bao 2016, 27, 3705–3711. [Google Scholar] [PubMed]
- Chawngthu, L.; Hnamte, R.; Lalfakzuala, R. Isolation and characterization of rhizospheric phosphate solubilizing bacteria from wetland paddy field of Mizoram, India. Geomicrobiol. J. 2020, 37, 366–375. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissoufi, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z.; Delaplace, P. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef]
- Zhang, H.; Han, L.; Jiang, B.; Long, C. Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J. Microbiol. Biotechnol. 2021, 37, 109. [Google Scholar] [CrossRef]
- Parastesh, F.; Alikhani, H.; Etesami, H. Vermicompost enriched with phosphate–solubilizing bacteria provides plant with enough phosphorus in a sequential cropping under calcareous soil conditions. J. Clean. Prod. 2019, 221, 27–37. [Google Scholar] [CrossRef]
- Trujillo-Narcía, A.; Rivera-Cruz, M.; Magaña-Aquino, M.; Trujillo-Rivera, E. The burning of sugarcane plantation in the tropics modifies the microbial and enzymatic processes in soil and rhizosphere. J. Soil Sci. Plant Nutr. 2019, 19, 906–919. [Google Scholar] [CrossRef]
- Paul, R.; Singh, R.; Patra, A.; Biswas, D.; Bhattacharyya, R.; Arunkumar, K. Phosphorus dynamics and solubilizing microorganisms in acid soils under different land uses of Lesser Himalayas of India. Agrofor. Syst. 2018, 92, 449–461. [Google Scholar] [CrossRef]
- Blanco-Vargas, A.; Rodríguez-Gacha, L.; Sánchez-Castro, N.; Garzón-Jaramillo, R.; Pedroza-Camacho, L.; Poutou-Piñales, R.; Rivera-Hoyos, C.; Díaz-Ariza, L.; Pedroza-Rodríguez, A. Phosphate-solubilizing Pseudomonas sp., and Serratia sp., co-culture for Allium cepa L. growth promotion. Heliyon 2020, 6, E05218. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wan, W. Phosphate-solubilizing bacterium Acinetobacter pittii gp-1 affects rhizosphere bacterial community to alleviate soil phosphorus limitation for growth of soybean (Glycine max). Front. Microbiol. 2021, 12, 737116. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Zhao, Y.; Guan, D.; Li, L.; Zhao, B.; Ma, M. Effects of Bradyrhizobium co-inoculated with Bacillus and Paenibacillus on the structure and functional genes of soybean rhizobacteria community. Genes 2022, 13, 1922. [Google Scholar] [CrossRef]
- Bagewadi, Z.; Yaraguppi, D.; Mulla, S.; Deshpande, S. Response Surface methodology based optimization, partial purification and characterization of alkaline phosphatase isolated from Pseudomonas asiatica Strain ZKB1 and its application in plant growth promotion. Mol. Biotechnol. 2022, 64, 984–1002. [Google Scholar] [CrossRef] [PubMed]
- Rombola, T.H.; Pedrinho, E.A.; de Macedo Lemos, E.G.; Gonçalves, A.M.; dos Santos, L.F.; Pizauro, J.M. Identification and enzymatic characterization of acid phosphatase from Burkholderia gladioli. BMC Res. Notes 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Dasila, H.; Sah, V.; Jaggi, V.; Kumar, A.; Tewari, L.; Taj, G.; Chaturvedi, S.; Perveen, K.; Bukhari, N.; Siang, T.; et al. Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front. Microbiol. 2023, 14, 1135693. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xing, Y.; Zhu, Y.; Gao, N.; Ying, Y. Diverse responses of pqqC- and phoD-harbouring bacterial communities to variation in soil properties of Moso bamboo forests. Microb. Biotechnol. 2022, 15, 2097–2111. [Google Scholar] [CrossRef]
- Feng, Q.; Guo, W.; Wang, T.; Cristina, M.A.L.; Luo, M.; Ge, R.; Zhou, C.; Zhang, Q.; Luo, J. Iron coupling with carbon fiber to stimulate biofilms formation in aerobic biological film systems for improved decentralized wastewater treatment: Performance, mechanisms and implications. Bioresour. Technol. 2021, 319, 124151. [Google Scholar] [CrossRef]
- Mónica, M.; Collavino, P.A.; Sansberro, L.A.; Mroginski, O.; Mario, A. Comparison of in vitro solubllization activity of diverse phosphate solubllizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils 2010, 46, 727–738. [Google Scholar]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.; Kaur, T.; Yadav, N.; Yadav, A.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.; Bertrand, I.; Barakat, A.; Zeroual, Y.; Oukarroum, A.; Plassard, C. Improved rock phosphate dissolution from organic acids is driven by nitrate assimilation of bacteria isolated from nitrate and CaCO3-rich soil. PLoS ONE 2023, 18, E0283437. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.; Mirza, M. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, E0204408. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil. Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Suleman, M.; Zaheer, A.; Reitz, T.; Tarkka, M.; Islam, E.; Mirza, M. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223–225, 1–12. [Google Scholar] [CrossRef]
- Sonnenburg, E.; Sonnenburg, J. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Nat. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef]
- Eeshita, B.; Renuka, D.; Yasmin, B.; Yasmin, B.; Sunil, K.M. Study of Pyrroloquinoline Quinine from phosphate-solubilizing microbes responsible for plant growth: In silico approach. Front. Agron. 2021, 3, 667339. [Google Scholar]
- Joshi, S.; Gangola, S.; Jaggi, V.; Sahgal, M. Functional characterization and molecular fingerprinting of potential phosphate solubilizing bacterial candidates from Shisham rhizosphere. Sci. Rep. 2023, 13, 7003. [Google Scholar] [CrossRef]
- Ding, Y.; Yi, Z.; Fang, Y.; He, S.; Li, Y.; He, K.; Zhao, H.; Jin, Y. Multi-omics reveal the efficient phosphate-solubilizing mechanism of bacteria on rocky soil. Front. Microbiol. 2021, 12, 761972. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wen, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, A.; Nguyen, T.; Posta, K. Metagenomic analysis of bacterial communities in agricultural soils from Vietnam with special attention to phosphate solubilizing bacteria. Microorganisms 2021, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria with Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Moe, L.; An, R. Regulation of pyrroloquinoline quinone-dependent glucose dehydrogenase activity in the model rhizosphere-dwelling bacterium Pseudomonas putida KT2440. Appl. Environ. Microbiol. 2016, 82, 4955–4964. [Google Scholar]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Maria, R.; Sumera, Y.; Mahreen, Y.; Claudia, B.; Mika, T.; Reitz, T. The wheat growth-promoting traits of Ochrobactrum and Pantoea species, responsible for solubilization of different P sources, are ensured by genes encoding enzymes of multiple P-releasing pathways. Microbiol. Res. 2021, 246, 126703. [Google Scholar]
- Cotta, S.R.; Cavalcante, F.D.; Seldin, L.A.; Andreote, F.D.; van Elsas, J.D. The diversity and abundance of phytase genes (β-propeller phytases) in bacterial communities of the maize rhizosphere. Lett. Appl. Microbiol. 2016, 62, 264–268. [Google Scholar] [CrossRef]
- Suleimanova, A.; Bulmakova, D.; Sokolnikova, L.; Egorova, E.; Itkina, D.; Kuzminova, O.; Gizatullina, A.; Sharipova, M. Phosphate solubilization and plant growth promotion by Pantoea brenneri soil isolates. Microorganisms 2023, 11, 1136. [Google Scholar] [CrossRef]
- Ducousso-Détrez, A.; Fontaine, J.; Lounès-Hadj Sahraoui, A.; Hijri, M. Diversity of phosphate chemical forms in soils and their contributions on soil microbial community structure changes. Microorganisms 2022, 10, 609. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Ma, L.; Luan, H.; Tang, J.; Li, R.; Li, M.; Huang, S.; Wang, L. Long-term partial substitution of chemical fertilizer by organic amendments influences soil microbial functional diversity of phosphorus cycling and improves phosphorus availability in greenhouse vegetable production. Agric. Ecosyst. Environ. 2023, 341, 108193. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, J.; Chen, H.; Zhang, Z.; Huang, J.; Lv, L.; Tan, J.; Jiang, X. The role of long-term mineral and manure fertilization on P species accumulation and phosphate-solubilizing microorganisms in paddy red soils. Soil 2023, 9, 101–116. [Google Scholar] [CrossRef]
- Kumar, A.; Ral, L. Soil organic carbon and phosphorus availability regulate abundance of culturable phosphate-solubilizing bacteria in paddy fields. Pedosphere 2020, 30, 405–413. [Google Scholar] [CrossRef]
- Kaur, G.; Sudhakara Reddy, M. Influence of P-solubilizing bacteria on crop yield and soil fertility at multilocational sites. Eur. J. Soil Biol. 2014, 61, 35–40. [Google Scholar] [CrossRef]
- Li, C.L.; Xun, Y.L.; Wang, Y.; Dang, Y.H.; Song, Y. Effects of long-term fertilization on soil nitrogen-transforming bacteria and phosphate-solubilizingbacteria in rainfed cropland of Loess Plateau, China. Chin. J. Plant Ecol. 2020, 39, 3658–3667. [Google Scholar]
- Zheng, B.; Hao, X.; Ding, K.; Zhou, G.; Chen, Q.; Zhang, J.; Zhu, Y. Long-term nitrogen fertilization decreased the abundance of inorganic phosphate solubilizing bacteria in an alkaline soil. Sci. Rep. 2017, 7, 42284. [Google Scholar] [CrossRef] [PubMed]
- Widdig, M.; Schleuss, P.; Weig, A.; Guhr, A.; Biederman, L.; Borer, E.; Crawley, M.; Kirkman, K.; Seabloom, E.; Wragg, P.; et al. Nitrogen and Phosphorus Additions Alter the Abundance of Phosphorus-Solubilizing Bacteria and Phosphatase Activity in Grassland Soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef]
- Tang, J.; Su, L.; Fang, Y.; Wang, C.; Meng, L.; Wang, J.; Zhang, J.; Xu, W. Moderate Nitrogen Reduction Increases Nitrogen Use Efficiency and Positively Affects Microbial Communities in Agricultural Soils. Agriculture 2023, 13, 796. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, D.; Wang, Y.; Hao, X.; Wadaan, M.; Hozzein, W.; Peñuelas, P.; Zhu, Y.; Yang, X. Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Sci. Rep. 2019, 9, 25. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, J.; Cao, Z.; Xue, K.; Quensen, J.; Guo, G.; Yang, Y.; Zhou, J.; Chu, H.; Tiedje, M. A buried Neolithic paddy soil reveals loss of microbial functional diversity after modern rice cultivation. Sci. Bull. 2016, 61, 1052–1060. [Google Scholar] [CrossRef]
- Xu, H.; Lv, J.; Yu, C. Combined phosphate-solubilizing microorganisms jointly promote Pinus massoniana growth by modulating rhizosphere environment and key biological pathways in seedlings. Ind. Crops Prod. 2023, 191, 116005. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.; Wang, J.; Zhang, K.; Wang, F.; Li, W.; Tian, P.; Li, Q. Inoculation of Escherichia coli enriched the key functional bacteria that intensified cadmium accumulation by halophyte Suaeda salsa in saline soils. J. Hazard. Mater. 2023, 458, 131922. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in phosphorus mobilization and community assembly of bacterial and fungal communities in rice rhizosphere under phosphate deficiency. Front. Microbiol 2022, 13, 953340. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, S. Prescience of endogenous regulation in Arabidopsis thaliana by Pseudomonas putida MTCC 5279 under phosphate starved salinity stress condition. Sci. Rep. 2020, 10, 5855. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S. Plant-microbe interactions ameliorate phosphate-mediated responses in the rhizosphere: A review. Front. Plant Sci. 2023, 14, 1074279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Fu, X. Integrated Effects of Co-Inoculation with Phosphate-Solubilizing Bacteria and N 2 -Fixing Bacteria on Microbial Population and Soil Amendment Under C Deficiency. Int. J. Environ. Res. Public Health 2019, 16, 2442. [Google Scholar] [CrossRef]
- Wang, S.; LV, H.R.; Zhang, H.; Wu, Z.W.; Xiao, C.H.; Sun, D.M. Whole-Genome Sequencing Identification of Phosphate-solubilizing Bacteria PSB-R and Analysis of Its Phosphate-solubilizing Properties. Biol. Bull. 2023, 39, 274–283. (In Chinese) [Google Scholar]
- Wang, K.X. Genomic Analysis of the Grassland soil Bacterium Baillus sp. SP1 and its Growth Promoting Effect on Forage; Inner Mongolia University: Neimenggu, Chian, 2022. (In Chinese) [Google Scholar]
- Xi, H. The Phosphate-Solubilizing Activity, Genome Analysis, Clone And Expression Of Genes Related to Phosphate Dissolution of a Strain A02; Nanjing University: Nanjing, China, 2017. (In Chinese) [Google Scholar]
- Li, S.S. Study on Phosphorus-Soluble Characteristics of Two Pseudomonas strains and Their Effects on the Soil Phosphorus Solublizing; Henan University of Technology: Luoyang, China, 2023. (In Chinese) [Google Scholar]
- Ma, Q.; He, S.; Wang, X.; Rengel, Z.; Chen, L.; Wang, X.; Zhang, X. Isolation and characterization of phosphate-solubilizing bacterium Pantoea rhizosphaerae sp. nov. from Acer truncatum rhizosphere soil and its effect on Acer truncatum growth. Front. Plant Sci. 2023, 14, 1218445. [Google Scholar] [CrossRef]
- Lv, H.; Yang, J.; Su, S.; Liu, Y.; Feng, J.; Sheng, Y.; Wang, T.; Pan, J.; Tang, L.; Chen, L.; et al. Distribution of Genes and Microbial Taxa Related to Soil Phosphorus Cycling across Soil Depths in Subtropical Forests. Forests 2023, 14, 1665. [Google Scholar] [CrossRef]
- Wei, L.Q. Isolation and Screening of Phosphate-solubilizing Bacteria and the Effect on Phosphorus Release from the Sediments in Rongcheng Swan Lake; Yantai University: Yantai, China, 2021. (In Chinese) [Google Scholar]
- Chen, D.Y.; Li, H.Q.; Zhang, B.H.; Dai, C.M.; Yang, J.Y. Phosphate solubilization activities and action mechanisms of two phosphate-solubilizing bacteria. Chin. J. Eco-Agric. 2017, 25, 410–418. (In Chinese) [Google Scholar]
- Wu, J.; Zhao, F. Machine learning: An effective technical method for future use in assessing the effectiveness of phosphorus-dissolving microbial agroremediation. Front. Bioeng. Biotechnol. 2023, 11, 1189166. [Google Scholar] [CrossRef] [PubMed]
| PSB Species | PSB Source | Secreted Enzymes | Reference |
|---|---|---|---|
| Pantoea sp. | Forest soil | Phytase | [5] |
| Serratia liquiefaciencs | Laboratory storage | ALP | [93] |
| Acinetobacter pittii gp-1 | Laboratory storage | ACP, phytase | [94] |
| aryabhattai | Laboratory storage | ACP | [95] |
| Pseudomonas asiatica | Ant hill soil | ALP | [96] |
| Burkholderia sp. | Zea mays rhizosphere soil | ACP | [97] |
| Pseudomonas sp. | Yellow sandalwood rhizosphere soil | ACP, ALP | [98] |
| Enterobacter sp. | rhizosphere soil of moso bamboo | ACP | [99] |
| PSB | Genome Size (bp) | G+C (%) | Predicted Number of Genes | Acidolysis of Associated Genes | Enzymatic Hydrolysis of Associated Genes | Genes Associated with Phosphorus Cycling | Related Pathways | Reference |
|---|---|---|---|---|---|---|---|---|
| Pseudomonas sp. | 5,617,746 | 62.86 | 5097 | GDH, ppq, gcd, gdh | ppa, PPX | PstS, PstC, PstA, PstB, PhoU | Entner–Doudoroff (ED) | [138] |
| Serratia marcescens | 5,061,510 | 59.75 | 4742 | gcd, PQQ, ipdC | ppx, ppa, phoA, phnX, chi | - | Secreted iron carrier-complete pathway | [139] |
| Bacillus subtilis | 4,173,570 | 43.25 | 4604 | pyruvate, carboxylase | phoD, phoR | pit, pstS, pstB | Carbon metabolism, biosynthesis of amino acids | [140] |
| Enterobacter cloacae | 4,608,301 | 54.78 | 4450 | ppq, gcd, gdh | - | phnG, PhnH, PhnJ, PhnM, PhnP, PhnI | - | [141] |
| Pseudomonas putida | 5,957,620 | 61.55 | 5535 | GDH, pqq, gcd, aceE, aceF, lpd, IDH3, idhA, dld, maeB | - | PstS, PstC, PstA, PstB, PhoU | Tricarboxylic acid cycle and pyruvate pathway | [142] |
| Pseudomonas fildesensis | 6,789,479 | 60.82 | 6028 | GDH, pqq, gcd, aceE, aceF, lpd, IDH3, idhA, dld, maeB | phoD | PstS, PstC, PstA, PstB, PhoU | Tricarboxylic acid cycle and pyruvate pathway | [142] |
| Pantoea | 7,989,160 | 51.3 | 4548 | pqq, GCD | - | phnN, phnM | Indole-3-pyruvate pathway | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. https://doi.org/10.3390/microorganisms11122904
Pan L, Cai B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms. 2023; 11(12):2904. https://doi.org/10.3390/microorganisms11122904
Chicago/Turabian StylePan, Lin, and Baiyan Cai. 2023. "Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects" Microorganisms 11, no. 12: 2904. https://doi.org/10.3390/microorganisms11122904
APA StylePan, L., & Cai, B. (2023). Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms, 11(12), 2904. https://doi.org/10.3390/microorganisms11122904






