Multiplex Real-Time PCR for the Detection of Tetracycline, Ciprofloxacin, and Erythromycin Resistance Determinants from Human and Foodborne Campylobacter jejuni and Campylobacter coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Campylobacter Isolates and Growth Conditions
2.2. Antibiotic Susceptibility Testing EUCAMP3
2.3. DNA Extraction and Quantification
2.4. Next-Generation Sequencing (NGS) and Assembly
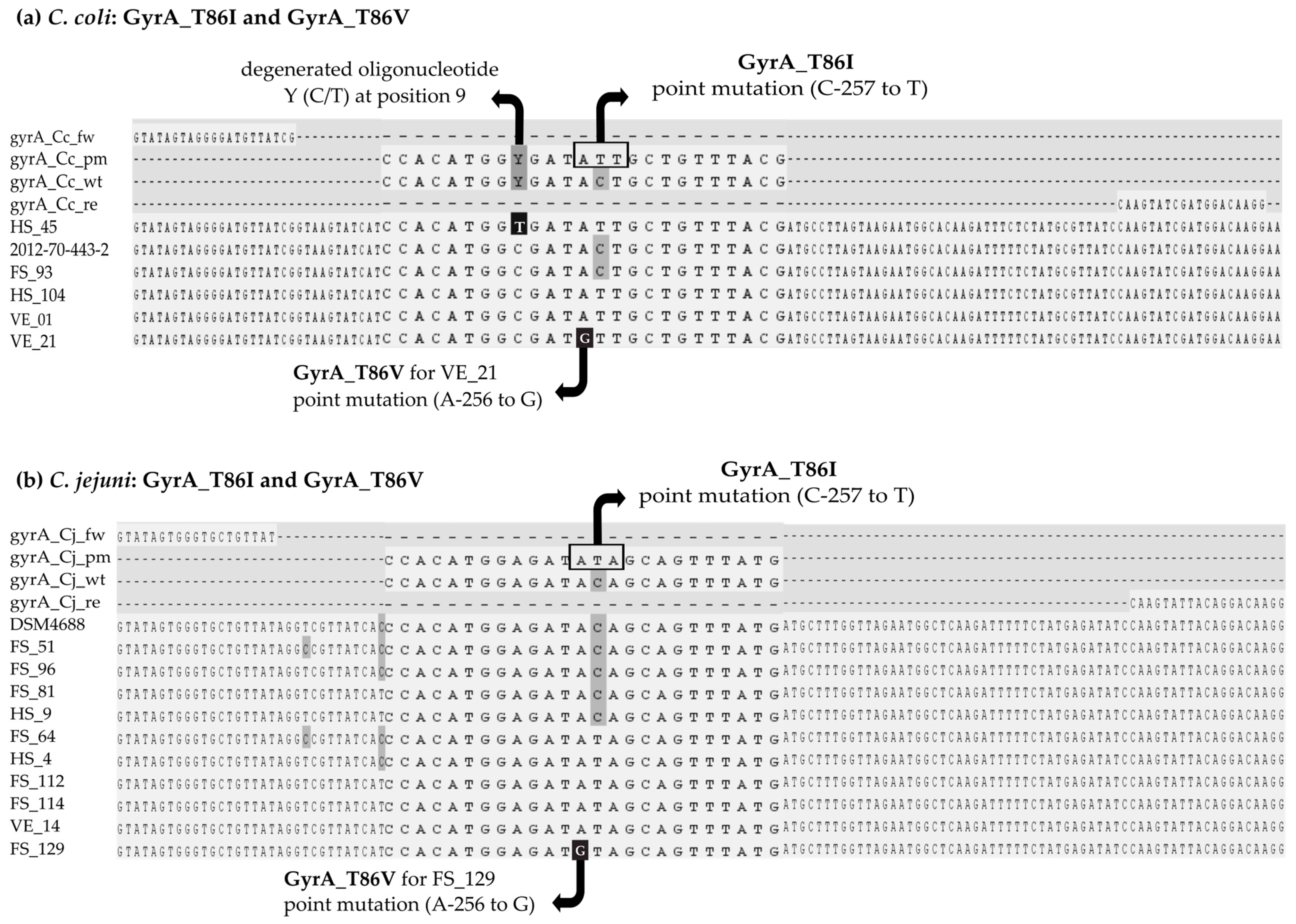
2.5. Design of Primers and Probes
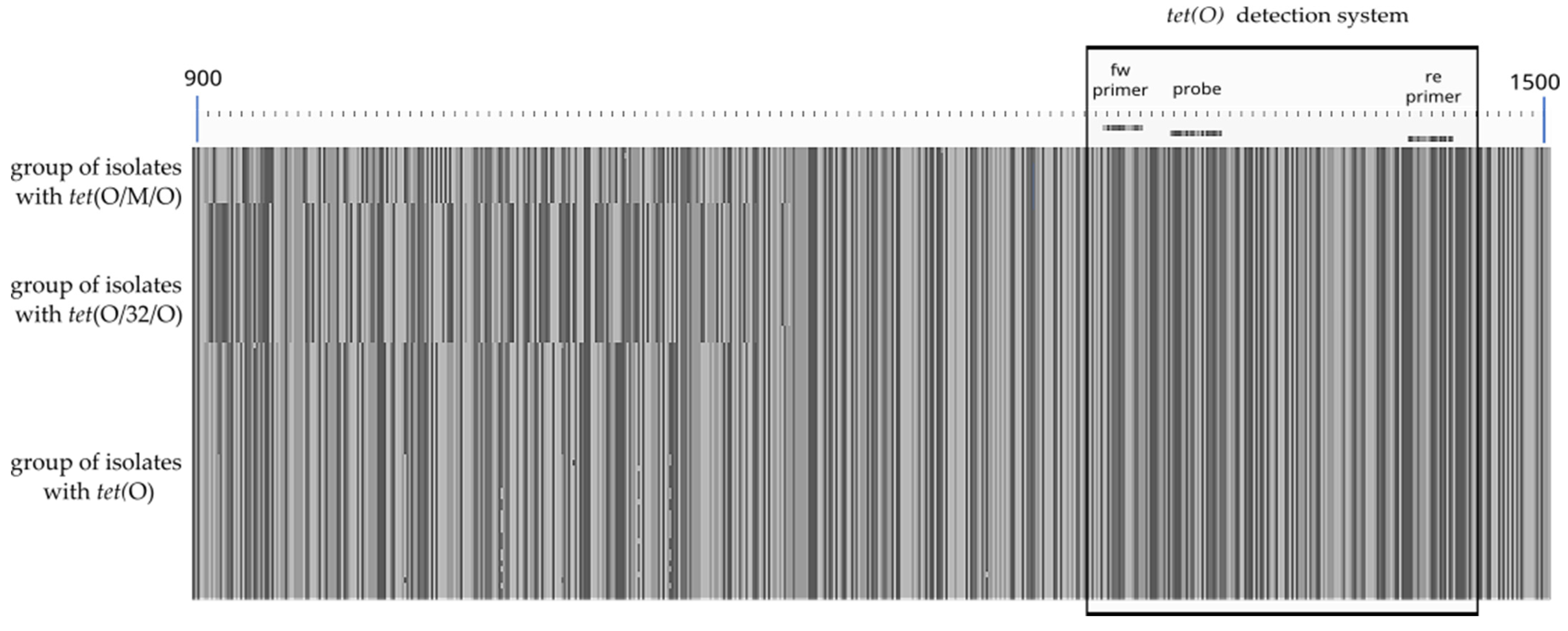
2.6. In Silico Screening for Primer Binding Sites and Gene Alignments
2.7. Multiplex Real Time PCR Assay for Detection of Resistance Determinants
2.8. In-House Validation of the Pentaplex Real-Time PCR Assay
2.8.1. Selectivity
2.8.2. Determination of Efficiency and LOD95%
2.8.3. Robustness
3. Results
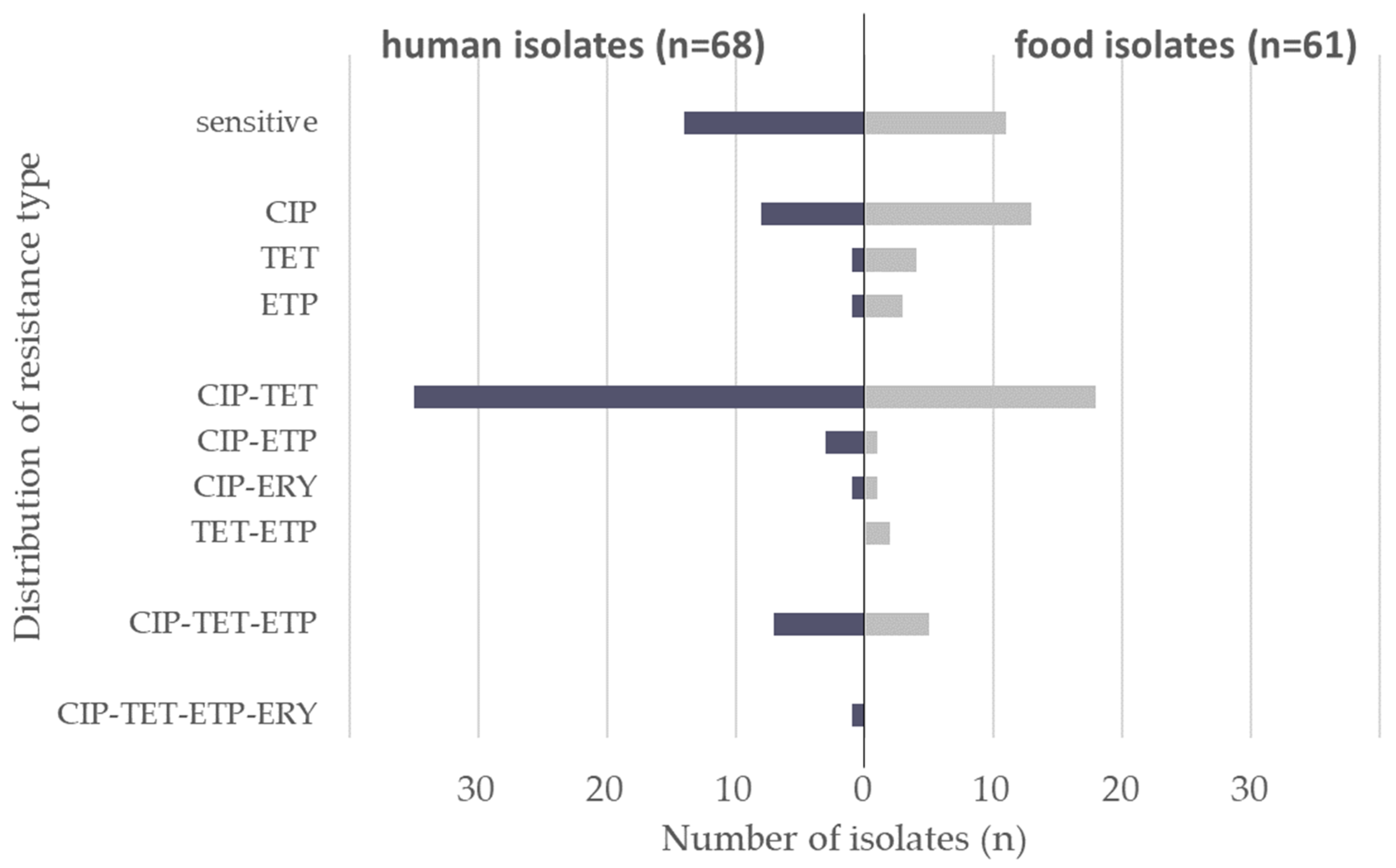
3.1. Antimicrobial Resistance Profiles
3.2. Design of Primer and Probes for Real-Time PCR Assays
3.3. In Silico Screening in Comparison to Phenotypic Results
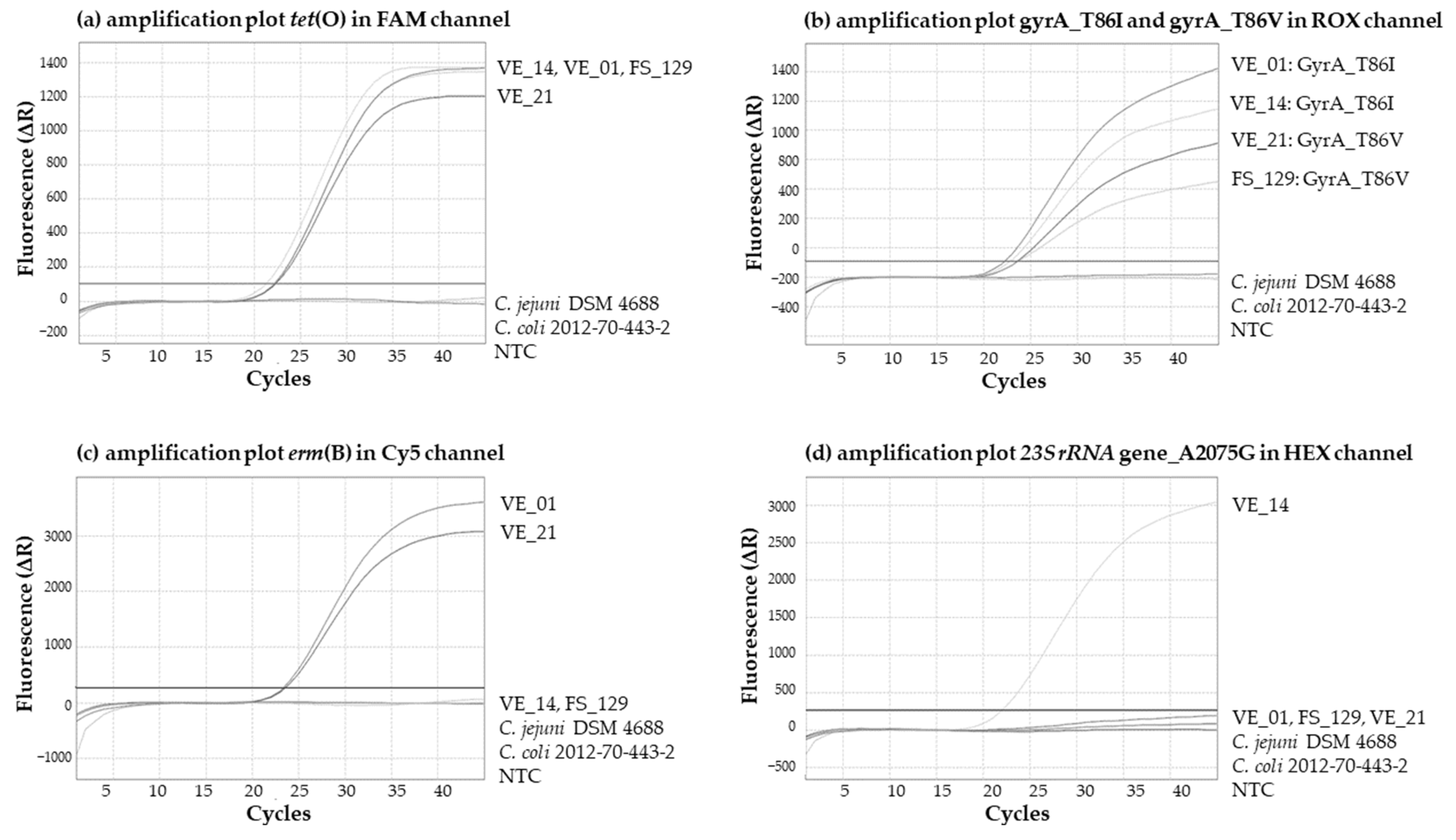
3.4. Multiplex Real-Time PCR Assay
3.5. In-House Validation of the Multiplex Real-Time PCR Assay
3.5.1. Specificity and Selectivity
3.5.2. Determination of Efficiency and LOD95%
3.5.3. Robustness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Nastasijevic, I.; Proscia, F.; Boskovic, M.; Glisic, M.; Blagojevic, B.; Sorgentone, S.; Kirbis, A.; Ferri, M. The European Union control strategy for Campylobacter spp. in the broiler meat chain. J. Food Saf. 2020, 40, e12819. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Levy, S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updat. 2000, 3, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC.OJ L 4, 7.1. 2019, pp. 43–167. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019R0006 (accessed on 29 November 2023).
- Qin, X.; Wang, X.; Shen, Z. The rise of antibiotic resistance in Campylobacter. Curr. Opin. Gastroenterol. 2022, 39, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, E.; Miljković-Selimović, B.; Tambur, Z.; Aleksić, N.; Biočanin, V.; Avramov, S. Resistance to Antibiotics in Thermophilic Campylobacters. Front. Med. 2021, 8, 763434. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, N.; Rojas, J.; Pollett, S.; Meza, R.; Patiño, L.; Leiva, M.; Camiña, M.; Bernal, M.; Reynolds, N.D.; Maves, R.; et al. Validation of the T86I mutation in the gyrA gene as a highly reliable real time PCR target to detect Fluoroquinolone-resistant Campylobacter jejuni. BMC Infect. Dis. 2020, 20, 518. [Google Scholar] [CrossRef] [PubMed]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q.; Blair, J.M.; Richmond, G.E.; Piddock, L.J.; de Lastours, V.; et al. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Futur. Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef]
- Whitehouse, C.A.; Zhao, S.; Mukherjee, S.; Tate, H.; Bodeis-Jones, S.; Young, S.; Gaines, S.; McDermott, P. Gyrase A Mutations in Campylobacter Associated with Decreased Susceptibility to Different Fluoroquinolones. J. Food Prot. 2017, 80, 1863–1866. [Google Scholar] [CrossRef]
- Rokney, A.; Valinsky, L.; Vranckx, K.; Feldman, N.; Agmon, V.; Moran-Gilad, J.; Weinberger, M. WGS-Based Prediction and Analysis of Antimicrobial Resistance in Campylobacter jejuni Isolates From Israel. Front. Cell. Infect. Microbiol. 2020, 10, 365. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Shen, Z.; Seng, V.W.; Sahin, O.; Jeon, B.; Liu, P.; Zhang, Q. Impaired Fitness and Transmission of Macrolide-Resistant Campylobacter jejuni in Its Natural Host. Antimicrob. Agents Chemother. 2012, 56, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Song, L.; Liang, H.; Gu, Y.; Zhang, C.; Liu, X.; Zhang, J.; Zhang, M. Molecular subtyping and erythromycin resistance of Campylobacter in China. J. Appl. Microbiol. 2016, 121, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Deng, F.; Shen, Z.; Wu, C.; Zhang, J.; Zhang, Q.; Shen, J. Emergence of Multidrug-Resistant Campylobacter Species Isolates with a Horizontally Acquired rRNA Methylase. Antimicrob. Agents Chemother. 2014, 58, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Florez-Cuadrado, D.; Ugarte-Ruiz, M.; Quesada, A.; Palomo, G.; Domínguez, L.; Porrero, M.C. Description of an erm(B)-carrying Campylobacter coli isolate in Europe. J. Antimicrob. Chemother. 2016, 71, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Tagg, K.A.; Joung, Y.J.; Bennett, C.; Watkins, L.F.; Eikmeier, D.; Folster, J.P. Report of erm (B)+ Campylobacter jejuni in the United States. Antimicrob. Agents Chemother. 2018, 62, e02615-17. [Google Scholar] [CrossRef] [PubMed]
- Dasti, J.I.; Groß, U.; Pohl, S.; Lugert, R.; Weig, M.; Schmidt-Ott, R. Role of the plasmid-encoded tet(O) gene in tetracycline-resistant clinical isolates of Campylobacter jejuni and Campylobacter coli. J. Med Microbiol. 2007, 56, 833–837. [Google Scholar] [CrossRef]
- Hormeño, L.; Campos, M.J.; Vadillo, S.; Quesada, A. Occurrence of tet(O/M/O) Mosaic Gene in Tetracycline-Resistant Campylobacter. Microorganisms 2020, 8, 1710. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.J.; Amodeo, N.; Roberts, A.P. Mosaic tetracycline resistance genes encoding ribosomal protection proteins. J. Antimicrob. Chemother. 2016, 71, 3333–3339. [Google Scholar] [CrossRef]
- Zarske, M.; Luu, H.Q.; Deneke, C.; Knüver, M.T.; Thieck, M.; Hoang, H.T.T.; Bretschneider, N.; Pham, N.T.; Huber, I.; Stingl, K. Systematic identification of knowledge gaps in whole-genome sequence analysis of multi-resistant thermotolerant Campylobacter spp., 24 October 2023, PREPRINT (Version 1) available at Research Square. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Hachesoo, B.A.; Khoshbakht, R.; Yazdi, H.S.; Tabatabaei, M.; Hosseinzadeh, S.; Asasi, K. Tetracycline Resistance Genes in Campylobacter jejuni and C. coli Isolated From Poultry Carcasses. Jundishapur J. Microbiol. 2014, 7, e12129. [Google Scholar] [CrossRef]
- Laprade, N.; Cloutier, M.; Lapen, D.R.; Topp, E.; Wilkes, G.; Villemur, R.; Khan, I.U. Detection of virulence, antibiotic resistance and toxin (VAT) genes in Campylobacter species using newly developed multiplex PCR assays. J. Microbiol. Methods 2016, 124, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Vacher, S.; Menard, A.; Bernard, E.; Santos, A.; Megraud, F.; Cha, W.; Mosci, R.; Wengert, S.L.; Singh, P.; Newton, D.W.; et al. Detection of Mutations Associated with Macrolide Resistance in Thermophilic Campylobacter spp. by Real-Time PCR. Microb. Drug Resist. 2005, 11, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zirnstein, G.; Li, Y.; Swaminathan, B.; Angulo, F. Ciprofloxacin Resistance in Campylobacter jejuni Isolates: Detection of gyrA Resistance Mutations by Mismatch Amplification Mutation Assay PCR and DNA Sequence Analysis. J. Clin. Microbiol. 1999, 37, 3276–3280. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.M.; Hotzel, H.; El-Adawy, H.; Tran, H.T.; Le, M.T.H.; Tomaso, H.; Neubauer, H.; Hafez, H.M. Genotyping and antibiotic resistance of thermophilic Campylobacter isolated from chicken and pig meat in Vietnam. Gut Pathog. 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Jalava, J.; Kataja, J.; Seppälä, H.; Huovinen, P. In Vitro Activities of the Novel Ketolide Telithromycin (HMR 3647) against Erythromycin-Resistant Streptococcus Species. Antimicrob. Agents Chemother. 2001, 45, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Boto, D.; López-Portolés, J.A.; Simón, C.; Valdezate, S.; Echeita, M.A. Study of the molecular mechanisms involved in high-level macrolide resistance of Spanish Campylobacter jejuni and Campylobacter coli strains. J. Antimicrob. Chemother. 2010, 65, 2083–2088. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, 7867. [Google Scholar] [CrossRef]
- ISO 10272-2:2017; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- Huber, I.; Pavlovic, M.; Maggipinto, M.; Konrad, R.; Busch, U. Interlaboratory Proficiency Test Using MALDI-TOF MS for Identification of Food-Associated Bacteria. Food Anal. Methods 2018, 11, 1068–1075. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Amore, G.; Beloeil, P.-A.; Garcia Fierro, R.; Guerra, B.; Papanikolaou, A.; Rizzi, V.; Stoicescu, A.-V. Manual for reporting 2022 antimicrobial resistance data within the framework of Directive 2003/99/EC and Decision 2020/1729/EU. EFSA Support. Publ. 2023, 20, EN-7826. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2020/1729 of 17 November 2020, on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU (Notified under Document C(2020) 7894). OJ L387. 19 November 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2020:387:TOC (accessed on 29 November 2023).
- EUCAST. European Committee on Antimicrobial Susceptibility Testing, Data from the EUCAST MIC Distribution Website 2023. Available online: https://mic.eucast.org/search/ (accessed on 1 March 2023).
- Deneke, C.; Brendebach, H.; Uelze, L.; Borowiak, M.; Malorny, B.; Tausch, S.H. Species-Specific Quality Control, Assembly and Contamination Detection in Microbial Isolate Sequences with AQUAMIS. Genes 2021, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Pietsch, K.; Zucker, R.; Mayr, A.; Müller-Hohe, E.; Messelhäusser, U.; Sing, A.; Busch, U.; Huber, I. Validation of a Duplex Real-Time PCR for the Detection of Salmonella spp. in Different Food Products. Food Anal. Methods 2011, 4, 259–267. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A.; Gupta, N.; Bharti, D.R.; Aggarwal, N.; Ravi, V. A two-step process for in silico screening to assess the performance of qRTPCR kits against variant strains of SARS-CoV-2. BMC Genom. 2022, 23, 755. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://github.com/dariober/bioinformatics-cafe/tree/master/fastaRegexFinder (accessed on 29 November 2023).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Govindaswamy, J.; Zeller-Péronnet, V.; Pavlovic, M.; Wirtz, D.; Murr, L.; Thärigen, D.; Colson, B.; Uhlig, S.; Busch, U.; Huber, I. Digital Droplet-PCR for Quantification of Viable Campylobacter jejuni and Campylobacter coli in Chicken Meat Rinses. Appl. Sci. 2022, 12, 5315. [Google Scholar] [CrossRef]
- QuoData Web Service ‘Validation of Qualitative PCR Methods within a Single Laboratory’. 2015. Available online: https://quodata.de/content/validation-qualitative-pcr-methods-single-laboratory (accessed on 29 November 2023).
- Bundesamts für Verbraucherschutz und Lebensmittelsicherheit (BVL). Guidelines for the Single-Laboratory Validation of Qualitative Real-Time PCR Methods; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit: Federal Office of Consumer Protection and Food Safety: Braunschweig, Germany, 2016; Available online: https://www.bvl.bund.de/SharedDocs/Downloads/07_Untersuchungen/Guidelines%20for%20the%20single%20laboratory.pdf?__blob=publicationFile&v=3 (accessed on 29 November 2023).
- Bundesamts für Verbraucherschutz und Lebensmittelsicherheit (BVL). Guidelines for the Validation of Qualitative Real-Time PCR Methods by Means of a Collaborative Study; Federal Office of Consumer Protection and Food Safety: Braunschweig, Germany, 2016; Available online: https://www.bvl.bund.de/SharedDocs/Downloads/07_Untersuchungen/Guidelines%20for%20the%20validation%20of%20qualitative.pdf?__blob=publicationFile&v=3 (accessed on 29 November 2023).
- Nakagawa, O.; Fujii, A.; Kishimoto, Y.; Nakatsuji, Y.; Nozaki, N.; Obika, S. 2′-O,4′-C -Methylene-Bridged Nucleic Acids Stabilize Metal-Mediated Base Pairing in a DNA Duplex. ChemBioChem 2018, 19, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Moreira, B.G.; Behlke, M.A.; Owczarzy, R. Design of LNA probes that improve mismatch discrimination. Nucleic Acids Res. 2006, 34, e60. [Google Scholar] [CrossRef]
- Tolstrup, N.; Nielsen, P.S.; Kolberg, J.G.; Frankel, A.M.; Vissing, H.; Kauppinen, S. OligoDesign: Optimal design of LNA (locked nucleic acid) oligonucleotide capture probes for gene expression profiling. Nucleic Acids Res. 2003, 31, 3758–3762. [Google Scholar] [CrossRef]
- Meinersmann, R.; Phillips, R.; Ladely, S. Inter- and intra-genomic heterogeneity of the intervening sequence in the 23S ribosomal RNA gene of Campylobacter jejuni and Campylobacter coli. Syst. Appl. Microbiol. 2009, 32, 91–100. [Google Scholar] [CrossRef]
- Broeders, S.; Huber, I.; Grohmann, L.; Berben, G.; Taverniers, I.; Mazzara, M.; Roosens, N.; Morisset, D. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci. Technol. 2014, 37, 115–126. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe. 2023–2021 data (2023) Stockholm: European Centre for Disease Prevention and Control (ECDC) and World Health Organization (WHO). Cataloguing-in-Publication (CIP) Data. 2023. Available online: http://apps.who.int/iris (accessed on 29 November 2023).
- Van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef] [PubMed]
- McGILL, K.; Cowley, D.; Moran, L.; Scates, P.; O’Leary, A.; Madden, R.H.; Carroll, C.; McNAMARA, E.; Moore, J.E.; Fanning, S.; et al. Antibiotic resistance of retail food and human Campylobacter isolates on the island of Ireland from 2001–2002. Epidemiol. Infect. 2006, 134, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, T.; Roasto, M.; Mäesaar, M.; Häkkinen, L.; Kisand, V.; Ivanova, M.; Valli, M.H.; Meremäe, K. Antibiotic Resistance in Campylobacter spp. Isolated from Broiler Chicken Meat and Human Patients in Estonia. Microorganisms 2022, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- GERMAP 2015 Antimicrobial Resistance and Consumption. Report on the Consumption of Antimicrobials and the Spread of Antimicrobial Resistance in Human and Veterinary Medicine in Germany; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL), Paul-Ehrlich-Gesellschaft für Chemotherapie: Rheinbach, Germany, 2016; ISBN 978-3-9818383-0-5. [Google Scholar]
- Tenhagen, B.-A.; Flor, M.; Alt, K.; Knüver, M.-T.; Buhler, C.; Käsbohrer, A.; Stingl, K. Association of Antimicrobial Resistance in Campylobacter spp. in Broilers and Turkeys with Antimicrobial Use. Antibiotics 2021, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Ghielmetti, G.; Seth-Smith, H.M.B.; Roloff, T.; Cernela, N.; Biggel, M.; Stephan, R.; Egli, A. Whole-genome-based characterization of Campylobacter jejuni from human patients with gastroenteritis collected over an 18 year period reveals increasing prevalence of antimicrobial resistance. Microb. Genom. 2023, 9, 000941. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, W.; Lu, Q.; Wen, G.; Zhao, Z.; Luo, Q.; Shao, H.; Zhang, T. Point Deletion or Insertion in CmeR-Box, A2075G Substitution in 23S rRNA, and Presence of erm(B) Are Key Factors of Erythromycin Resistance in Campylobacter jejuni and Campylobacter coli Isolated From Central China. Front. Microbiol. 2020, 11, 203. [Google Scholar] [CrossRef]
| Antimicrobial | MIC [mg/L] Resistant > C. jejuni | MIC [mg/L] Resistant > C. coli | Reference |
|---|---|---|---|
| ciprofloxacin | 0.5 | 0.5 | ECOFFs for C. spp. [32,33,34] |
| tetracycline | 1 | 2 | |
| ertapenem | 0.5 | 0.5 | |
| erythromycin | 4 | 8 | |
| chloramphenicol | 16 | 16 | |
| gentamicin | 2 | 2 |
| Percentage of German Isolates Resistant to Antimicrobials Tested (%) | ||||||
|---|---|---|---|---|---|---|
| Antibiotic | Human Isolates (n = 68) | Food Isolates (n = 61) | ||||
| C. jejuni (n = 44) | C. coli (n = 24) | C. jejuni + C. coli | C. jejuni (n = 41) | C. coli (n = 20) | C. jejuni + C. coli | |
| ciprofloxacin | 81.8 | 79.2 | 80.9 | 68.3 | 50.0 | 62.3 |
| tetracycline | 65.9 | 62.5 | 64.7 | 43.9 | 55.0 | 47.5 |
| ertapenem | 6.8 | 37.5 | 17.6 | 7.3 | 40.0 | 18.0 |
| erythromycin | 0.0 | 8.3 | 2.9 | 0.0 | 5.0 | 1.6 |
| chloramphenicol | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| gentamicin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Antimicrobial Resistance and Target | Primer/Probe Name | Oligonucleotide Sequence 5’→ 3’ | Amplicon Size [bp] | Final Concentration in qPCR [nM] | Reference |
|---|---|---|---|---|---|
| Tetracycline tet(O) 1 | tet(O)-fw | AAGTCCCGCCAAATCCT | 157 (Acc. No. NG_048257.1) | 150 nM | the current study |
| tet(O)-re | TGCTCGCAGCCATAAAGAA | 150 nM | |||
| tet(O)-probe | 6-FAM 6— TCGGGTTGT*CCATAGAGCCG —IABkFQ 12 | 100 nM | |||
| Ciprofloxacin for C.jejuni GyrA_T86I 2 | gyrA_Cj_fw | GTATAGTGGGTGCTGTTAT | 118 (Acc. No. wt AB104527.1, pm CP053659.1 ) | 400 nM | the current study |
| gyrA_Cj_re | CCTTGTCCTGTAATACTTG | 400 nM | [8] | ||
| gyrA_Cj_wt | CCACATGGAGAT+A+C+A+GCAGTTTATG | 600 nM | the current study | ||
| gyrA_Cj_pm | ROX 7—CCACATGGAGAT+A+T+A+GCAGTTTATG —BHQ2 13 | 200 nM | |||
| Ciprofloxacin for C.coli GyrA_T86I 2 | gyrA_Cc_fw | GTATAGTAGGGGATGTTATCG | 118 (Acc. No. wt CP092026.1 pm CP091310.1 CP082881.1) | 400 nM | the current study |
| gyrA_Cc_re | CCTTGTCCATCGATACTTG | 400 nM | |||
| gyrA_Cc_wt | CCACATGGYGAT+A+C+T+GCTGTTTACG 17 | 600 nM | |||
| gyrA_Cc_pm | ROX 7—CCACATGGYGAT+A+T+T+GCTGTTTACG —BHQ2 13, 17 | 200 nM | |||
| Erythromycin erm(B) 3 | erm(B)-fw | AGGGTTGCTCTTGCACACTC | 125 (Acc. No. MF134831.1) | 400 nM | the current study |
| erm(B)-re | GAACATCTGTGGTATGGCGG | 400 nM | |||
| erm(B)-probe | Cy5 8—AGCTGCCAG*CGGAATGCTTTCA —IAbRQSp 14 | 200 nM | |||
| Erythromycin 23S rRNA_ A2075G 4 | 23S_A2075G_fw | GTGGAGGTGAAAATTCCTC | 113 (Acc. No. wt CP020776 pm GU384931.1) | 400 nM | the current study |
| 23S_A2075G_re | CAAAGCCTCCCACCTATC | 400 nM | |||
| 23S_A2075G_wt | CAAGACGG+A+A+A+GACCCCGTG | 600 nM | |||
| 23S_A2075G_pm | HEX 9— CAAGACGG+A+G+A+GACCCCGTG —BHQ1 15 | 200 nM | |||
| Internal PCR control (target gene ntb2 5) | IPC-ntb2-fw | ACCACAATGCCAGAGTGACAAC | 125 | 300 nM | [36] |
| IPC-ntb2-re | TACCTGGTCTCCAGCTTTCAGTT | 300 nM | |||
| IPC-ntb2 probe | AriaMx: ATTO425 10—CACGCGCAT*GAAGTTAGGGGACCA —IABkFQ 12 QuantStudio5 and CFX96: Cy5.5 11— CACGCGCAT*GAAGTTAGGGGACCA —NFQ-2 16 | 150 nM |
| Tetracycline | Ciprofloxacin | Erythromycin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tet(O) | gyrA_T86I | gyrA_T86I Cc | gyrA_T86I Cj | 23S rRNA_ A2075G | erm(B) | |||||||
| S | R | S | R | S | R | S | R | S | R | |||
| DE | pheno | 32 | 29 | 23 | 38 | 10 | 10 | 13 | 28 | 60 | 1 | |
| (food) | geno | 32 | 29 | 23 | 38 | 10 | 10 | 13 | 27 +1* | 60 | 1 | 0 |
| DE | pheno | 24 | 44 | 13 | 55 | 5 | 19 | 8 | 36 | 66 | 2 | |
| (human) | geno | 24 | 44 | 13 | 55 | 5 | 19 ** | 8 | 36 | 66 | 2 | 0 |
| VN | pheno | 0 | 21 | 0 | 21 | 0 | 18 | 0 | 3 | 2 | 19 | |
| (food) | geno | 0 | 21 | 0 | 21 | 0 | 17 +1* | 0 | 3 | 2 | 9 | 10 |
| Antimicrobial Resistance | Resistance Determinant/Gene | Channel | Prevalence of Positive Signals in PCR | Mean Cq-Value ± Standard Deviation | Range of Cq-Values |
|---|---|---|---|---|---|
| tetracycline | tet(O) | FAM | n = 94 | 23.36 ± 1.23 | 21.06–26.45 |
| ciprofloxacin C. coli | GyrA_T86I Cc | ROX | n = 47 | 23.87 ± 1.26 | 22.12–26.92 |
| ciprofloxacin C. jejuni | GyrA_T86I Cj | ROX | n = 67 | 24.05 ± 0.97 | 21.94–26.14 |
| erythromycin | 23S rRNA_A2075G | HEX | n = 12 | 22.48 ± 0.75 | 21.54–23.92 |
| erythromycin | erm(B) | Cy5 | n = 10 | 24.10 ± 1.11 | 23.24–26.62 |
| IAC | ntb2 | ATTO425 | n = 150 | 31.56 ± 0.46 | 30.67–33.10 |
| BfR-CA-16092 (VE_14, C. jejuni) | BfR-CA-15062 (VE_01, C. coli) | |||
|---|---|---|---|---|
| LOD95% | 95% Confidence Interval | LOD95% | 95% Confidence Interval | |
| tet(O) | 1.460 cp/µL | [0.961, 2.219] | 2.533 cp/µL | [2.028, 5.229] |
| gyrA_T86I C. jejuni | 6.115 cp/µL | [4.134, 9.093] | ||
| gyrA_T86I C. coli | 1.696 cp/µL | [1.119, 2.565] | ||
| 23S rRNA_A2075G | 1.214 cp/µL | [0.928, 2.265] | ||
| erm(B) | 5.835 cp/µL | [3.938, 8.663] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeller-Péronnet, V.; Bretschneider, N.; Lausch, J.; Hanifi, N.; Pavlovic, M.; Zarske, M.; Luu, H.Q.; Busch, U.; Stingl, K.; Huber, I. Multiplex Real-Time PCR for the Detection of Tetracycline, Ciprofloxacin, and Erythromycin Resistance Determinants from Human and Foodborne Campylobacter jejuni and Campylobacter coli. Microorganisms 2023, 11, 2927. https://doi.org/10.3390/microorganisms11122927
Zeller-Péronnet V, Bretschneider N, Lausch J, Hanifi N, Pavlovic M, Zarske M, Luu HQ, Busch U, Stingl K, Huber I. Multiplex Real-Time PCR for the Detection of Tetracycline, Ciprofloxacin, and Erythromycin Resistance Determinants from Human and Foodborne Campylobacter jejuni and Campylobacter coli. Microorganisms. 2023; 11(12):2927. https://doi.org/10.3390/microorganisms11122927
Chicago/Turabian StyleZeller-Péronnet, Véronique, Nancy Bretschneider, Johanna Lausch, Nadera Hanifi, Melanie Pavlovic, Michael Zarske, Huong Quynh Luu, Ulrich Busch, Kerstin Stingl, and Ingrid Huber. 2023. "Multiplex Real-Time PCR for the Detection of Tetracycline, Ciprofloxacin, and Erythromycin Resistance Determinants from Human and Foodborne Campylobacter jejuni and Campylobacter coli" Microorganisms 11, no. 12: 2927. https://doi.org/10.3390/microorganisms11122927





