Improved Biohythane Production from Rice Straw in an Integrated Anaerobic Bioreactor under Thermophilic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum Characteristics
2.1.1. Substrate
2.1.2. Inoculum
2.2. Batch Biohythane Production Tests for Optimizing Substrate Concentration
2.3. Reactor Configuration and Operation
2.3.1. Reactor Configuration
2.3.2. Reactor Start-Up and Operation
2.3.3. Energy Conversion Efficiency (ECE) Calculation
2.4. Analytical Methods
3. Results and Discussion
3.1. Effect of Substrate Concentration on Biohythane Production
3.2. Performance of Biohythane Production from Rice Straw
3.2.1. Continuous Gas Production
3.2.2. Specific H2, CH4, and Biohythane Production Yield
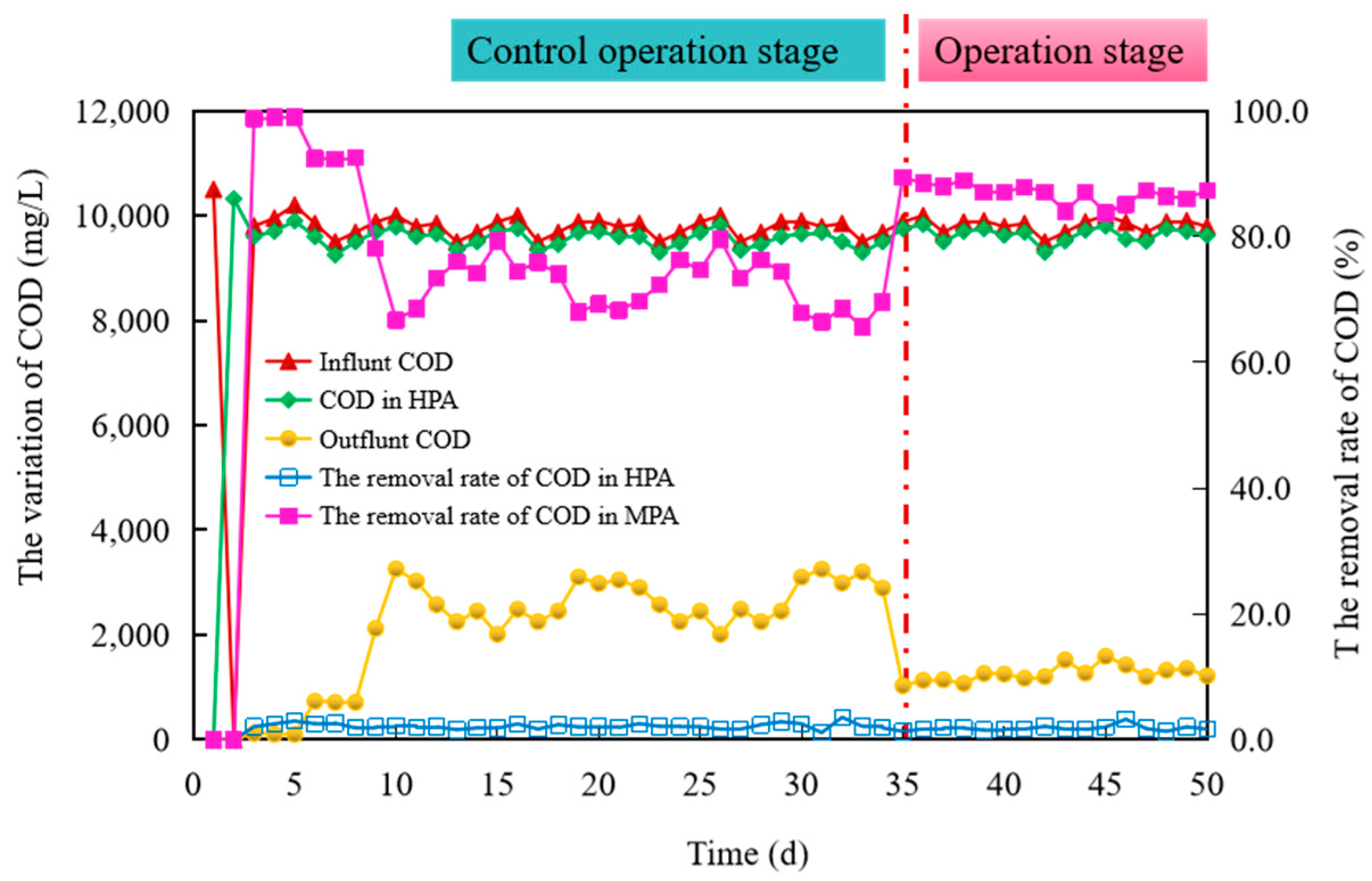
3.2.3. COD Removal in Biohythane Production Process
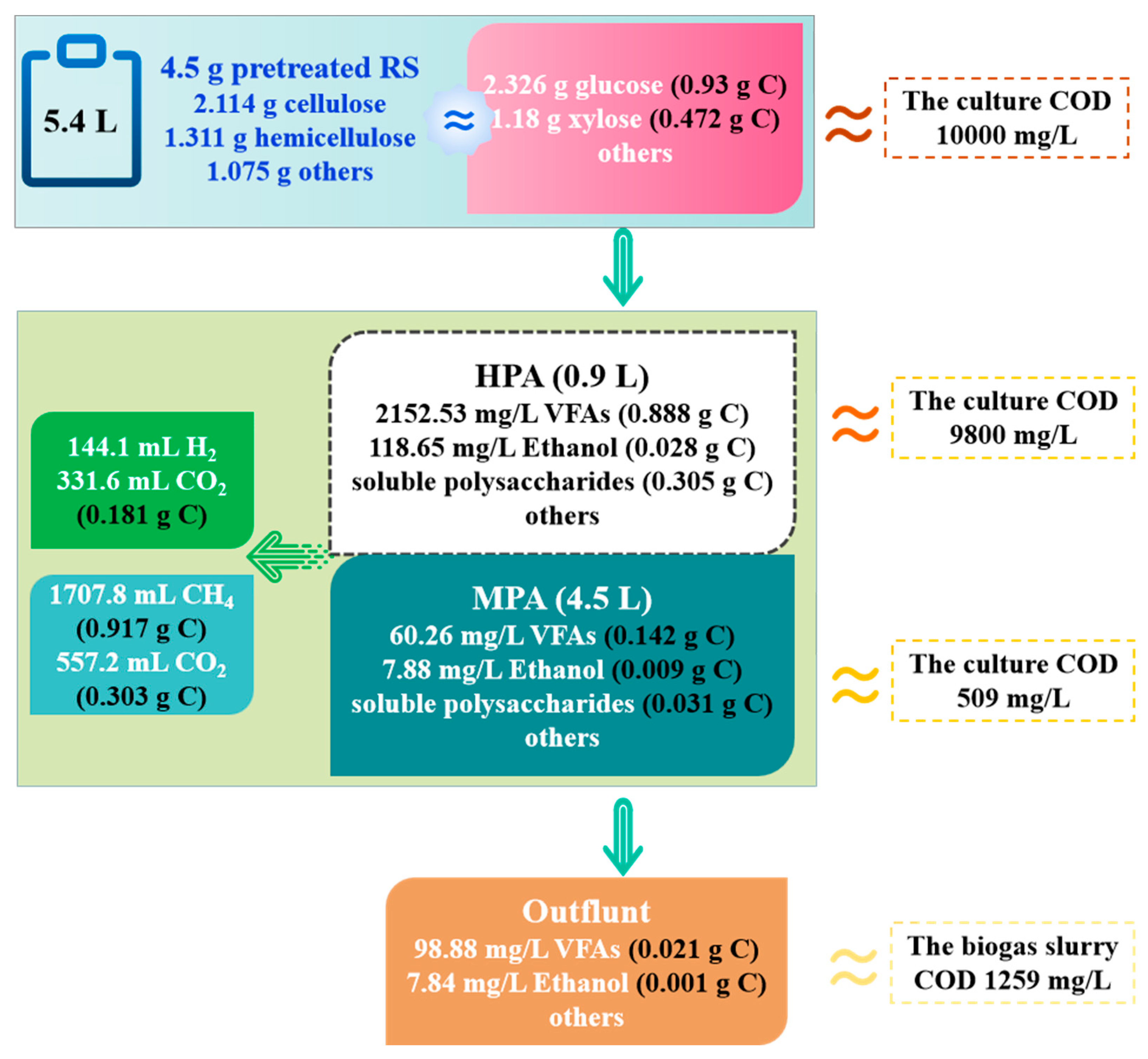
3.3. Carbon Distribution Analysis during Biohythane Production
3.4. Prospects and Challenges of Biohythane Production from Straw
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Wang, E.Z.; Li, X.; Cui, X.; Guo, J.B.; Dong, R.J. Potential biomethane production from crop residues in China: Contributions to carbon neutrality. Renew. Renew. Sustain. Energy Rev. 2021, 148, 111360. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Lu, Y.; Wu, X.; Wang, L.; Wang, L.; Han, B.; Xing, X.-H. States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour. Technol. 2013, 135, 292–303. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renew. Sustain. Energy Rev. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- BP World Energy Statistics Yearbook. 2021. Available online: https://yearbook.enerdata.net/ (accessed on 5 February 2023).
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresour. Technol. 2018, 267, 71–76. [Google Scholar] [CrossRef]
- Roy, S.; Das, D. Biohythane production from organic wastes: Present state of art. Environ. Sci. Pollut. Res. 2015, 23, 9391–9410. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Biohythane production from sugarcane bagasse and water hyacinth: A way towards promising green energy production. J. Clean. Prod. 2019, 207, 689–701. [Google Scholar] [CrossRef]
- Kongjan, P.; O-Thong, S.; Angelidaki, I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour. Technol. 2011, 102, 4028–4035. [Google Scholar] [CrossRef]
- Fulton, J.; Marmaro, R.; Egan, G. System for Producing a Hydrogen Enriched Fuel. U.S. Patent Application No. 7721682, 25 May 2010. [Google Scholar]
- Algapani, D.E.; Qiao, W.; di Pumpo, F.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R. Long-term bio-H2 and bio-CH4 production from food waste in a continuous two-stage system: Energy efficiency and conversion pathways. Bioresour. Technol. 2017, 248, 204–213. [Google Scholar] [CrossRef]
- Ren, N.Q.; Wang, A.J.; Cao, G.L.; Xu, J.F.; Gao, L.F. Bioconversion of lignocellulosic biomass to hydrogen: Potential and challenges. Biotechnol. Adv. 2009, 27, 1051–1060. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Song, W.; Xia, A.; Zhou, J.; Cen, K. Cogeneration of hydrogen and methane from Arthrospira maxima biomass with bacteria domestication and enzymatic hydrolysis. Int. J. Hydrogen Energy 2011, 36, 1474–1481. [Google Scholar] [CrossRef]
- Dong, L.; Wu, X.; Wang, Q.; Cao, G.; Wu, J.; Zhou, C.; Ren, N. Evaluation of a novel pretreatment of NaOH/Urea at outdoor cold-winter conditions for enhanced enzymatic conversion and hythane production from rice straw. Sci. Total. Environ. 2020, 744, 140900. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, N.L.P.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigün, O. Two-phase anaerobic digestion processes: A review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Liu, D.W.; Liu, D.P.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Si, B.C.; Li, J.M.; Zhu, Z.B.; Zhang, Y.H.; Lu, J.W.; Shen, R.H.; Zhang, C.; Xing, X.H.; Liu, Z.D. Continuous production of biohythane from hydrothermal liquefied cornstalk biomass via two-stage high-rate anaerobic reactors. Biotechnol. Biofuels 2016, 9, 254. [Google Scholar] [CrossRef]
- Ghimire, A.; Kumar, G.; Sivagurunathan, P.; Shobana, S.; Saratale, G.D.; Kim, H.W.; Luongo, V.; Esposito, G.; Munoz, R. Bio-hythane production from microalgae biomass: Key challenges and potential opportunities for algal bio-refineries. Bioresour. Technol. 2017, 241, 525–536. [Google Scholar] [CrossRef]
- Ramos, L.; Silva, E.L. Thermophilic hydrogen and methane production from sugarcane stillage in two-stage anaerobic fluidized bed reactors. Int. J. Hydrogen Energy 2020, 45, 5239–5251. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.-L.; Sheng, T.; Ren, H.-Y.; Wang, A.-J.; Zhang, J.; Zhong, Y.-J.; Ren, N.-Q. Bio-immobilization of dark fermentative bacteria for enhancing continuous hydrogen production from cornstalk hydrolysate. Bioresour. Technol. 2017, 243, 548–555. [Google Scholar] [CrossRef]
- Robert, A.A. Physical Chemistry; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Haloua, F.; Foulon, E.; Allard, A.; Hay, B.; Filtz, J.R. Traceable measurement and uncertainty analysis of the gross calorific value of methane determined by isoperibolic calorimetry. Metrologia 2015, 52, 741–755. [Google Scholar] [CrossRef]
- Prakasham, R.; Brahmaiah, P.; Sathish, T.; Rao, K.S. Fermentative biohydrogen production by mixed anaerobic consortia: Impact of glucose to xylose ratio. Int. J. Hydrogen Energy 2009, 34, 9354–9361. [Google Scholar] [CrossRef]
- Ntaikou, I.; Gavala, H.N.; Lyberatos, G. Effect of the substrate concentration on the final hydrogen yield and distribution of metabolites during the growth of the hydrogen—Producing bacterium Ruminococcus albus on glucose. In Proceedings of the International Conference on Advances in Biotechnology-Industrial Microbial Biotechnology, Thessaloniki, Greece, 3–5 November 2010. [Google Scholar]
- Dong, L.; Cao, G.; Wu, J.; Yang, S.; Ren, N. Reflux of acidizing fluid for enhancing biomethane production from cattle manure in plug flow reactor. Bioresour. Technol. 2019, 284, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Rowse, L.E. Design of Small Scale Anaerobic Digesters for Application in Rural Developing Countries; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Zacharia, K.M.B.; Yadav, S.; Machhirake, N.P.; Kim, S.H.; Lee, B.D.; Kumar, R. Bio-hydrogen and bio-methane potential analysis for production of bio-hythane using various agricultural residues. Bioresour. Technol. 2020, 309, 123297. [Google Scholar]
- Urbaniec, K.; Grabarczyk, R. Raw materials for fermentative hydrogen production. J. Clean. Prod. 2009, 17, 959–962. [Google Scholar] [CrossRef]
- Sapporo Breweries Ltd. Commercial Biohydrogen Production Project in 2013; Sapporo Breweries Ltd.: Tokyo, Japan, 2009. [Google Scholar]
- Zheng, H.S.; Wang, Y.; Feng, X.C.; Li, S.; Leong, Y.K.; Chang, J.S. Renewable biohydrogen production from straw biomass e Recent advances in pretreatment/hydrolysis technologies and future development. Int. J. Hydrogen Energy 2022, 47, 37359–37373. [Google Scholar] [CrossRef]
- Kim, M.; Liu, C.; Noh, J.-W.; Yang, Y.; Oh, S.; Shimizu, K.; Lee, D.-Y.; Zhang, Z. Hydrogen and methane production from untreated rice straw and raw sewage sludge under thermophilic anaerobic conditions. Int. J. Hydrogen Energy 2013, 38, 8648–8656. [Google Scholar] [CrossRef]
- Guo, Y.-C.; Dai, Y.; Bai, Y.-X.; Li, Y.-H.; Fan, Y.-T.; Hou, H.-W. Co-producing hydrogen and methane from higher-concentration of corn stalk by combining hydrogen fermentation and anaerobic digestion. Int. J. Hydrogen Energy 2014, 39, 14204–14211. [Google Scholar] [CrossRef]




| Substrates | Pretreatment Method | Conditions | Microorganism | T, °C | YH2 | YCH4 | YHythane | References |
|---|---|---|---|---|---|---|---|---|
| Rice straw | Mechanical crushing under 2 mm. SS and rice straw codigestion | B | SS | 55 | 21 mL/g VS | 266 mL/g VS | 287 mL/g VS | [32] |
| Corn stalk | 0.5% H2SO4 and 10% w/w at 121 °C for 60 min | B | Bacillus sp. FS2011and CDS | 55 | 88.1 mL/g VS | 227 mL/g COD | 306.8 mL/g COD | [33] |
| Cornstalk | Hydrothermal liquefaction, 260 °C for 0 min, vacuum filtration | UASB to PBR | SS | 37 | 146 mL H2/g COD | 302 mL CH4/g COD | 448 mL/g COD | [17] |
| Wheat straw hydrolysate | Provided by Risø DTU (Denmark) | UASB to UASB | Mixed-culture and methonogenic granules and BS | 55 and 70 | 89 mL/g VS | 307 mL/g VS | 396 mL/g VS | [8] |
| Rice straw enzymatic hydrolyzate | NU pretreatment at outdoor winter and 100% w/v for 3 months | B | Thermoanaerobacterium thermosaccharolyticum W16 and BS | 60 | 155.5 mL/g VS | 63.1 mL/g VS | 218.6 mL/g VS | [13] |
| Rice straw | NU pretreatment at outdoor winter and 100% w/v for 3 months | IABR | BS | 55–60 | 104.1 mL/g VS | 508.4 mL/g VS | 612.5 mL/g VS | This research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Cao, G.; Wang, W.; Luo, G.; Yang, F.; Ren, N. Improved Biohythane Production from Rice Straw in an Integrated Anaerobic Bioreactor under Thermophilic Conditions. Microorganisms 2023, 11, 474. https://doi.org/10.3390/microorganisms11020474
Dong L, Cao G, Wang W, Luo G, Yang F, Ren N. Improved Biohythane Production from Rice Straw in an Integrated Anaerobic Bioreactor under Thermophilic Conditions. Microorganisms. 2023; 11(2):474. https://doi.org/10.3390/microorganisms11020474
Chicago/Turabian StyleDong, Lili, Guangli Cao, Wanqing Wang, Geng Luo, Fei Yang, and Nanqi Ren. 2023. "Improved Biohythane Production from Rice Straw in an Integrated Anaerobic Bioreactor under Thermophilic Conditions" Microorganisms 11, no. 2: 474. https://doi.org/10.3390/microorganisms11020474





