Differential Effects of Viruses on the Growth Efficiency of Freshwater Bacterioplankton in Eutrophic Relative to Non-Eutrophic Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Limnological Variables
2.3. Flow Cytometry Analyses: Enumeration of Viral and Bacterial Abundances
2.4. Bacterial Production (BP), Respiration (BR) and Growth Efficiency (BGE)
2.5. Viral Lytic Infection
2.6. Induction of Lysogens
2.7. Data Analyses
3. Result
3.1. In Situ Environmental Parameters or Limnological Characteristics
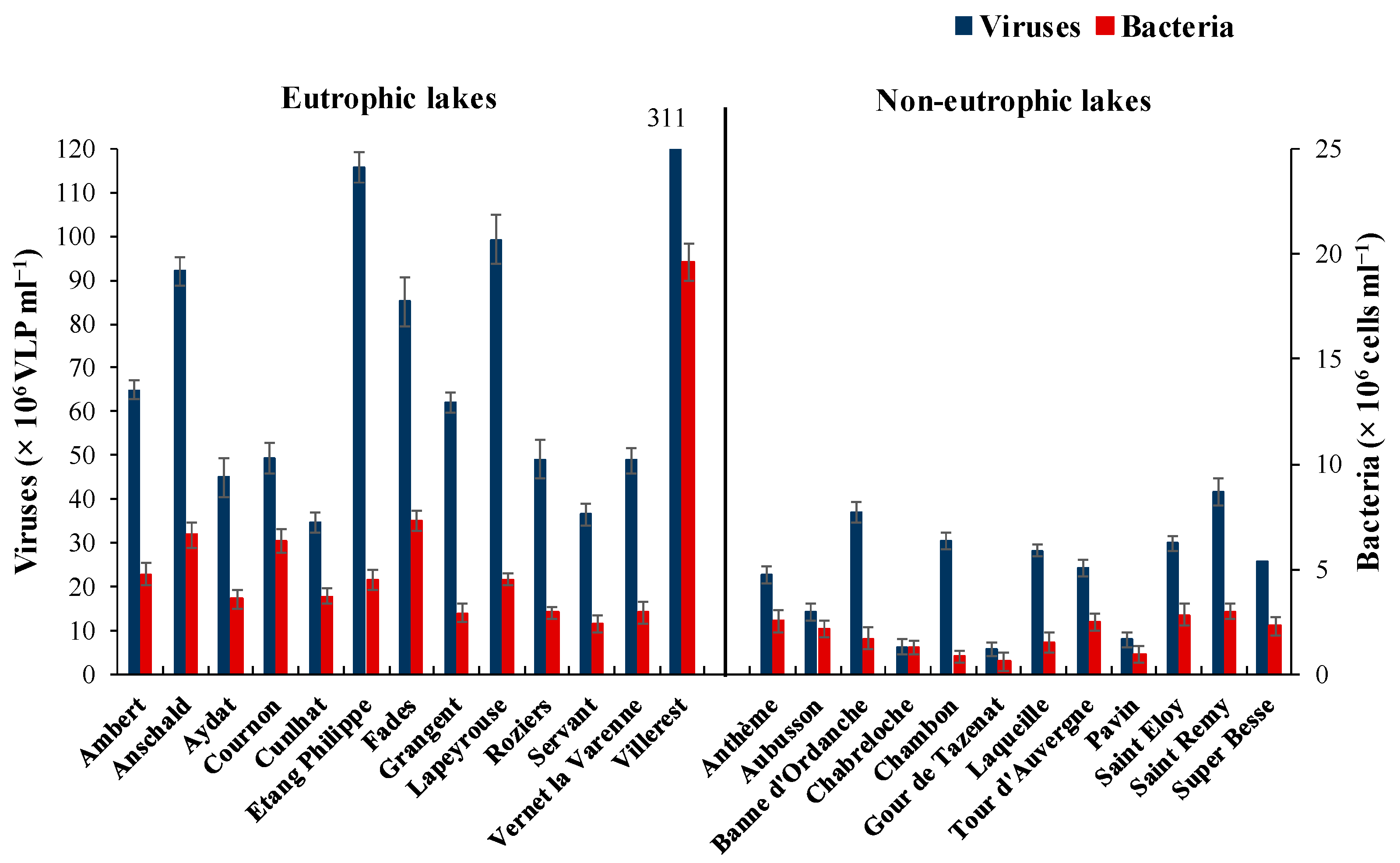
3.2. Flow Cytometry Counts
3.3. Bacterial Growth Efficiency
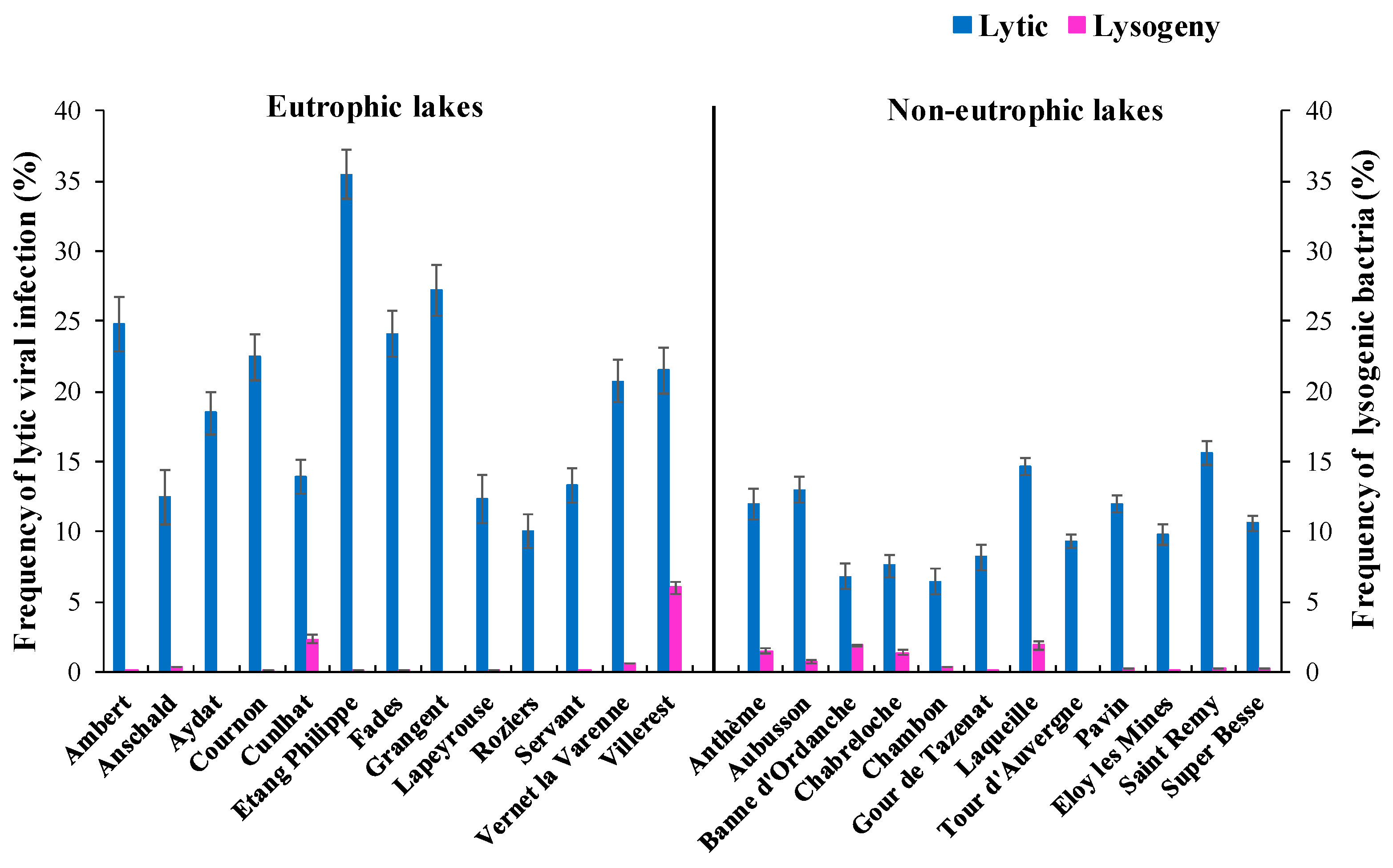
3.4. Phage Life Strategy
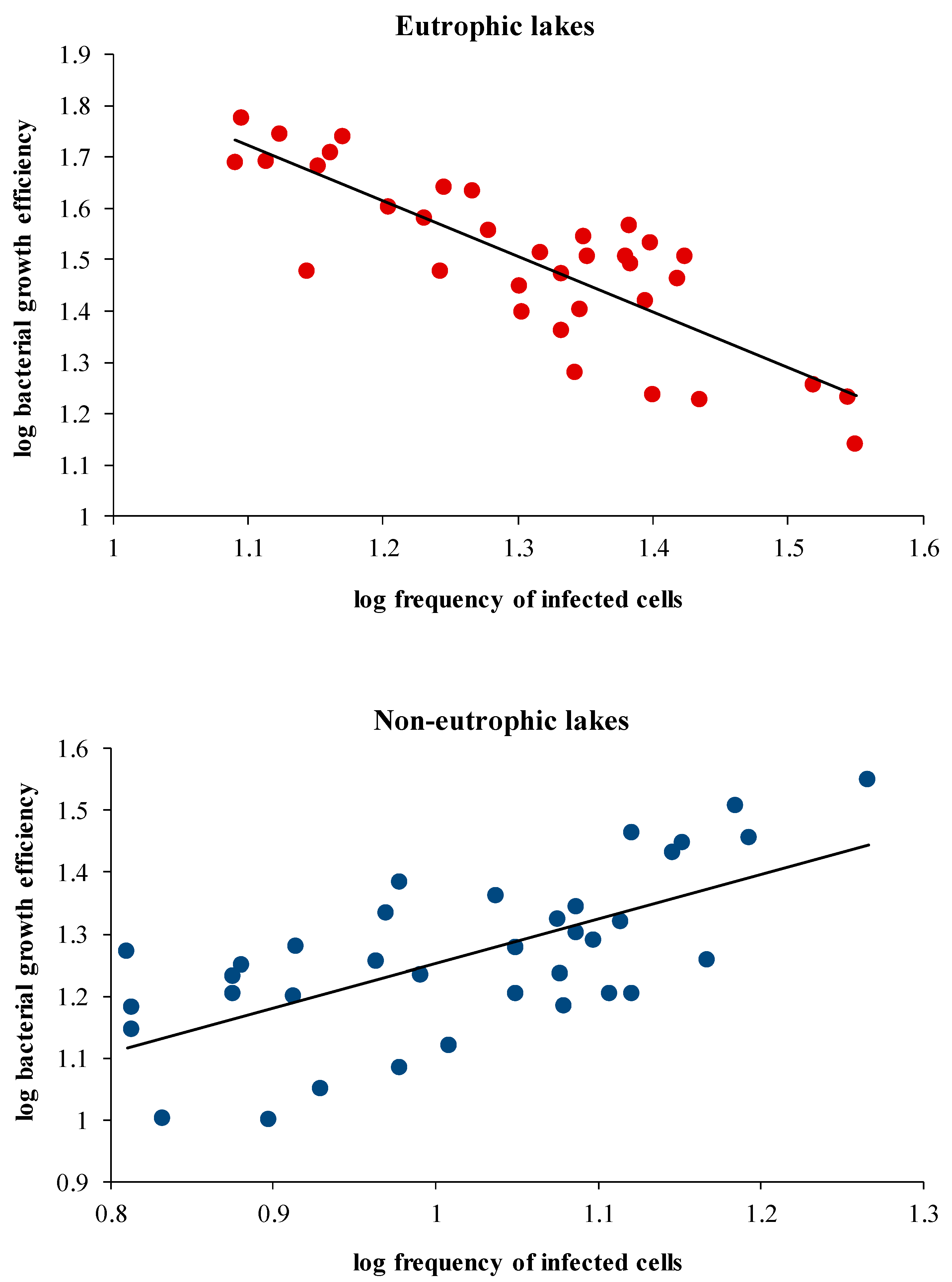
3.5. Relationships between Variables
4. Discussion
4.1. Microbial Environment
4.2. Variable Impact of Viral Lysis on BGE in the Classified Lakes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Gobet, A.; Pommiers, T. Two decades of describing the unseen majority of aquatic microbial diversity. Mol. Ecol. 2021, 21, 1878–1896. [Google Scholar] [CrossRef]
- Yadav, A.N.; Yadav, N.; Kour, D.; Kumar, A.; Yadav, K.; Kumar, A.; Rastegari, A.A.; Sachan, S.G.; Singh, B.; Chauhan, V.S.; et al. Bacterial community composition in lakes. In Freshwater Microbiology; Bandh, S.A., Shafi, S., Shameem, N., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 1; pp. 1–71. [Google Scholar]
- Kritzberg, E.S.; Langenheder, S.; Lindstrom, E.S. Influence of dissolved organic matter source on lake bacterioplankton structure and function-implications for seasonal dynamics of community composition. FEMS Microb. Ecol. 2006, 56, 406–417. [Google Scholar] [CrossRef]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Neilson, N.J.; Andersson, A.F.; Laudon, H.; Lindstrom, E.S.; Kritzberg, E.S. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 2015, 10, 533–545. [Google Scholar] [CrossRef]
- del Giorgio, P.A.; Cole, J.J. Bacterial growth efficiency in natural aquatic ecosystems. Annu. Rev. Ecol. Syst. 1998, 29, 503–541. [Google Scholar] [CrossRef]
- Cotner, J.B.; Biddanda, B.A. Small players, Large role: Microbial influence on biogeochemical processes in pelagic aquatic systems. Ecosystems 2002, 5, 105–121. [Google Scholar] [CrossRef]
- Smith, E.M.; Prairie, Y.T. Bacterial metabolism and growth efficiency in lakes: The importance of phosphorus availability. Limnol. Oceanogr. 2004, 49, 137–147. [Google Scholar] [CrossRef]
- Vidal, L.O.; Graneli, W.; Daniel, C.B.; Heiberg, L.; Roland, F. Carbon and phosphorus regulating bacterial metabolism in oligotrophic boreal lakes. J. Plank. Res. 2011, 33, 1747–1756. [Google Scholar] [CrossRef]
- Hewson, J.; Chow, C.; Fuhrman, J.A. Ecological Role of Viruses in Aquatic Systems; John Wiley and Sons: New York, NY, USA, 2010. [Google Scholar]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Palesse, S.; Colombet, J.; Thouvenot, A.; Sime-Ngando, T. The relative importance of viral lysis and nanoflagellate grazing for prokaryote mortality in temperate lakes. Freshw. Biol. 2014, 59, 300–311. [Google Scholar]
- Kopylov, A.I.; Zabotkina, Z.A. Virioplankton as an important component of plankton in the Volga reservoirs. Biosyst. Divers. 2021, 29, 151–159. [Google Scholar] [CrossRef]
- Sime-Ngando, T. Environmental phages: Viruses of microbes in aquatic systems. Front. Microbiol. 2014, 5, 355. [Google Scholar] [CrossRef]
- Thingstad, T. Elements of a theory for the mechanisms controlling abundance, diversity and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 2000, 45, 1320–1328. [Google Scholar] [CrossRef]
- Amado, A.M.; Meirelles-Pereira, F.; Vidal, L.O.; Sarmento, H.; Suhett, A.L.; Farjalla, V.F.; Cotner, J.B.; Ronand, F. Tropical freshwater ecosystems have lower growth efficiency than temperate ones. Front. Microbiol. 2013, 4, 167. [Google Scholar] [CrossRef]
- Llirós, M.; Inceoğlu, Ö.; García-Armisen, T.; Anzil, A.; Leporcq, B.; Pigneur, J.-P.; Viroux, L.; Darchambeau, F.; Descy, J.-P.; Servais, P. Bacterial community composition in three freshwater reservoirs of different alkalinity and trophic status. PLoS ONE 2014, 9, e116145. [Google Scholar] [CrossRef] [PubMed]
- Motegi, C.; Nagata, T.; Miki, T.; Weinbauer, M.G.; Legendre, L.; Rassoulzadegan, F. Viral control of bacterial growth efficiency in marine pelagic environments. Limnol. Oceanogr. 2009, 54, 1901–1910. [Google Scholar] [CrossRef]
- Xu, J.; Jing, H.; Sun, M.; Harrison, P.J.; Liu, H. Regulation of bacterial metabolic activity by dissolved organic carbon and viruses. J. Geophys. Res. Biogeosci. 2013, 118, 1573–1583. [Google Scholar] [CrossRef]
- Maurice, C.F.; Bouvier, T.; Comte, J.; Guillemette, F.; del Giorgio, P.A. Seasonal variation of phage life strategies and bacterial physiological states in the three northern temperate lakes. Environ. Microbiol. 2010, 12, 628–641. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Mauduit, M.-E.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. Top-down controls of bacterial metabolism: A case study from a temperate freshwater lake ecosystem. Microorganisms 2022, 10, 175. [Google Scholar] [CrossRef]
- Bana, Z.; Abad, N.; Uranga, A.; Azua, I.; Artolozaga, I.; Unanue, M.; Irriberi, J.; Arrieta, J.M.; Ayo, B. Recurrent seasonal changes in bacterial growth efficiency, metabolism and community composition in coastal waters. Environ. Microbiol. 2020, 22, 369–380. [Google Scholar] [CrossRef]
- Keshri, J.; Pradeep Ram, A.S.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. Differential impact of lytic viruses on the taxonomic resolution of freshwater bacterioplankton community structure. Water Res. 2017, 124, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Davis, C.E.; Blackbird, S.; Wolff, G.; Woodward, M.; Mahaffey, C. Seasonal organic matter dynamics in a temperate shelf area. Prog. Oceanogr. 2019, 177, 101925. [Google Scholar] [CrossRef]
- Hu, C.; Muller-Karger, F.E.; Zepp, R.G. Absorbance, Absorption Coefficient, and Apparent Quantum Yield: A Comment on Common Ambiguity in the Use of These Optical Concepts. Limnol. Oceanogr. 2002, 47, 1261–1267. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Brussaard, C.P.D. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 2004, 70, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Dobbs, F.C.; Cowen, R.K. Short term temporal and spatial dynamics of bacterioplankton near Barbados in the Caribbean Sea. Aquat. Microb. Ecol. 2001, 25, 45–53. [Google Scholar] [CrossRef]
- Carignan, R.; Blais, A.M.; Vis, C. Measurement of primary production and community respiration in oligotrophic lakes using the Winkler method. Can. J. Fish. Aquat. Sci. 1998, 55, 1078–1084. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Winter, C.; Höfle, M.G. Reconsidering transmission electron microscopy based estimates of viral infection of bacterioplankton using conversion factors derived from natural communities. Aquat. Microb. Ecol. 2002, 27, 103–110. [Google Scholar] [CrossRef]
- Binder, B. Reconsidering the relationship between virally induced bacterial mortality and frequency of infected cells. Aquat. Microb. Ecol. 1999, 18, 207–215. [Google Scholar] [CrossRef]
- Paul, J.H.; Jiang, S. Lysogeny and transduction. In Methods in Microbiology; Paul, J.H., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 30, pp. 105–125. [Google Scholar]
- Baki, M.A.; Motegi, C.; Shibata, A.; Fukuda, H.; Shimode, S.; Kikuchi, T. Temporal changes in chlorophyll a concentrations and bacterial, viral and heterotrophic nanoflagellate abundances in the coastal zone of Sagami Bay, Japan: Implications of top-down and bottom-up effects. Coast. Mar. Sci. 2009, 32, 29–38. [Google Scholar]
- Tsuchiya, K.; Sano, T.; Tomioka, N.; Kohzu, A.; Komatsu, K.; Shinohara, R.; Takamura, N.; Nagakawa, M.; Sugai, Y.; Kuwahara, V.S.; et al. Seasonal variability and regulation of bacterial production in a shallow eutrophic lake. Limnol. Oceanogr. 2019, 64, 2441–2454. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Höfle, M.G. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 1998, 64, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Hanson, A.M.; Berges, J.A.; Young, E.B. Viral morphological diversity and relationship to bacteria and chlorophyll across a freshwater trophic gradient in the Lake Michigan watershed. Hydrobiologia 2017, 794, 93–108. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef]
- Schweichhart, J.S. Prokaryotic viruses: Intriguing players in the aquatic stream. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- Lymer, D.; Lindstrom, E.S.; Vrede, K. Variable importance of viral-induced bacterial mortality along gradients of trophic status and humic content in lakes. Freshw. Biol. 2008, 53, 1101–1113. [Google Scholar] [CrossRef]
- Parvathi, A.; Zhong, X.; Pradeep Ram, A.S.; Jacquet, S. Dynamics of auto- and heterotrophic picoplankton and associated viruses in Lake Geneva. Hydrol. Earth Syst. Sci. 2014, 18, 1073–1087. [Google Scholar] [CrossRef]
- Shen, S.; Shimizu, Y. Seasonal variation in viral infection rates and cell sizes of infected prokaryotes in a large and deep freshwater lake (Lake Biwa, Japan). Front. Microbiol. 2021, 12, 624980. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Shiemer, F. Bacteria and viruses in the water column of a tropical freshwater reservoirs. Environ. Microbiol. 2004, 6, 707–715. [Google Scholar] [CrossRef]
- Fischer, U.; Velimirov, B. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquat. Microb. Ecol. 2002, 27, 1–12. [Google Scholar] [CrossRef]
- Eissler, Y.; Galvez, M.-J.; Dorador, C.; Hengst, M.; Molina, V. Active microbiome structure and its association with environmental factors and viruses at different aquatic sites of a high altitude wet-land. Microbiol. Open 2019, 8, e667. [Google Scholar] [CrossRef]
- Palesse, S.; Colombet, J.; Pradeep Ram, A.S.; Sime-Ngando, T. Linking host prokaryotic physiology to viral life style dynamics in a temperate freshwater lake (Lake Pavin, France). Microb. Ecol. 2014, 68, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Cook, G.M. Energetics of bacterial growth: Balance of anabolic and catabolic reactions. Microbiol. Rev. 1995, 59, 48–62. [Google Scholar] [CrossRef]
- Berggren, M.; Laudon, H.; Jonsson, A.; Jansson, M. Nutrients constraints on metabolism affect the temperature regulation of aquatic bacterial growth efficiency. Microb. Ecol. 2010, 60, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Middelboe, M.; Lyck, P.G. Regeneration of dissolved organic matter by viral lysis in marine microbial communities. Aquat. Microb. Ecol. 2002, 27, 187–194. [Google Scholar] [CrossRef]
- Maurice, C.F.; Bouvier, C.; de Wit, R.; Bouvier, T. Linking the lytic and lysogenic bacteriophage cycles to environmental conditions, host physiology and their variability in coastal lagoons. Environ. Microbiol. 2013, 15, 2463–2475. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Chaibi-Slouma, S.; Keshri, J.; Colomber, J.; Sime-Ngando, T. Functional responses of bacterioplankton diversity and metabolism to experimental bottom-up and top-down forcings. Microb. Ecol. 2016, 72, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Sandaa, R.-A.; Gomez-Consarnau, L.; Pinhassi, J.; Riemann, L.; Maltis, A.; Weinbauer, M.G.; Gasol, J.M.; Thingstad, T.F. Viral control of bacterial biodiversity-evidence from a nutrient enriched marine mesocosm experiment. Environ. Microbiol. 2009, 11, 2585–2597. [Google Scholar] [CrossRef]
- Storesund, J.E.; Erga, S.R.; Ray, J.L.; Thingstad, T.F.; Sandaa, R.-A. Top-down and bottom-up control on bacterial diversity in a western Norwegian deep-silled fjord. FEMS Microb. Ecol. 2015, 91, 7. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Lignell, R. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat. Microb. Ecol. 1997, 13, 19–27. [Google Scholar] [CrossRef]
- Bonilla-Findji, O.; Maltis, A.; Lefèvre, D.; Rochelle-Newall, E.; Lemée, R.; Weinbauer, M.; Gattuso, J.-P. Viral effects on bacterial respiration, production and growth efficiency: Consistent trends in the Southern Ocean and the Mediterranean Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 790–800. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Nishimura, Y.; Tomaru, Y.; Nagasaki, K.; Nagata, T. Seasonal variation in viral induced mortality of bacterioplankton in the water column of a large mesotrophic lake (Lake Biwa, Japan). Aquat. Microb. Ecol. 2010, 58, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Šimek, K.; Pernthaler, J.; Weinbauer, M.G.; Hornak, K.; Dolan, J.R.; Nedoma, J.; Mašín, M.; Amann, R. Changes in bacterial community composition, dynamics and viral mortality rates associated with enhanced flagellate grazing in a meso-eutrophic reservoir. Appl. Environ. Microbiol. 2001, 67, 2723–2733. [Google Scholar] [CrossRef]
- Bana, Z.; Ayo, B.; Marrasé, C.; Gasol, J.M.; Iriberri, J. Changes in bacterial metabolism in response to dissolved organic matter modification during protozoan grazing in coastal Cantabrian Mediterranean waters. Environ. Microbiol. 2014, 16, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Middelboe, M.; Jorgensen, N.O.G. Viral lysis of bacteria: An important source of amino acids and cell wall compounds. J. Mar. Biol. Assoc. UK 2006, 86, 605–612. [Google Scholar] [CrossRef]
- Perez, M.T.; Pausz, C.; Herndl, G.J. Major shift in bacterioplankton utilization of enantiomeric amino acids between surface waters and the ocean’s interior. Limnol. Oceanogr. 2003, 48, 755–763. [Google Scholar] [CrossRef]
- Zhao, Z.; Gonsior, M.; Schmitt-Kopplin, P.; Zhan, Y.; Zhang, R.; Jiao, N.; Chen, F. Microbial transformation of virus-induced dissolved organic matter from picocyanobacteria: Coupling of bacterial diversity and DOM chemodiversity. ISME J. 2019, 13, 2551–2565. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Xiao, X.; Wallace, D.; Hu, C.; Zhang, L.; Batt, J.; Liu, J.; Gonsior, M.; Zhang, Y.; et al. Heterogenous viral contribution to dissolved organic matter processing in a long-tern macrocosm experiment. Environ. Int. 2019, 158, 106950. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Goode, A.G.; Fields, D.M.; Archer, S.D.; Martınez, J.M. Physiological responses of Oxyrrhis marina to a diet of virally infected Emiliania huxleyi. PeerJ 2019, 7, e6722. [Google Scholar] [CrossRef] [PubMed]
- Pourtois, J.; Tarnita, C.E.; Bonachela, J.A. Impact of lytic phages on phosphorous- vs. Nitrogen-limited marine microbes. Front. Microbiol. 2020, 11, 221. [Google Scholar] [CrossRef] [Green Version]


| Lake | Water Temperature (°C) | pH | CDOM (m−1) | TOC (mg L−1) | DOC (mg L−1) | TN (mg L−1) | Chlorophyll a (µg L−1) |
|---|---|---|---|---|---|---|---|
| Antheme | 20.4 ± 0.1 | 8.7 ± 0.1 | 1.8 ± 0.2 | 7.9 ± 0.8 | 5.2 ± 0.5 | 0.4 ± 0.04 | 13.6 ± 0.9 |
| Aubusson | 22.4 ± 0.2 | 7.7 ± 0.2 | 2.8 ± 0.3 | 8.2 ± 0.9 | 7.8 ± 0.6 | 0.7 ± 0.05 | 6.3 ± 0.8 |
| Banne d’ordanche | 19.3 ± 0.2 | 8.2 ± 0.1 | 0.7 ± 0.1 | 5.6 ± 0.4 | 3.8 ± 0.2 | 0.4 ± 0.02 | 5.6 ± 0.5 |
| Chabreloche | 20.5 ± 0.1 | 7.3 ± 0.2 | 2.8 ± 0.2 | 5.8 ± 0.5 | 5.3 ± 0.4 | 0.5 ± 0.05 | 3.7 ± 0.2 |
| Chambon | 21.3 ± 0.2 | 7.6 ± 0.2 | 0.9 ± 0.1 | 8.2 ± 0.9 | 4.7 ± 0.3 | 0.2 ± 0.02 | 4.1 ± 0.4 |
| Gour de Tazenat | 24.5 ± 0.3 | 8.5 ± 0.2 | 0.5 ± 0.06 | 6.4 ± 0.5 | 5.3 ± 0.4 | 0.3 ± 0.03 | 2.0 ± 0.2 |
| Laqueille | 17.5 ± 0.2 | 7.7 ± 0.3 | 0.6 ± 0.05 | 4.3 ± 0.3 | 3.2 ± 0.3 | 1.1 ± 0.09 | 10.9 ± 0.8 |
| Tour d’Auvergne | 21.6 ± 0.1 | 7.9 ± 0.1 | 0.5 ± 0.03 | 7.6 ± 0.5 | 5.4 ± 0.5 | 0.2 ± 0.02 | 13.1 ± 0.9 |
| Pavin | 20.9 ± 0.2 | 7.4 ± 0.1 | 0.5 ± 0.03 | 3.7 ± 0.2 | 2.5 ± 0.2 | 0.1 ± 0.01 | 1.2 ± 0.2 |
| St.Eloy les Mines | 24.5 ± 0.1 | 8.2 ± 0.1 | 1.2 ± 0.1 | 8.6 ± 0.8 | 7.4 ± 0.5 | 0.3 ± 0.02 | 3.8 ± 0.2 |
| St. Remy | 23.2 ± 0.2 | 8.2 ± 0.2 | 1.6 ± 0.2 | 8.4 ± 0.9 | 6.3 ± 0.4 | 0.7 ± 0.03 | 9.4 ± 0.8 |
| Super Besse | 18.8 ± 0.2 | 7.9 ± 0.2 | 0.7 ± 0.07 | 4.5 ± 0.3 | 3.5 ± 0.2 | 0.1 ± 0.03 | 9.2 ± 0.7 |
| Ambert | 23.9 ± 0.2 | 7.7 ± 0.1 | 1.2 ± 0.2 | 14.3 ± 1.1 | 10.3 ± 0.7 | 0.4 ± 0.02 | 8.8 ± 0.7 |
| Anschald | 21.9 ± 0.2 | 7.2 ± 0.2 | 1.2 ± 0.1 | 8.1 ± 0.7 | 8.5 ± 0.8 | 0.4 ± 0.02 | 11.2 ± 0.9 |
| Aydat | 23.1 ± 0.3 | 9.1 ± 0.2 | 0.7 ± 0.08 | 9.8 ± 0.9 | 6.6 ± 0.4 | 0.5 ± 0.02 | 15.6 ± 1.0 |
| Cournon | 24.2 ± 0.1 | 8.9 ± 0.3 | 0.5 ± 0.05 | 11.7 ± 0.9 | 7.7 ± 0.5 | 0.3 ± 0.01 | 9.5 ± 0.7 |
| Cunlhat | 21.6 ± 0.2 | 8.1 ± 0.2 | 3.0 ± 0.2 | 14.6 ± 0.9 | 9.2 ± 0.5 | 0.5 ± 0.02 | 19.9 ± 1.1 |
| Etang Philippe | 23.3 ± 0.1 | 7.5 ± 0.1 | 0.7 ± 0.05 | 13.7 ± 0.8 | 10.6 ± 0.5 | 0.5 ± 0.01 | 48.5 ± 1.5 |
| Fades | 23.4 ± 0.3 | 9.7 ± 0.2 | 2.3 ± 0.08 | 11.2 ± 0.8 | 8.5 ± 0.7 | 0.6 ± 0.02 | 17.0 ± 1.2 |
| Grangent | 22.3 ± 0.2 | 7.4 ± 0.2 | 1.8 ± 0.1 | 7.4 ± 0.5 | 5.8 ± 0.5 | 1.0 ± 0.08 | 11.6 ± 0.9 |
| Lepeyrouse | 24.4 ± 0.2 | 8.2 ± 0.1 | 0.7 ± 0.05 | 13.0 ± 0.8 | 11.2 ± 0.9 | 0.7 ± 0.05 | 21.4 ± 1.3 |
| Servant | 25.3 ± 0.3 | 7.6 ± 0.2 | 1.2 ± 0.02 | 12.1 ± 0.9 | 10.4 ± 0.7 | 0.6 ± 0.03 | 7.2 ± 0.5 |
| Vernet la Varenne | 22.8 ± 0.1 | 8.1 ± 0.2 | 1.4 ± 0.2 | 13.6 ± 0.7 | 9.7 ± 0.6 | 0.4 ± 0.02 | 13.3 ± 0.9 |
| Villerest | 24.8 ± 0.2 | 9.8 ± 0.2 | 1.6 ± 0.2 | 10.2 ± 0.8 | 8.6 ± 0.4 | 0.5 ± 0.02 | 25.8 ± 1.1 |
| Roziers | 21.8 0.3 | 7.1 ± 0.1 | 8.3 ± 0.7 | 15.1 ± 0.9 | 13.6 ± 1.0 | 0.5 ± 0.01 | 15.5 ± 1.3 |
| Parameters | Temp | CDOM | DOC | Chl | VA | BA | FIC | BR | BP |
|---|---|---|---|---|---|---|---|---|---|
| Temp | |||||||||
| CDOM | NS | ||||||||
| DOC | NS/NS 0.61 *** | NS/0.88 *** 0.51 *** | |||||||
| Chl | NS | NS | NS/NS 0.51 *** | ||||||
| VA | NS/NS 0.38 * | NS | NS/NS 0.38 * | NS/NS 0.49 *** | |||||
| BA | NS/NS 0.41 * | NS | NS/0.53 * NS | NS/0.53 * 0.51 *** | 0.94 ***/0.56 * 0.94 *** | ||||
| FIC | NS/NS 0.37 * | NS | NS/NS 0.37 * | NS/0.61 0.47 ** | NS/NS 0.45 ** | NS/0.58 * 0.42 * | |||
| BR | NS | NS/−0.60 * −0.38 * | NS/−0.68 ** NS | NS | NS | NS | 0.77 ***/NS NS | ||
| BP | NS/NS 0.48 ** | NS | NS/NS 0.51 *** | NS/0.68 ** 0.56 *** | NS | NS/0.69 *** 0.38 * | −0.87 ***/0.87 *** NS | −0.75 ***/NS NS | |
| BGE | NS/NS 0.47 ** | NS | NS/0.61 * 0.56 *** | NS/0.60 * 0.50 *** | NS | NS/0.70 *** NS | −0.85 ***/0.66 ** NS | −0.94 ***/−0.72 *** −0.66 *** | 0.92 ***/0.69 ** 0.91 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeep Ram, A.S.; Sime-Ngando, T. Differential Effects of Viruses on the Growth Efficiency of Freshwater Bacterioplankton in Eutrophic Relative to Non-Eutrophic Lakes. Microorganisms 2023, 11, 384. https://doi.org/10.3390/microorganisms11020384
Pradeep Ram AS, Sime-Ngando T. Differential Effects of Viruses on the Growth Efficiency of Freshwater Bacterioplankton in Eutrophic Relative to Non-Eutrophic Lakes. Microorganisms. 2023; 11(2):384. https://doi.org/10.3390/microorganisms11020384
Chicago/Turabian StylePradeep Ram, Angia Sriram, and Télesphore Sime-Ngando. 2023. "Differential Effects of Viruses on the Growth Efficiency of Freshwater Bacterioplankton in Eutrophic Relative to Non-Eutrophic Lakes" Microorganisms 11, no. 2: 384. https://doi.org/10.3390/microorganisms11020384








