Development and Application of an Assay to Evaluate the Anti-Parasitic Effect of Humoral Responses against Trypanosoma cruzi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample Collection
2.3. Cell and Parasite Cultures
2.4. Design and Validation of the Serum Parasite Inhibition Assay
2.5. Determination of Glycerol Toxicity
2.6. In-House ELISA to Detect Anti-T. cruzi Antibodies
2.7. Data Analysis
3. Results
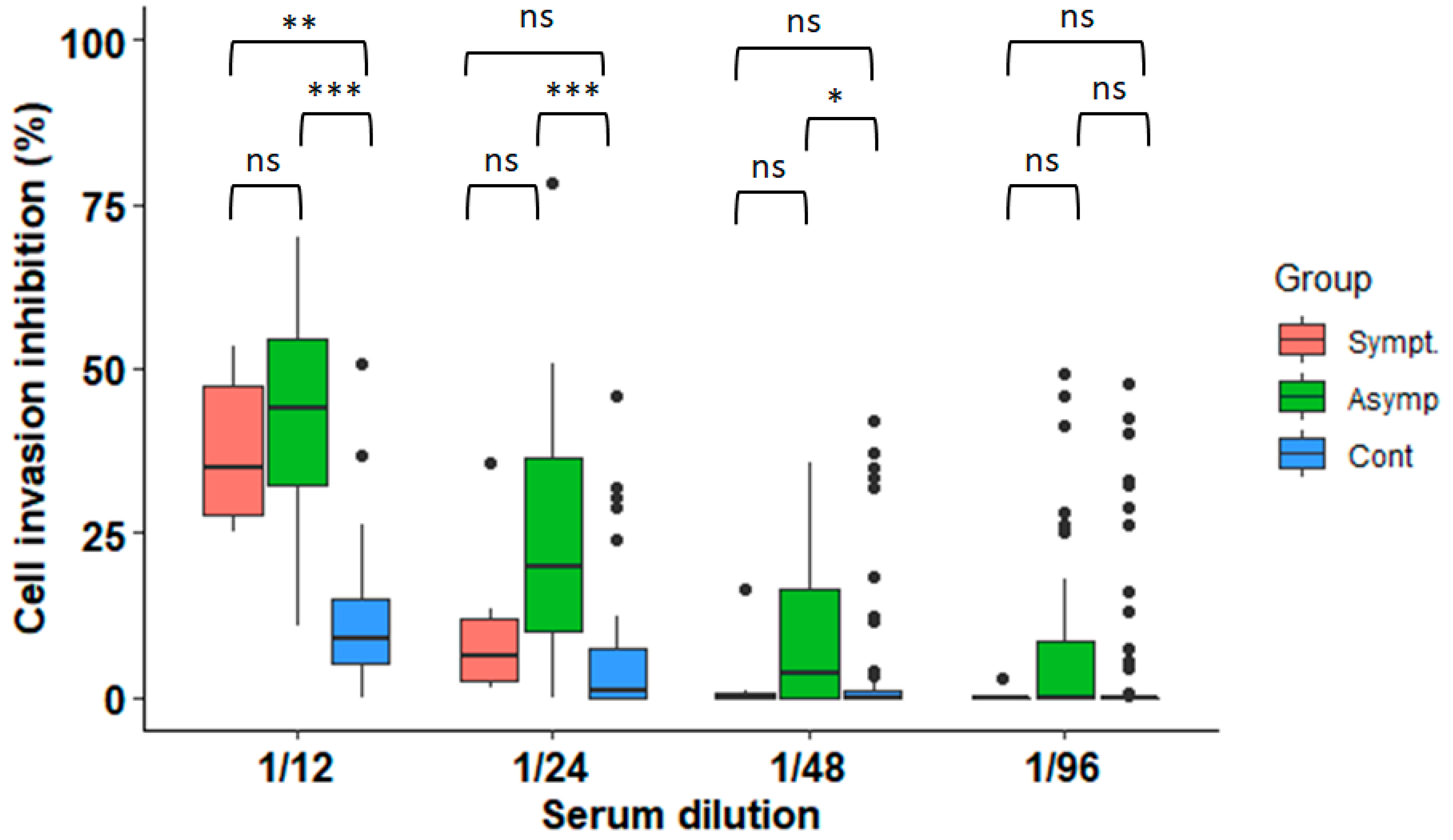
3.1. Anti-Parasitic Effect of Serum from Subjects Infected with T. cruzi
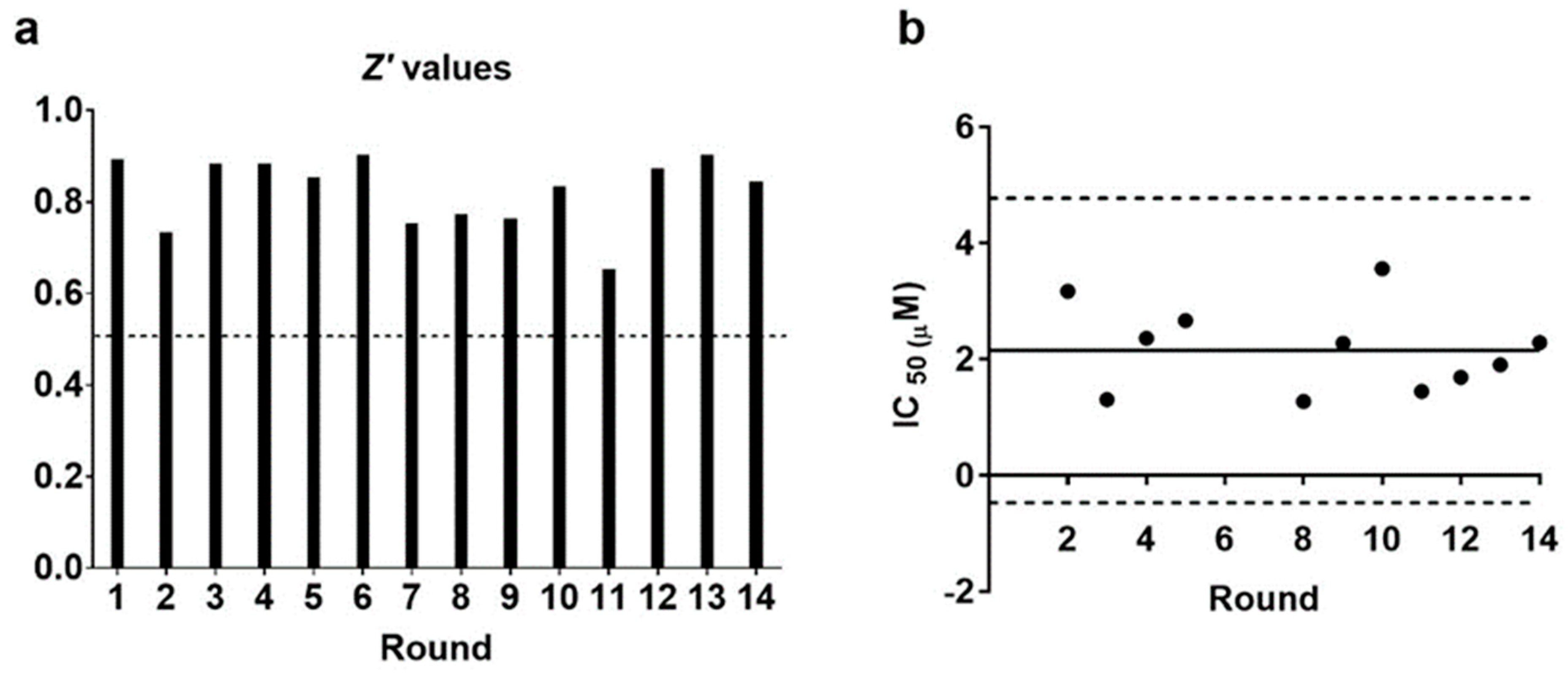
3.2. The Serum Parasite Inhibition Assay Is Statistically Robust
3.3. Inhibition of Parasite Invasion Is Associated with Higher Anti-T. cruzi Antibody Titres
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Fact Sheets, Chagas Disease. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis (accessed on 28 December 2022).
- Alonso-Padilla, J.; Abril, M.; Alarcón de Noya, B.; Almeida, I.C.; Angheben, A.; Araujo Jorge, T.; Chatelain, E.; Esteva, M.; Gascón, J.; Grijalva, M.J.; et al. Target product profile for a test for the early assessment of treatment efficacy in Chagas disease patients: An expert consensus. PLoS Negl. Trop. Dis. 2020, 14, e0008035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan American Health Organization. Guidelines for the Diagnosis and Treatment of Chagas Disease; PAHO: Washington, DC, USA, 2019; Available online: https://iris.paho.org/bitstream/handle/10665.2/49653/9789275120439_eng.pdf (accessed on 6 December 2022).
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Tanowitz, H.B.; Malvestio, L.M.; Celes, M.R.; Campos, E.C.; Blefari, V.; Prado, C.M. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS Negl. Trop. Dis. 2010, 4, e674. [Google Scholar] [CrossRef] [PubMed]
- Monje-Rumi, M.M.; Brandán, C.P.; Ragone, P.G.; Tomasini, N.; Lauthier, J.J.; Alberti D’Amato, A.M.; Cimino, R.O.; Orellana, V.; Basombrío, M.A.; Diosque, P. Trypanosoma cruzi diversity in the Gran Chaco: Mixed infections and differential host distribution of TcV and TcVI. Infect. Genet. Evol. 2015, 29, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Arce-Fonseca, M.; Rios-Castro, M.; Carrillo-Sánchez, S.d.C.; Martínez-Cruz, M.; Rodríguez-Morales, O. Prophylactic and therapeutic DNA vaccines against Chagas disease. Parasit. Vectors 2015, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.A.; Wang, Q.; Jones, K.M.; Heffernan, M.J.; Buhaya, M.H.; Beaumier, C.M.; Keegan, B.P.; Zhan, B.; Dumonteil, E.; Bottazzi, M.E.; et al. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Hum. Vaccines Immunother. 2016, 12, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Michel-Todó, L.; Reche, P.A.; Bigey, P.; Pinazo, M.J.; Gascón, J.; Alonso-Padilla, J. In silico design of an epitope-based vaccine ensemble for Chagas disease. Front. Immunol. 2019, 10, 2698. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife 2018, 7, e34039. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef]
- Bryan, M.A.; Guyach, S.E.; Norris, K.A. Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice. PLoS Negl. Trop. Dis. 2010, 4, e733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, J.; Neira, I.; Gutiérrez, B.; Anacona, D.; Manque, P.; Silva, X.; Marín, S.; Sagua, H.; Vergara, U. Serum antibodies to Trypanosoma cruzi antigens in Atacameños patients from highland of northern Chile. Acta Trop. 1996, 60, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Andrews, N.W.; Kuwajima, V.Y.; Colli, W. Cell surface antigens of Trypanosoma cruzi: Possible correlation with the interiorization process in mammalian cells. Mol. Biochem. Parasitol. 1982, 6, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Bivona, A.E.; Alberti, A.S.; Cerny, N.; Trinitario, S.N.; Malchiodi, E.L. Chagas disease vaccine design: The search for an efficient Trypanosoma cruzi immune-mediated control. Biochim Biophys Acta Mol. Basis Dis. 2020, 1866, 165658. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Peinado, N.; Cortes-Serra, N.; Torras-Claveria, L.; Pinazo, M.J.; Gascon, J.; Bastida, J.; Alonso-Padilla, J. Amaryllidaceae alkaloids with anti-Trypanosoma cruzi activity. Parasit. Vectors 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Acevedo, G.R.; Girard, M.C.; Gómez, K.A. The unsolved jigsaw puzzle of the immune response in Chagas disease. Front. Immunol. 2018, 9, 1929. [Google Scholar] [CrossRef]
- Dumonteil, E.; Herrera, C. The case for the development of a Chagas disease vaccine: Why? how? when? Trop. Med. Infect. Dis. 2021, 6, 16. [Google Scholar] [CrossRef]
- Pereira-Chioccola, V.L.; Acosta-Serrano, A.; Correia de Almeida, I.; Ferguson, M.A.; Souto-Padron, T.; Rodrigues, M.M.; Travassos, L.R.; Schenkman, S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 2000, 113, 1299–1307. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Bavia, L.; Ambrosio, A.R.; de Messias-Reason, I.J. The complement system: A prey of Trypanosoma cruzi. Front Microbiol. 2017, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, S.M.; Kolliker-Frers, R.A.; Sanchez, M.S.; Razzitte, G.; Britos, R.D.; Fuentes, M.E.; Schwarcz de Tarlovsky, M.N. Antiproliferative effect of sera from chagasic patients on Trypanosoma cruzi epimastigotes. Involvement of xanthine oxidase. Acta Trop. 2009, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Soto, J.C.; Lasso, P.; Guzmán, F.; Forero-Shelton, M.; Thomas, M.d.C.; López, M.C.; Guhl, F.; Cuellar, A.; Puerta, C.J.; González, J.M. Rabbit serum against K1 peptide, an immunogenic epitope of the Trypanosoma cruzi KMP-11, decreases parasite invasion to cells. Acta Trop. 2012, 123, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Finkelsztein, E.J.; Diaz-Soto, J.C.; Vargas-Zambrano, J.C.; Suesca, E.; Guzmán, F.; López, M.C.; Thomas, M.C.; Forero-Shelton, M.; Cuellar, A.; Puerta, C.J.; et al. Altering the motility of Trypanosoma cruzi with rabbit polyclonal anti-peptide antibodies reduces infection to susceptible mammalian cells. Exp. Parasitol. 2015, 150, 36–43. [Google Scholar] [CrossRef]
- Martín, F.; Puertas, C.; Thomas, M.C.; Marañón, C.; Patarroyo, M.E.; Martín, J.; Alonso, C.; López, M.C. Identification of a Trypanosoma cruzi antigenic epitope implicated in the infectivity of fibroblast LLC-MK2 cells. Parasitol. Res. 1997, 83, 226–232. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Poveda, C.; Martinez-Perez, A.; Guhl, F.; Monge-Maillo, B.; Fresno, M.; López-Velez, R.; Ramírez, J.D.; Girones, N. Distribution of Trypanosoma cruzi discrete typing units in Bolivian migrants in Spain. Infect. Genet. Evol. 2014, 21, 440–442. [Google Scholar] [CrossRef]
- Zingales, B.; Bartholomeu, D.C. Trypanosoma cruzi genetic diversity: Impact on transmission cycles and Chagas disease. Memórias Inst. Oswaldo Cruz 2022, 117, e210193. [Google Scholar] [CrossRef]

| Sex | |
|---|---|
| Male (%) | 29 (27.9%) |
| Female (%) | 75 (72.1%) |
| Median age (IQR) | 42.5 (36.7–52) |
| Nationality | |
| Bolivia (%) | 94 (90.4%) |
| Paraguay (%) | 2 (1.9%) |
| Argentina (%) | 1 (1%) |
| Brazil (%) | 1 (1%) |
| Peru (%) | 1 (1%) |
| Ecuador (%) | 1 (1%) |
| Spain (%) | 1 (1%) |
| Unknown (%) | 3 (2.9%) |
| Clinical manifestations | |
| Cardiovascular (%) | 4 (3.8%) |
| Digestive (%) | 1 (1%) |
| Cardiovascular and digestive (%) | 1 (1%) |
| Indeterminate stage (asymptomatic) (%) | 39 (37.5%) |
| Total infected | 45 |
| Total non-infected | 59 |
| Total | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Peinado, N.; Gabaldon-Figueira, J.C.; Martinez-Añon, I.; Rodríguez-Gordo, C.; Robleda-Castillo, R.; Pinazo, M.-J.; Bigey, P.; Gascon, J.; Alonso-Padilla, J. Development and Application of an Assay to Evaluate the Anti-Parasitic Effect of Humoral Responses against Trypanosoma cruzi. Microorganisms 2023, 11, 241. https://doi.org/10.3390/microorganisms11020241
Martinez-Peinado N, Gabaldon-Figueira JC, Martinez-Añon I, Rodríguez-Gordo C, Robleda-Castillo R, Pinazo M-J, Bigey P, Gascon J, Alonso-Padilla J. Development and Application of an Assay to Evaluate the Anti-Parasitic Effect of Humoral Responses against Trypanosoma cruzi. Microorganisms. 2023; 11(2):241. https://doi.org/10.3390/microorganisms11020241
Chicago/Turabian StyleMartinez-Peinado, Nieves, Juan Carlos Gabaldon-Figueira, Ignacio Martinez-Añon, Cristian Rodríguez-Gordo, Raquel Robleda-Castillo, Maria-Jesus Pinazo, Pascal Bigey, Joaquim Gascon, and Julio Alonso-Padilla. 2023. "Development and Application of an Assay to Evaluate the Anti-Parasitic Effect of Humoral Responses against Trypanosoma cruzi" Microorganisms 11, no. 2: 241. https://doi.org/10.3390/microorganisms11020241





