Luteibacter flocculans sp. nov., Isolated from a Eutrophic Pond and Isolation and Characterization of Luteibacter Phage vB_LflM-Pluto
Abstract
:1. Introduction
2. Materials and Methods
2.1. Luteibacter flocculans EIF3 Strain Isolation, DNA Extraction and 16S rRNA Gene Sequencing
2.2. Sequencing, Assembly and Annotation of Bacterial and Phage Genome
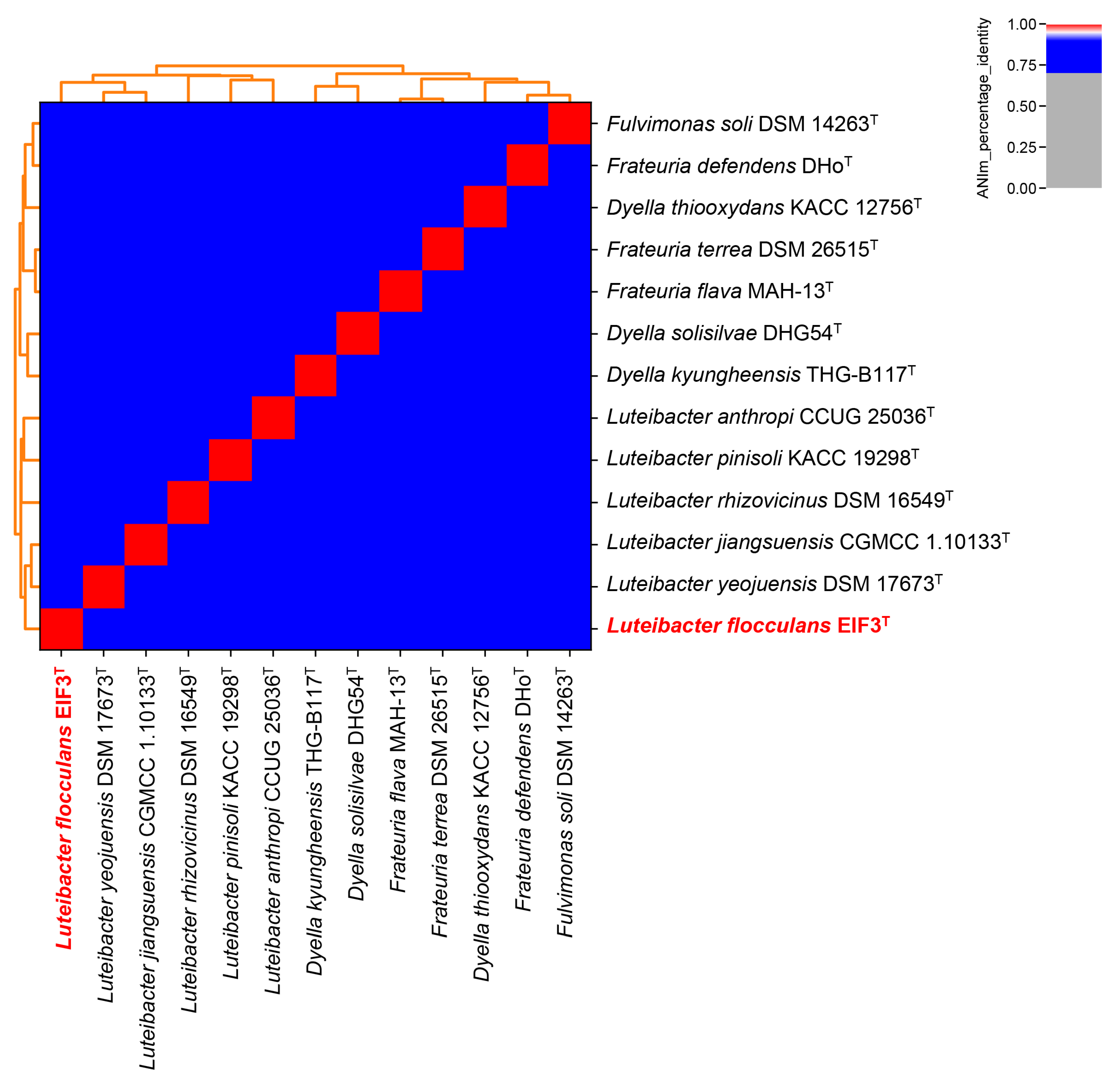
2.3. Luteibacter flocculans sp. nov. EIF3T Phylogenetic Classification
2.4. Genomic Characterization
2.5. Cell Morphology and Gram Staining Techniques
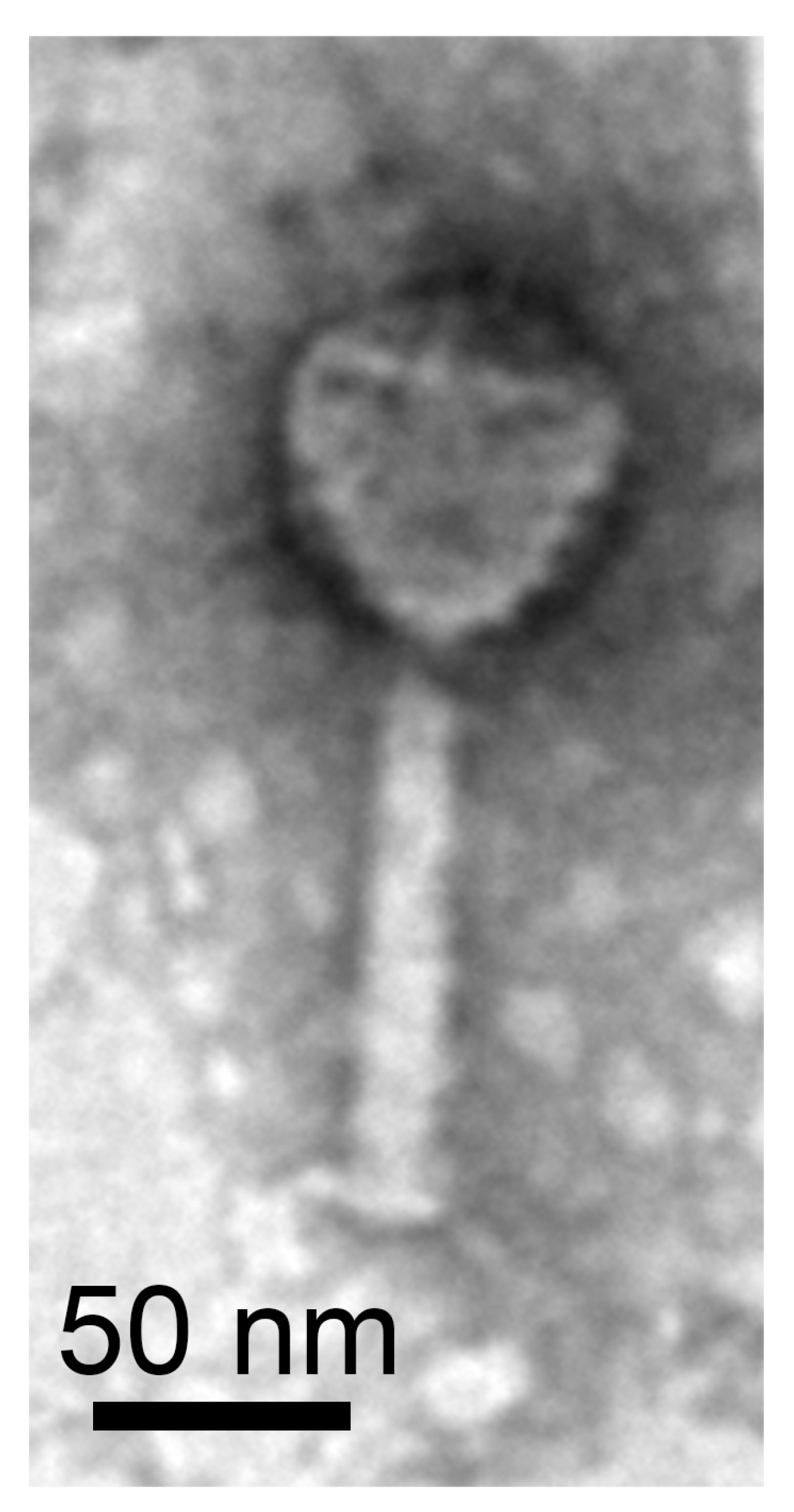
2.6. Bacterial and Phage Isolate Transmission Electron Microscopy
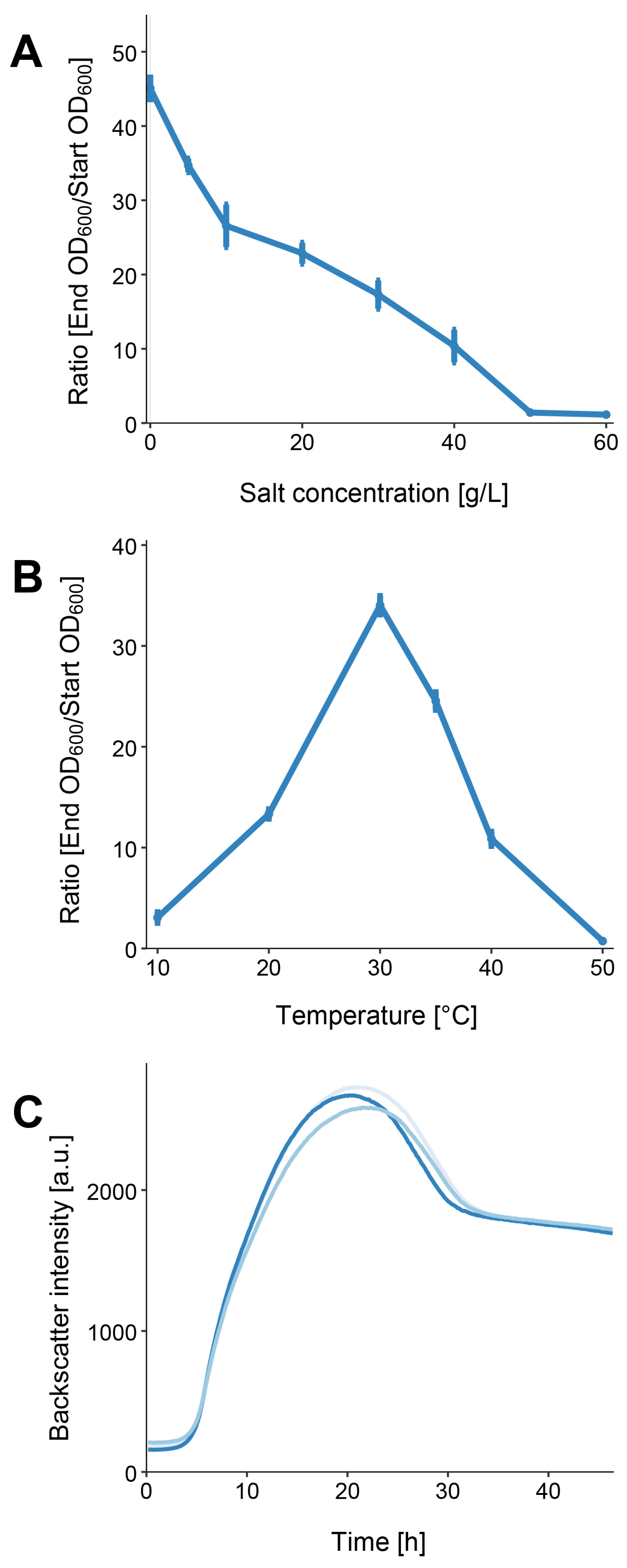
2.7. Determination of Salt Tolerance and Optimal Temperature
2.8. Growth Kinetics Determination
2.9. Antibiotic Resistances and Metabolic Activity
2.10. Examination of Plaques
2.11. Naming of Bacteriophage Isolate
3. Results and Discussion
3.1. Morphological Characterization
3.2. Physiological Characterization
3.3. Genome Characterization
3.4. Prophages Analysis
3.5. Phage Isolation and Characterization
4. Conclusions
Description of Luteibacter flocculans sp. nov.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.E.; Binnerup, S.J.; Kroer, N.; Mølbak, L. Luteibacter rhizovicinus gen. nov., sp. nov., a Yellow-Pigmented Gammaproteobacterium Isolated from the Rhizosphere of Barley (Hordeum vulgare L.). Int. J. Syst. Evol. Microbiol. 2005, 55, 2285–2291. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Weon, H.-Y.; Lee, K.-H.; Seok, S.-J.; Kwon, S.-W.; Go, S.-J.; Stackebrandt, E. Dyella yeojuensis sp. nov., Isolated from Greenhouse Soil in Korea. Int. J. Syst. Evol. Microbiol. 2006, 56, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Lodders, N.; Falsen, E. Luteibacter Anthropi Sp. Nov., Isolated from Human Blood and Reclassification of Dyella yeojuensis Kim et al. 2006 as Luteibacter yeojuensis comb. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, G.-L.; Li, S.-P.; Jiang, J.-D. Luteibacter jiangsuensis sp. nov.: A Methamidophos-Degrading Bacterium Isolated from a Methamidophos-Manufacturing Factory. Curr. Microbiol. 2011, 62, 289–295. [Google Scholar] [CrossRef]
- Akter, S.; Huq, A. Luteibacter pinisoli sp. nov., a Casein Degrading Bacterium Isolated from Rhizospheric Soil of Pinus koraiensis. Arch. Microbiol. 2018, 200, 1017–1023. [Google Scholar] [CrossRef]
- Kohm, K.; Hertel, R. The Life Cycle of SPβ and Related Phages. Arch. Virol. 2021, 166, 2119–2130. [Google Scholar] [CrossRef]
- Friedrich, I.; Klassen, A.; Neubauer, H.; Schneider, D.; Hertel, R.; Daniel, R. Living in a Puddle of Mud: Isolation and Characterization of Two Novel Caulobacteraceae Strains Brevundimonas pondensis sp. nov. and Brevundimonas goettingensis sp. nov. Appl. Microbiol. 2021, 1, 38–59. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an Update of CRISRFinder, Includes a Portable Version, Enhanced Performance and Integrates Search for Cas Proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Nayfach, S.; Camargo, A.P.; Schulz, F.; Eloe-Fadrosh, E.; Roux, S.; Kyrpides, N.C. CheckV Assesses the Quality and Completeness of Metagenome-Assembled Viral Genomes. Nat. Biotechnol. 2021, 39, 578–585. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Arnold, K.; Gosling, J.; Holmes, D. The Java Programming Language, 4th ed.; Addison-Wesley Professional: Lebanon, IN, USA, 2005; ISBN 978-0-321-34980-4. [Google Scholar]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Conesa, A.; García-Alcalde, F. Qualimap 2: Advanced Multi-Sample Quality Control for High-Throughput Sequencing Data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kieft, K.; Zhou, Z.; Anantharaman, K. VIBRANT: Automated Recovery, Annotation and Curation of Microbial Viruses and Evaluation of Viral Community Function from Genomic Sequences. Microbiome 2020, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Zdobnov, E.M.; Apweiler, R. InterProScan—An Integration Platform for the Signature-Recognition Methods in InterPro. Bioinformatics 2001, 17, 847–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A Toolkit to Classify Genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and Taxonomy in Diagnostics for Food Security: Soft-Rotting Enterobacterial Plant Pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [Green Version]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved Annotation of Antibiotic Resistance Determinants Reveals Microbial Resistomes Cluster by Ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Claus, D. A Standardized Gram Staining Procedure. World J. Microbiol. Biotechnol. 1992, 8, 451–452. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.-R.; Strompl, C.; Meyer, H.; Lindholst, S.; Moore, E.R.B.; Christ, R.; Vancanneyt, M.; Tindall, B.J.; Bennasar, A.; Smit, J.; et al. Phylogeny and Polyphasic Taxonomy of Caulobacter Species. Proposal of Maricaulis gen. nov. with Maricaulis maris (Poindexter) comb. nov. as the Type Species and Emended Description of the Genera Brevundimonas and Caulobacter. Int. J. Syst. Evol. Microbiol. 1999, 49, 1053–1073. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2—Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; Volume 77, p. 3. ISBN 978-0-387-98140-6. [Google Scholar]
- Bruder, S.; Reifenrath, M.; Thomik, T.; Boles, E.; Herzog, K. Parallelized Online Biomass Monitoring in Shake Flasks Enables Efficient Strain and Carbon Source Dependent Growth Characterization of Saccharomyces cerevisiae. Microb. Cell Factories 2016, 15, 127. [Google Scholar] [CrossRef] [Green Version]
- Clarke, P.H.; Cowan, S.T. Biochemical Methods for Bacteriology. Microbiol. Soc. 1952, 6, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Willms, I.M.; Hertel, R. Phage VB_BsuP-Goe1: The Smallest Identified Lytic Phage of Bacillus subtilis. FEMS Microbiol. Lett. 2016, 363, fnw208. [Google Scholar] [CrossRef] [Green Version]
- Willms, I.; Hoppert, M.; Hertel, R. Characterization of Bacillus subtilis Viruses VB_BsuM-Goe2 and VB_BsuM-Goe3. Viruses 2017, 9, 146. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Brister, J.R. How to Name and Classify Your Phage: An Informal Guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Naushad, S.; Adeolu, M.; Wong, S.; Sohail, M.; Schellhorn, H.E.; Gupta, R.S. A Phylogenomic and Molecular Marker Based Taxonomic Framework for the Order Xanthomonadales: Proposal to Transfer the Families Algiphilaceae and Solimonadaceae to the Order Nevskiales ord. nov. and to Create a New Family within the Order Xanthomonadales, the Family Rhodanobacteraceae fam. nov., Containing the Genus Rhodanobacter and Its Closest Relatives. Antonie Leeuwenhoek 2015, 107, 467–485. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, X.; Wang, P.; He, L.; Huang, Z.; Sheng, X. Dyella jiangningensis sp. nov., a γ-Proteobacterium Isolated from the Surface of Potassium-Bearing Rock. Int. J. Syst. Evol. Microbiol. 2013, 63, 3154–3157. [Google Scholar] [CrossRef] [Green Version]
- Reimer, L.C.; Vetcininova, A.; Carbasse, J.S.; Söhngen, C.; Gleim, D.; Ebeling, C.; Overmann, J. BacDive in 2019: Bacterial Phenotypic Data for High-Throughput Biodiversity Analysis. Nucleic Acids Res. 2019, 47, D631–D636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, L.; Sundari, C.S.; Balasubramanian, D.; Sonti, R.V. The Bacterial Pigment Xanthomonadin Offers Protection against Photodamage. FEBS Lett. 1997, 415, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S.; et al. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R. Shifting the Genomic Gold Standard for the Prokaryotic Species Definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.; Le, P.; Zurla, C.; Finzi, L.; Adhya, S. Multilevel Autoregulation of λ Repressor Protein CI by DNA Looping in Vitro. Proc. Natl. Acad. Sci. USA 2011, 108, 14807–14812. [Google Scholar] [CrossRef] [Green Version]
- Gruenig, M.C.; Lu, D.; Won, S.J.; Dulberger, C.L.; Manlick, A.J.; Keck, J.L.; Cox, M.M. Creating Directed Double-Strand Breaks with the Ref Protein. J. Biol. Chem. 2011, 286, 8240–8251. [Google Scholar] [CrossRef] [Green Version]
- Yeo, A.; Feiss, M. Specific Interaction of Terminase, the DNA Packaging Enzyme of Bacteriophage γ, with the Portal Protein of Theeh Prohead. J. Mol. Biol. 1995, 245, 141–150. [Google Scholar] [CrossRef]
- Mahony, J.; Alqarni, M.; Stockdale, S.; Spinelli, S.; Feyereisen, M.; Cambillau, C.; van Sinderen, D. Functional and Structural Dissection of the Tape Measure Protein of Lactococcal Phage TP901-1. Sci. Rep. 2016, 6, 36667. [Google Scholar] [CrossRef] [Green Version]
- Holtappels, D.; Fortuna, K.J.; Moons, L.; Broeckaert, N.; Bäcker, L.E.; Venneman, S.; Rombouts, S.; Lippens, L.; Baeyen, S.; Pollet, S.; et al. The Potential of Bacteriophages to Control Xanthomonas campestris pv. campestris at Different Stages of Disease Development. Microb. Biotechnol. 2022, 15, 1762–1782. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [Green Version]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am Nat. 1972, 106, 645–668. [Google Scholar]

| Characteristics | L. flocculans EIF3T | L. yeojuensis DSM 17673T | L. jiangsuensis CGMCC 1.10133T | L. anthropi CCUG 25036T | L. rhizovicinus DSM 16549T |
|---|---|---|---|---|---|
| Source of isolation | Eutrophic pond | Rhizosphere soil | Soil | Human blood | Rhizosphere soil |
| Motility | + | + | – | + | + |
| Temperature (°C) | |||||
| Range | 10–45 | 5–37 | 4–42 | 15–37 | 5–30 |
| Optimum | 30 | 28 | 25–30 | 28 | 17.5 |
| NaCl (g/L) | |||||
| Range | 0–40 | 0–50 | 0–40 | n/a | 0–30 |
| Optimum | 0 | n/a | n/a | n/a | 15 |
| Enzymatic activity | |||||
| Alkaline phosphatase | + | + | + | n/a | + |
| Esterase | + | + | n/a | n/a | – |
| Esterase lipase | + | + | n/a | + | – |
| Lipase | + | – | + | n/a | – |
| Leucine arylamidase | + | + | n/a | n/a | + |
| Valine arylamidase | + | + | n/a | n/a | + |
| Cysteine arylamidase | + | + | n/a | n/a | – |
| Trypsin | – | – | n/a | n/a | – |
| α-Chymotrypsin | – | – | n/a | n/a | – |
| Acid phosphatase | + | + | n/a | + | |
| Naphthol-AS-BI-phosphohydrolase | + | + | + | n/a | + |
| α-Galactosidase | – | + | n/a | n/a | + |
| β-Galactosidase | + | + | + | + | + |
| β-Glucuronidase | – | – | n/a | n/a | – |
| α-Glucosidase | + | + | + | n/a | + |
| β-Glucosidase | + | + | n/a | n/a | + |
| N-Acetyl-β-glucosaminidase | + | + | + | n/a | – |
| α-Mannosidase | – | – | n/a | n/a | – |
| α-Fucosidase | – | – | n/a | n/a | – |
| Utilization of | |||||
| Potassium nitrate | – | – | + | n/a | – |
| L-Tryptophane | – | – | n/a | n/a | – |
| D-Glucose (fermentation) | – | – | n/a | – | – |
| L-Arginine | – | – | + | n/a | – |
| Urea | – | – | – | n/a | – |
| Esculin/ferric citrate | + | + | + | n/a | + |
| Gelatin | – | + | + | – | + |
| 4-Nitrophenyl-β-D-galacto-pyranoside | – | – | n/a | n/a | – |
| D-Glucose (assimilation) | + | + | – | + | + |
| L-Arabinose | – | – | + | n/a | – |
| D-Mannose | + | + | + | + | + |
| D-Mannitol | – | – | – | + | – |
| N-Acetyl-D-glucosamine | + | + | n/a | + | + |
| D-Maltose | – | + | + | n/a | – |
| Potassium gluconate | – | – | n/a | + | – |
| Capric acid | – | – | n/a | n/a | – |
| Adipic acid | – | – | n/a | n/a | – |
| Malic acid | + | – | – | + | – |
| Trisodium citrate | – | – | n/a | n/a | – |
| Phenylacetic acid | – | – | n/a | n/a | – |
| Oxidase | + | + | + | + | + |
| Catalase | + | + | + | – | + |
| Resistance to | |||||
| Ampicillin | – | n/a | n/a | n/a | n/a |
| Erythromycin | + | n/a | n/a | n/a | n/a |
| Kanamycin | – | n/a | n/a | n/a | n/a |
| Oxytetracycline | – | n/a | n/a | n/a | n/a |
| Rifampicin | – | n/a | n/a | n/a | n/a |
| Tetracycline | + | n/a | n/a | n/a | n/a |
| Streptomycin | – | n/a | n/a | n/a | n/a |
| Vancomycin | + | n/a | n/a | n/a | n/a |
| G + C % | 64.8 | 63.0 | 63.6 | 65.3 | 63.0 |
| Fatty acid | L. flocculans EIF3T | L. yeojuensis DSM 17673T | L. jiangsuensis CGMCC 1.10133T | L. anthropi CCUG 25036T | L. rhizovicinus DSM 16549T |
|---|---|---|---|---|---|
| Unknown 11.799 | – | 2.3 | – | 0.8 | 2.2 |
| iso-C11:0 | 4.3 | 3.8 | 4.7 | 3.6 | 4.0 |
| iso-C11:0 3-OH | 4.1 | 4.2 | 1.6 | 2.9 | 3.9 |
| iso-C13:0 | 0.2 | – | – | 0.4 | 0.5 |
| iso-C12:0 3-OH | 0.1 | 1.0 | – | – | – |
| iso-C14:0 | 0.2 | 1.1 | – | – | – |
| C14:0 | 0.1 | – | – | 0.5 | 0.4 |
| iso-C13:0 3-OH | 3.2 | 2.4 | 2.6 | 1.2 | 2.7 |
| iso-C15:0 | 18.3 | 14.5 | 24.0 | 21.7 | 17.0 |
| anteiso-C15:0 | 8.0 | 6.9 | 9.7 | 2.4 | 4.0 |
| iso-C16:0 | 2.8 | 21.3 | 2.2 | 0.5 | 0.8 |
| Summed feature 3 * | 5.8 | 5.2 | 4.1 | 6.5 | 9.2 |
| C16:0 | 2.1 | 1.8 | 4.2 | 5.6 | 6.5 |
| iso-C17:1 ω9c | 29.4 | 26.5 | 20.3 | 23.8 | 24.4 |
| iso-C17:0 | 18.2 | 14.9 | 20.2 | 27.0 | 22.4 |
| anteiso-C17:0 | 1.3 | 1.6 | 1.2 | 0.9 | 0.6 |
| C18:0 | 0.1 | – | 0.8 | 0.5 | – |
| iso-C17:0 3-OH | 0.7 | 0.8 | – | – | 0.5 |
| Features | Luteibacter flocculans sp. nov. EIF3T |
|---|---|
| Genome size (bp) | 4,299,254 |
| GC content (%) | 64.82 |
| Coverage | 280.1-fold |
| Coding sequence (CDS) | 3672 |
| rRNA genes | 59 |
| tRNA genes | 49 |
| ncRNA | 4 |
| CRISPR | 0 |
| Prophage(s) | 2 |
| Completeness estimate * | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, I.; Kuritsyn, A.; Hertel, R.; Daniel, R. Luteibacter flocculans sp. nov., Isolated from a Eutrophic Pond and Isolation and Characterization of Luteibacter Phage vB_LflM-Pluto. Microorganisms 2023, 11, 307. https://doi.org/10.3390/microorganisms11020307
Friedrich I, Kuritsyn A, Hertel R, Daniel R. Luteibacter flocculans sp. nov., Isolated from a Eutrophic Pond and Isolation and Characterization of Luteibacter Phage vB_LflM-Pluto. Microorganisms. 2023; 11(2):307. https://doi.org/10.3390/microorganisms11020307
Chicago/Turabian StyleFriedrich, Ines, Alisa Kuritsyn, Robert Hertel, and Rolf Daniel. 2023. "Luteibacter flocculans sp. nov., Isolated from a Eutrophic Pond and Isolation and Characterization of Luteibacter Phage vB_LflM-Pluto" Microorganisms 11, no. 2: 307. https://doi.org/10.3390/microorganisms11020307






