Magnetite Nanoparticles and Carbon Nanotubes for Improving the Operation of Mesophilic Anaerobic Digesters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biowaste and Inoculum
2.2. Additives
2.3. Biochemical Methane Potential Experiments and Analytical Methods
2.4. Microbial Community Structure Analysis
3. Results and Discussion
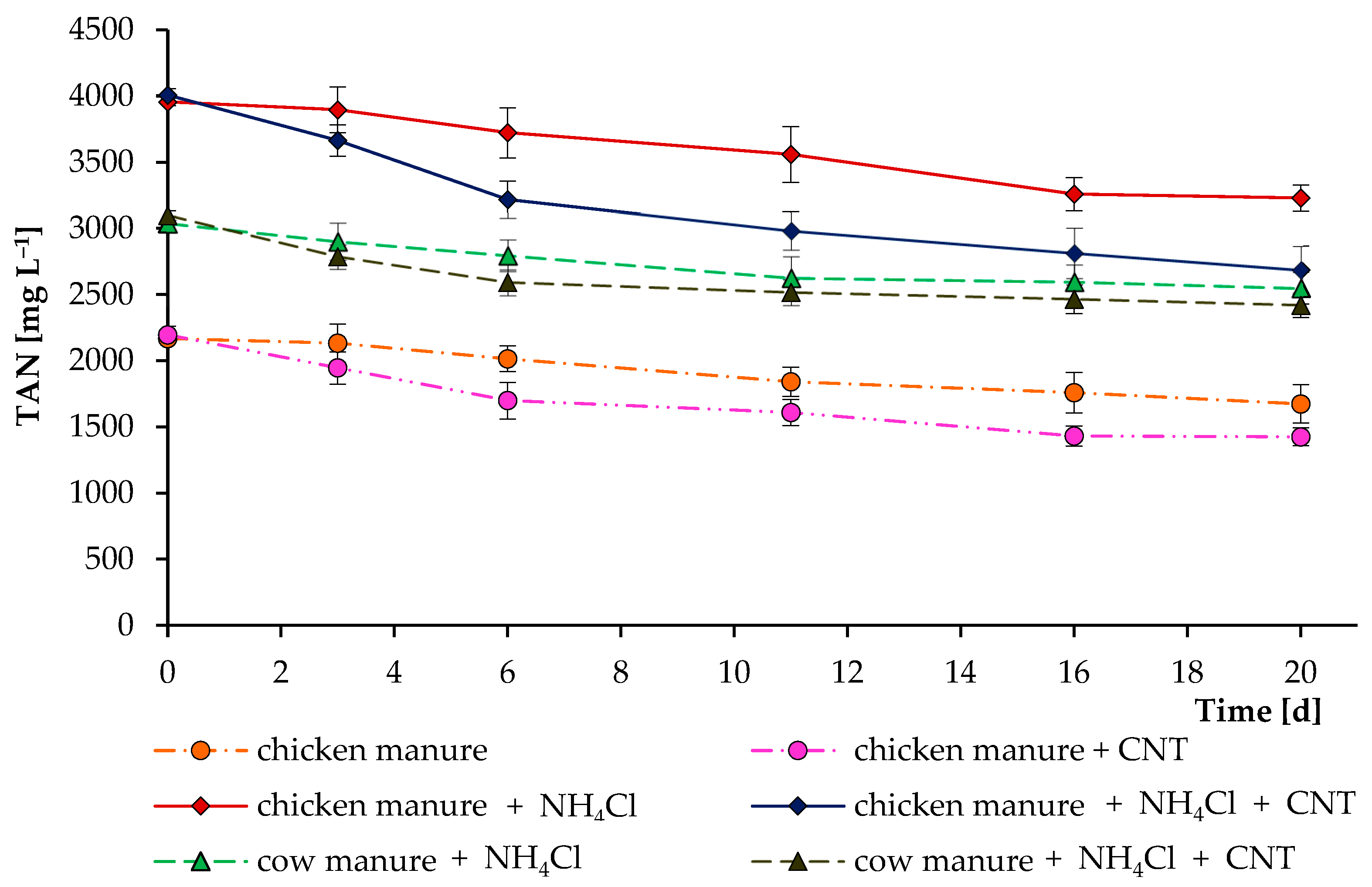
3.1. Process Stability and Methane Production
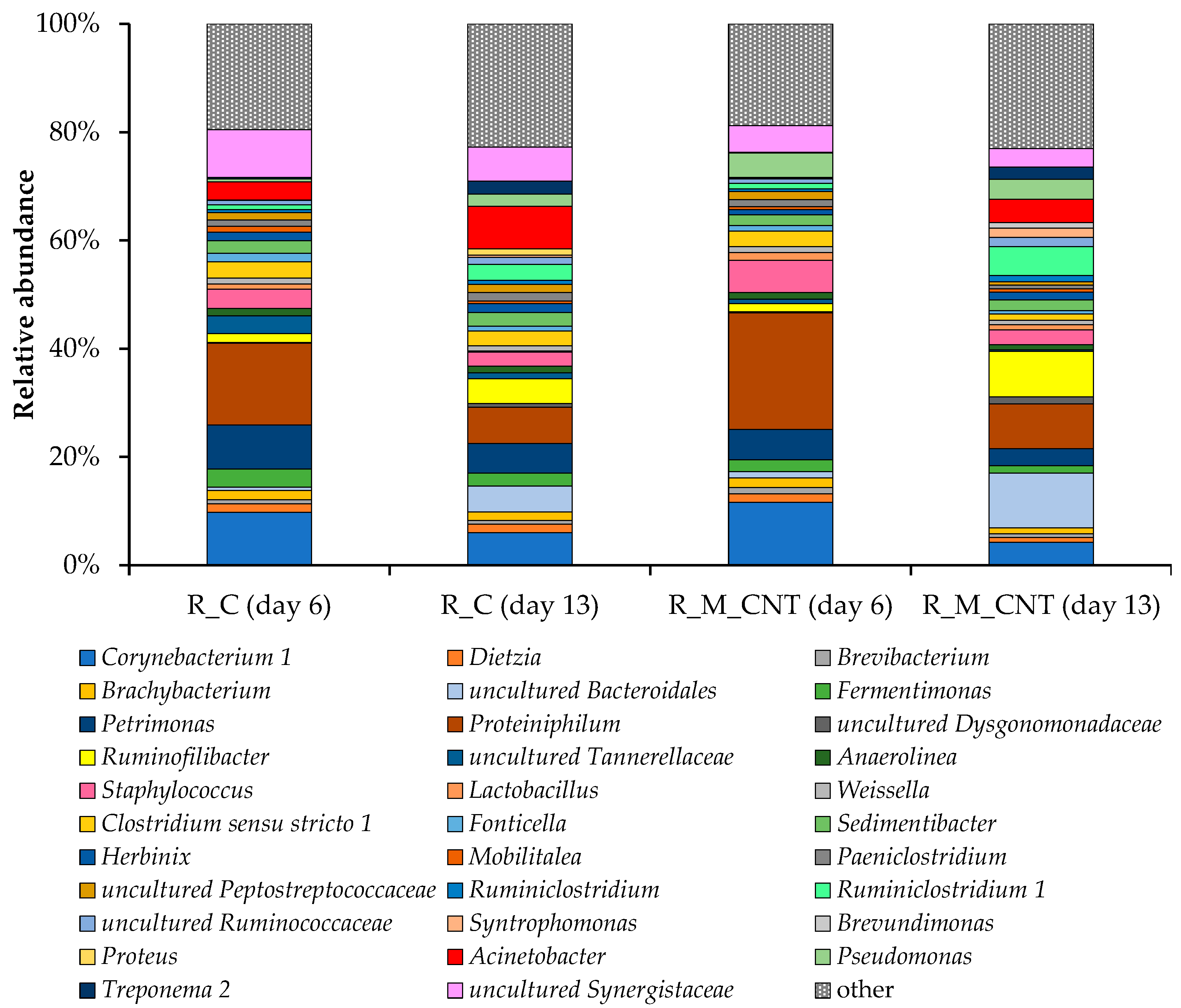
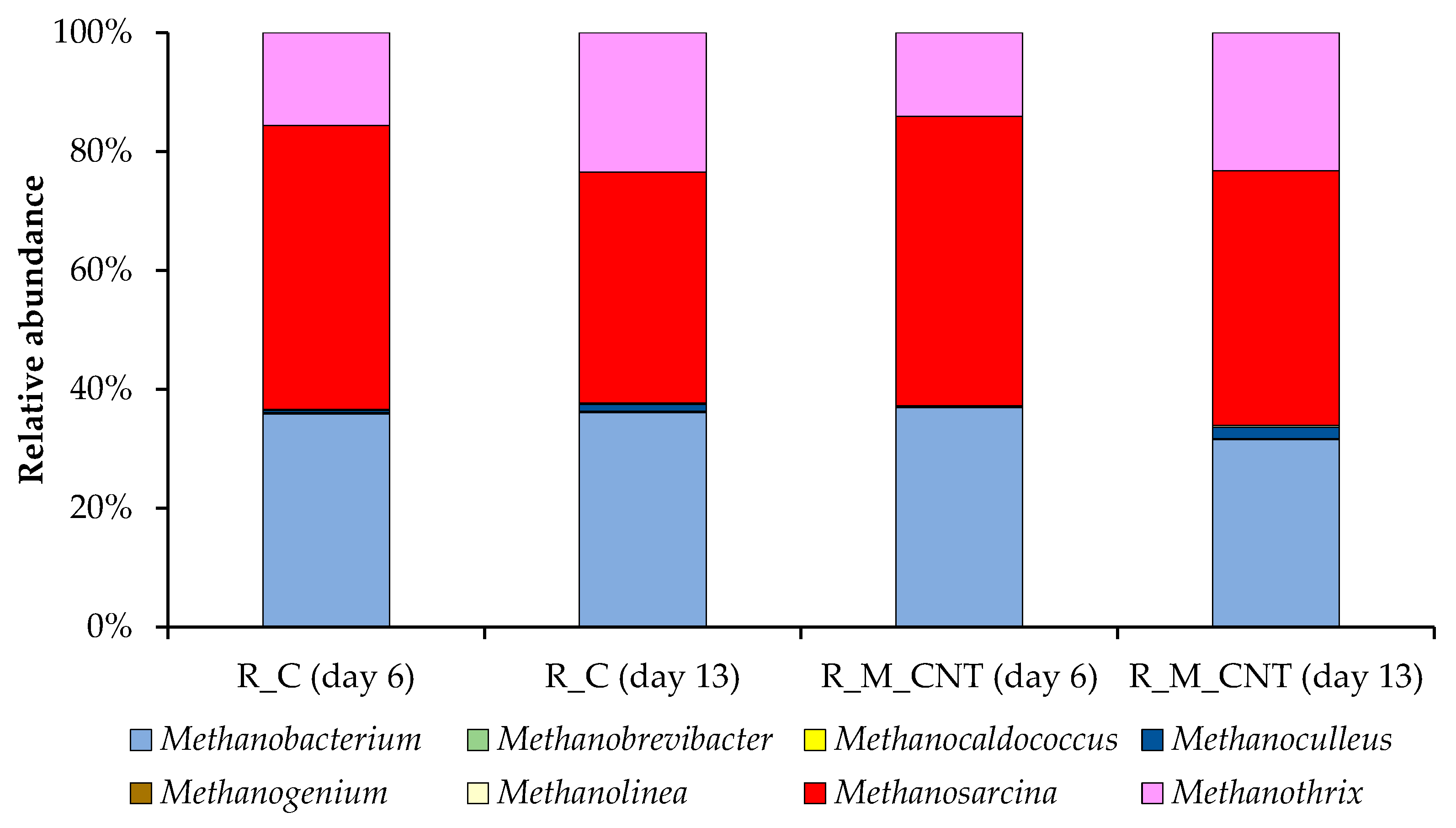
3.2. Variation in Microbial Communities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, A.H.; Tao, L. Economic perspectives of biogas production via anaerobic digestion. Bioengineering 2020, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.K.; Manikandan, S.; Oviyapriya, M.; Selvaraj, M.; Assiri, M.A.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; et al. Recent advances in biogas production using agro-industrial waste: A comprehensive review outlook of techno-economic analysis. Bioresour Technol. 2022, 363, 127871. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, R.; Chiumenti, A.; da Borso, F.; Limina, S. Anaerobic Digestion of Swine Manure in Conventional and Hybrid Pilot Scale Plants: Performance and Gaseous Emissions Reduction; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009; Volume 4. [Google Scholar]
- Lee, M.; Steiman, M.; St. Angelo, S. Biogas digestate as a renewable fertilizer: Effects of digestate application on crop growth and nutrient composition. Renew. Agric. Food Syst. 2021, 36, 173–181. [Google Scholar] [CrossRef]
- Czekala, W.; Jasinski, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas plant operation: Digestate as the valuable product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of microalgae on liquid anaerobic digestate for depollution, biofuels and cosmetics: A review. Environ. Chem. Lett. 2022, 20, 3631–3656. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Growth parameters of various green microalgae species in effluent from biogas reactors: The importance of effluent concentration. Plants 2022, 11, 3583. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Detman, A.; Chojnacka, A.; Błaszczyk, M.K. Anaerobic digestion: I. A common process ensuring energy flow and the circulation of matter in ecosystems. II. A tool for the production of gaseous biofuels. In Fermentation Processes; Jozala, A.F., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Kurth, J.M.; Op den Camp, H.J.M.; Welte, C.U. Several ways one goal—Methanogenesis from unconventional substrates. Appl. Microbiol. Biotechnol. 2020, 104, 6839–6854. [Google Scholar] [CrossRef]
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Chang, J.; Liang, J.; Zhang, P.; Zhang, G. Improvement of direct interspecies electron transfer via adding conductive materials in anaerobic digestion: Mechanisms, performances, and challenges. Front. Microbiol. 2022, 13, 860749. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Gu, M.; Wu, G. Inhibition mitigation of methanogenesis processes by conductive materials: A critical review. Bioresour Technol. 2020, 317, 123977. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Ziganshin, A.M. Anaerobic digestion of chicken manure in the presence of magnetite, granular activated carbon, and biochar: Operation of anaerobic reactors and microbial community structure. Microorganisms 2022, 10, 1422. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Anaerobic digestion of chicken manure assisted by carbon nanotubes: Promotion of volatile fatty acids consumption and methane production. Fermentation 2022, 8, 641. [Google Scholar] [CrossRef]
- Barrena, R.; Vargas-García, M.D.C.; Catacora-Padilla, P.; Gea, T.; Abo Markeb, A.; Moral-Vico, J.; Sánchez, A.; Font, X.; Aspray, T.J. Magnetite-based nanoparticles and nanocomposites for recovery of overloaded anaerobic digesters. Bioresour Technol. 2023, 372, 128632. [Google Scholar] [CrossRef]
- Yan, Y.; Du, Z.; Zhang, L.; Feng, L.; Sun, D.; Dang, Y.; Holmes, D.E.; Smith, J.A. Identification of parameters needed for optimal anaerobic co-digestion of chicken manure and corn stover. RSC Adv. 2019, 9, 29609–29618. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Di, L.; Zhang, Q.; Wang, F.; Wang, H.; Liu, H.; Yi, W.; Zhang, Z.; Zhang, D. Effect of nano-Fe3O4 biochar on anaerobic digestion of chicken manure under high ammonia nitrogen concentration. J. Clean. Prod. 2022, 375, 134107. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon nanotubes—The promising adsorbent in wastewater treatment. J. Phys. Conf. Ser. 2007, 61, 698–702. [Google Scholar] [CrossRef]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas production from undiluted chicken manure and maize silage: A study of ammonia inhibition in high solids anaerobic digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapovalov, Y.; Zhadan, S.; Bochmann, G.; Salyuk, A.; Nykyforov, V. Dry anaerobic digestion of chicken manure: A review. Appl. Sci. 2020, 10, 7825. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sánchez, E.; Milán, Z.; Cortés, I.; Rubia, M. Application of natural zeolites in anaerobic digestion processes: A review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour Technol. 2021, 325, 124697. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Sagitov, I.I.; Akhmetova, R.F.; Saleeva, G.T.; Kiassov, A.P.; Gogoleva, N.E.; Shagimardanova, E.I.; Ziganshin, A.M. Comparison of the microbiota and inorganic anion content in the saliva of patients with gastroesophageal reflux disease and gastroesophageal reflux disease-free individuals. BioMed Res. Intern. 2020, 2020, 2681791. [Google Scholar] [CrossRef]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef]
- Kombarakkarana, J.; Clewett, C.F.M.; Pietraß, T. Ammonia adsorption on multi-walled carbon nanotubes. Chem. Phys. Let. 2007, 441, 282–285. [Google Scholar] [CrossRef]
- Yan, W.; Lu, D.; Liu, J.; Zhou, Y. The interactive effects of ammonia and carbon nanotube on anaerobic digestion. Chem. Eng. J. 2019, 372, 332–340. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Im, S.; Song, Y.-C.; Kang, S.; Kim, D.-H. Enhanced anaerobic digestion of long chain fatty acid by adding magnetite and carbon nanotubes. Microorganisms 2020, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.L.; Tong, Z.H.; Fang, C.Y.; Chu, J.; Yu, H.Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Salvador, A.F.; Martins, G.; Mellefranco, M.; Serpa, R.; Ajm, S.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.Y. Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manag. 2015, 43, 114–122. [Google Scholar] [CrossRef]
- Mei, R.; Nobu, M.K.; Narihiro, T.; Liu, W.T. Metagenomic and metatranscriptomic analyses revealed uncultured Bacteroidales populations as the dominant proteolytic amino acid degraders in anaerobic digesters. Front Microbiol. 2020, 11, 593006. [Google Scholar] [CrossRef]
- Morrison, M.; Miron, J. Adhesion to cellulose by Ruminococcus albus: A combination of cellulosomes and Pil-proteins? FEMS Microbiol. Lett. 2000, 185, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Hahnke, S.; Abendroth, C.; Langer, T.; Codoñer, F.M.; Ramm, P.; Porcar, M.; Luschnig, O.; Klocke, M. Complete genome sequence of a new Ruminococcaceae bacterium isolated from anaerobic biomass hydrolysis. Genome Announc. 2018, 6, e00030-18. [Google Scholar] [CrossRef] [Green Version]
- Ntaikou, I.; Gavala, H.N.; Kornaros, M.; Lyberatos, G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int. J. Hydrogen Energy 2007, 33, 1153–1163. [Google Scholar] [CrossRef]
- Sträuber, H.; Schröder, M.; Kleinsteuber, S. Metabolic and microbial community dynamics during the hydrolytic and acidogenic fermentation in a leach-bed process. Energ. Sustain. Soc. 2012, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Krieg, N.R.; Staley, J.T.; Brown, D.R.; Hedlund, B.P.; Paster, B.J.; Ward, N.L.; Ludwig, W.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Detman, A.; Bucha, M.; Treu, L.; Chojnacka, A.; Pleśniak, Ł.; Salamon, A.; Łupikasza, E.; Gromadka, R.; Gawor, J.; Gromadka, A.; et al. Evaluation of acidogenesis products’ effect on biogas production performed with metagenomics and isotopic approaches. Biotechnol Biofuels. 2021, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Ziels, R.M.; Karlsson, A.; Beck, D.A.C.; Ejlertsson, J.; Yekta, S.S.; Bjorn, A.; Stensel, H.D.; Svensson, B.H. Microbial community adaptation influences long-chain fatty acid conversion during anaerobic codigestion of fats, oils, and grease with municipal sludge. Water Res. 2016, 103, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, L.; Michaelsen, T.Y.; Singleton, C.M.; Dottorini, G.; Kirkegaard, R.H.; Albertsen, M.; Nielsen, P.H.; Dueholm, M.S. Novel syntrophic bacteria in full-scale anaerobic digesters revealed by genome-centric metatranscriptomics. ISME J. 2020, 14, 906–918. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 1316. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Zeng, Y.; Wang, H.Z.; Zheng, D.; Kamagata, Y.; Narihiro, T.; Nobu, M.K.; Tang, Y.-Q. Different interspecies electron transfer patterns during mesophilic and thermophilic syntrophic propionate degradation in chemostats. Microb. Ecol. 2020, 80, 120–132. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour Technol. 2020, 296, 122342. [Google Scholar] [CrossRef]
- Vrieze, J.D.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2013, 7, 408–415. [Google Scholar] [CrossRef]
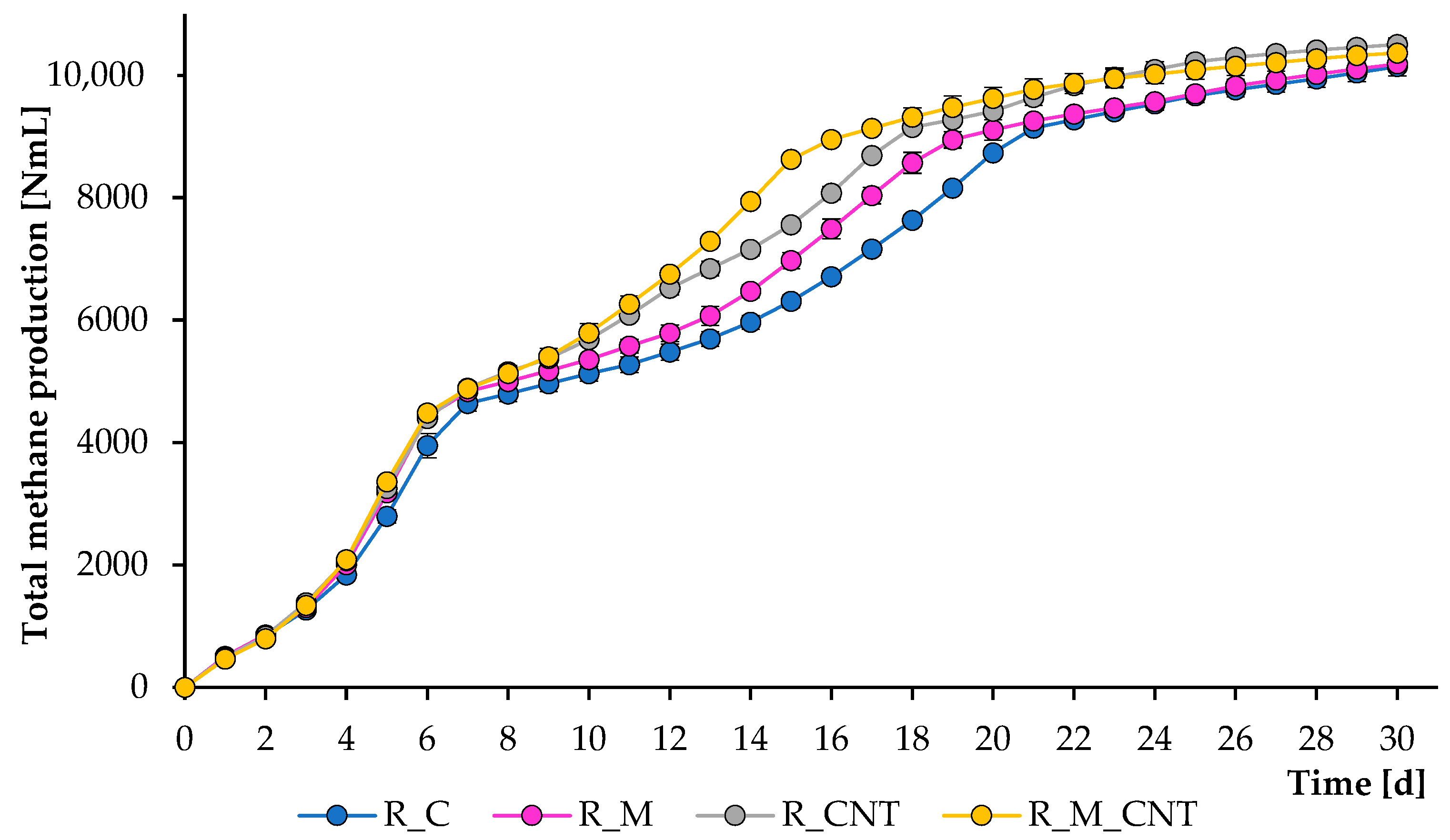



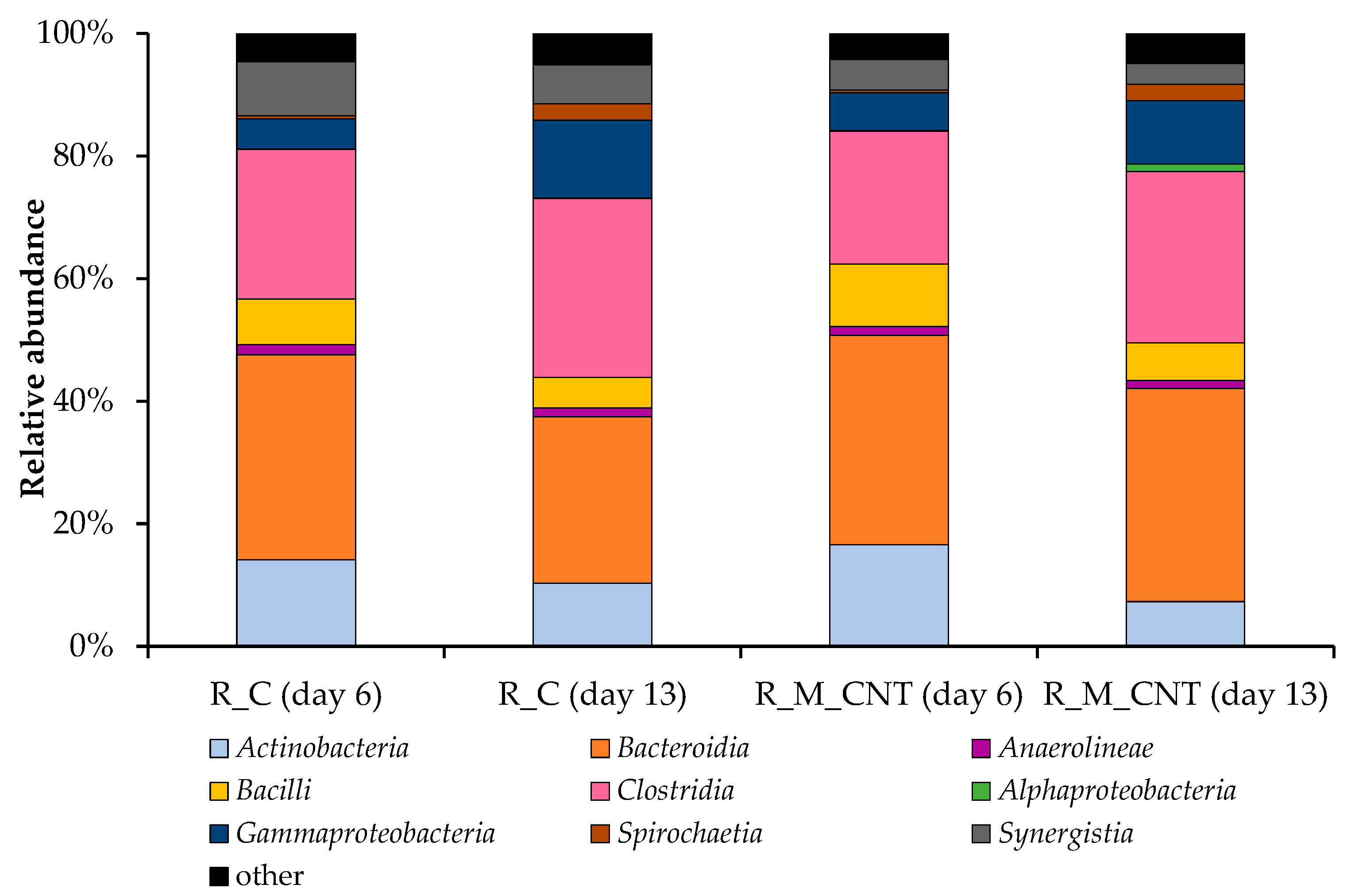
| Sample | Bacteria | Methanogenic Archaea | ||||||
|---|---|---|---|---|---|---|---|---|
| OTUs | Chao1 | Shannon | Simpson | OTUs | Chao1 | Shannon | Simpson | |
| R_C (day 6) | 609 | 641 | 6.76 | 0.97 | 24 | 25 | 2.40 | 0.76 |
| R_C (day 13) | 661 | 673 | 7.10 | 0.98 | 25 | 27 | 2.46 | 0.77 |
| R_M_CNT (day 6) | 603 | 645 | 6.72 | 0.97 | 22 | 24 | 2.33 | 0.75 |
| R_M_CNT (day 13) | 624 | 664 | 6.84 | 0.97 | 27 | 32 | 2.47 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Ziganshin, A.M. Magnetite Nanoparticles and Carbon Nanotubes for Improving the Operation of Mesophilic Anaerobic Digesters. Microorganisms 2023, 11, 938. https://doi.org/10.3390/microorganisms11040938
Ziganshina EE, Ziganshin AM. Magnetite Nanoparticles and Carbon Nanotubes for Improving the Operation of Mesophilic Anaerobic Digesters. Microorganisms. 2023; 11(4):938. https://doi.org/10.3390/microorganisms11040938
Chicago/Turabian StyleZiganshina, Elvira E., and Ayrat M. Ziganshin. 2023. "Magnetite Nanoparticles and Carbon Nanotubes for Improving the Operation of Mesophilic Anaerobic Digesters" Microorganisms 11, no. 4: 938. https://doi.org/10.3390/microorganisms11040938




