Ligilactobacillus murinus Strains Isolated from Mice Intestinal Tract: Molecular Characterization and Antagonistic Activity against Food-Borne Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Selection of LAB
2.2. Strain Handling
2.3. Microbiological Tests
2.4. Molecular Identification
2.4.1. Genomic DNA Extraction
2.4.2. Primer Sequences Design
2.4.3. PCR Conditions
2.4.4. Sequence Analysis and Identity Percentage Matrix
2.4.5. GenBank Submission
2.4.6. Phylogenetic Tree Building
2.5. Antagonistic Activity by Agar Diffusion Tests
2.6. Bacterial Growth Parameters
2.7. Supernatant Bacteriolytic Activity
2.7.1. Obtention of Supernatants Culture
2.7.2. Protein Quantification
2.7.3. Electrophoretic Profile of Supernatants Proteins (SDS-PAGE)
2.7.4. Zymograms
2.7.5. UPLC-MS Fingerprinting of L. murinus B1 Lytic Proteins
2.8. Statistical Analysis
3. Results
3.1. LAB Isolation and Microbiological Tests
3.2. Molecular Identification
3.2.1. Identity Percentage Matrix
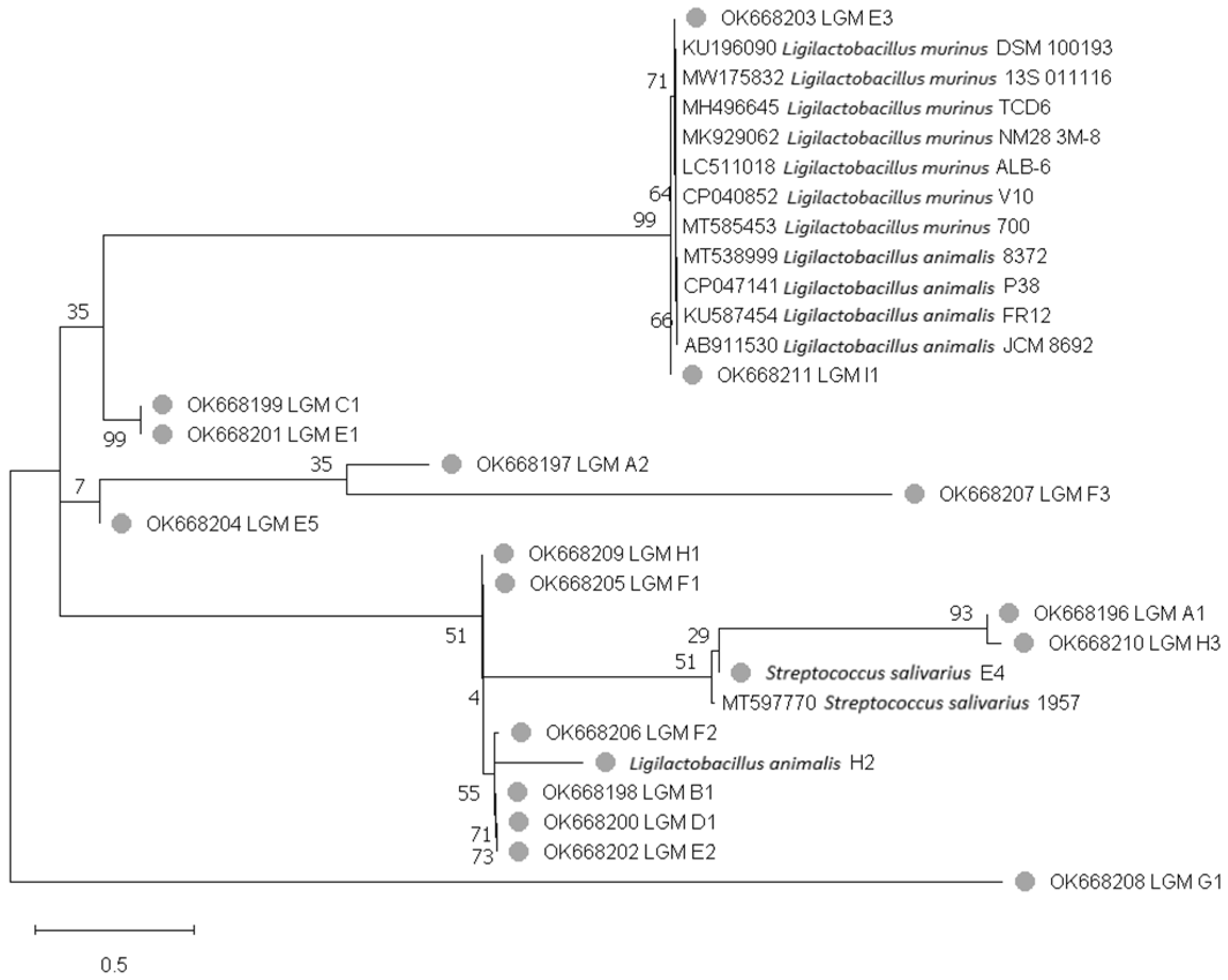
3.2.2. Phylogenetic Tree
3.3. Bacterial Growth Parameters
3.4. Agar Diffusion Tests
3.5. SDS-PAGE and Zymograms
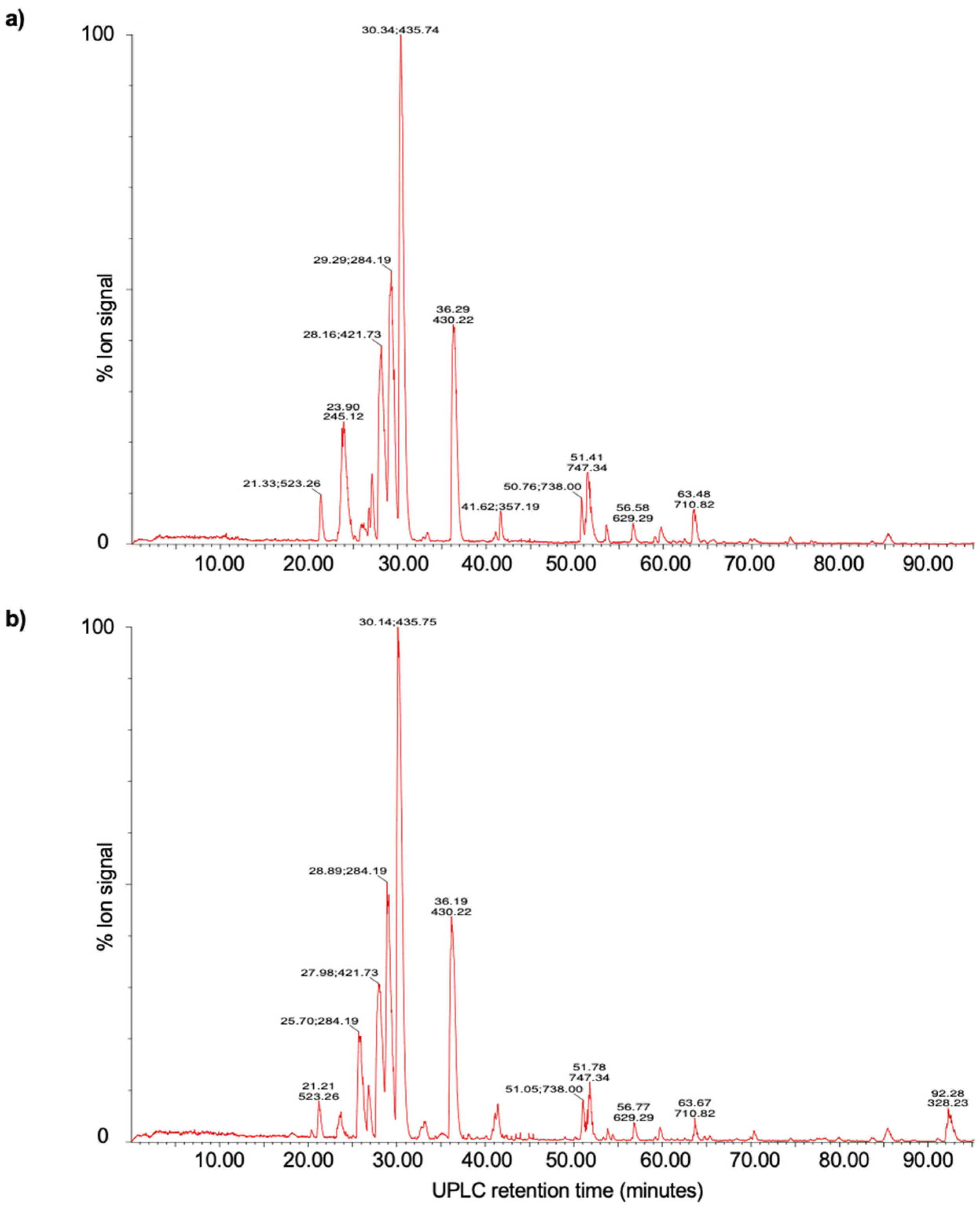
3.6. UPLC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organization for Animal Health. Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic DISEASES in Countries. WHO 2019. Available online: https://apps.who.int/iris/handle/10665/325620 (accessed on 2 November 2022).
- Oliver-Gonzalez, R.; García-Tovar, C.; Juárez-Mosqueda, L.; Navarro-Garcia, F. Infection of rabbit kidney cells (RK13) by enteropathogenic Escherichia coli as a model to study the dynamics of actin cytoskeleton. Can. J. Microbiol. 2008, 54, 748–757. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Veter. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization PUBLISHES list of BACTERIA for Which New Antibiotics Are Urgently Needed. WHO 2021. Available online: https://www.paho.org/es/noticias/4-3-2021-patogenos-multirresistentes-que-son-prioritarios-para-oms (accessed on 3 March 2022).
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A Review of Significant European Foodborne Outbreaks in the Last Decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Sustainable Development Goals. 17 Goals to Transform Our World. 2015. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 3 March 2022).
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations and World Health Organization; FAO/WHO Working Group: London, ON, Canada, 2002. [Google Scholar]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, B.; Cotter, P.D.; Colin, H.; Ross, R.P. Applications of Lactic Acid Bacteria-Produced Bacteriocins in Biotechnology of Lactic Acid Bacteria: Novel Applications; John Wiley & Sons: New York, NY, USA, 2016; pp. 89–100. [Google Scholar]
- Federal Register. Nisin preparation: Affirmation of GRAS status as a direct human food ingredient. Fed. Regist. 1988, 54, 11247–11251. [Google Scholar]
- Magnusson, J. Antifungical Activity of Lactic Acid Bacteria. Uppsala. Sveriges Lanthbruksuniversitet. Acta Universitatis Agriculturae Sueciae. Agraria 2003, 397. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-31 (accessed on 3 March 2022).
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef]
- Gao, Z.; Daliri, E.B.-M.; Wang, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Inhibitory Effect of Lactic Acid Bacteria on Foodborne Pathogens: A Review. J. Food Prot. 2019, 82, 441–453. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Garcia, E.F.; Araújo, A.D.O.; Magnani, M.; Saarela, M.; de Souza, E.L. In Vitro Characterization of Lactobacillus Strains Isolated from Fruit Processing By-Products as Potential Probiotics. Probiotics Antimicrob. Proteins 2018, 10, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, O.; Maria, C.; Tope, P.; Funmi, O. In vitro Study of Potential Probiotic Lactic Acid Bacteria Isolated from The Gut of Chickens in Abeokuta, Nigeria. Alex. J. Veter. Sci. 2018, 59, 73–84. [Google Scholar] [CrossRef]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef] [Green Version]
- Yanagida, F.; Chen, Y.-S.; Shinohara, T. Searching for bacteriocin-producing lactic acid bacteria in soil. J. Gen. Appl. Microbiol. 2006, 52, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Elayaraja, S.; Annamalai, N.; Mayavu, P.; Balasubramanian, T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014, 4, S305–S311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, M.; Tsujibe, S.; Kiyoshima-Shibata, J.; Watanabe, Y.; Kato-Nagaoka, N.; Shida, K.; Matsumoto, S. Microbiota of the Small Intestine Is Selectively Engulfed by Phagocytes of the Lamina Propria and Peyer’s Patches. PLoS ONE 2016, 11, e0163607. [Google Scholar] [CrossRef] [Green Version]
- Norma Oficial Mexicana. Especificaciones TéCnicas Para la ProduccióN, Cuidado Y Uso de Los Animales de Laboratorio; [NOM-062- ZOO-1999]. 6 December 1999; Diario Oficial de la Federación: Ciudad de México, México, 1999. [Google Scholar]
- MacFaddin, J.F. Biochemical Test for Identification of Medical Bacteria; Medica Panamericana: Madrid, Spain, 2003; pp. 344–353. [Google Scholar]
- Burbano, E.; Sierra, S.; Torres, K.; Mercado, M.; Carrascal, A.; Poutou, R. Rapid DNA extraction and PCR validation for direct detection of Listeria monocytogenes in raw milk. Rev. MVZ Córdoba 2006, 11, 715–724. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Orihuel, A.; Terán, L.; Renaut, J.; Vignolo, G.M.; De Almeida, A.M.; Saavedra, M.L.; Fadda, S. Differential Proteomic Analysis of Lactic Acid Bacteria—Escherichia coli O157:H7 Interaction and Its Contribution to Bioprotection Strategies in Meat. Front. Microbiol. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kotrsová, V.; Dordević, D.; Buňková, L.; Vítězová, M.; Amedei, A. Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. Biomolecules 2019, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Llorente, B.A. Caracterización de la Actividad Antibacteriana de Pediococcus Acidilactici ATCC 8042. Tesis de Doctorado en Ciencias Biológicas. Universidad Nacional Autónoma de México, UNAM. 2008. Available online: http://132.248.9.195/ptd2008/junio/0628529/Index.html (accessed on 3 March 2022).
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; Musacchio, F.; Fortina, M.; Senini, L.; Manachini, P. Autolytic activity and pediocin-induced lysis in Pediococcus acidilactici and Pediococcus pentosaceus strains. J. Appl. Microbiol. 2003, 94, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Valence, F.; Lortal, S. Zymogram and Preliminary Characterization of Lactobacillus helveticus Autolysins. Appl. Environ. Microbiol. 1995, 61, 3391–3399. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.M.; Herrera, D.J.; Dinkova, T.D. Caracterización de patrones de hordeínas en variedades mexicanas de cebada maltera. Tip. Rev. Esp. Cienc. Quím. Biol. 2015, 18, 43–51. [Google Scholar]
- Bos, D.H.; Posada, D. Using models of nucleotide evolution to build phylogenetic trees. Dev. Comp. Immunol. 2005, 29, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Söhngen, C.; Bunk, B.; Podstawka, A.; Gleim, D.; Overmann, J. BacDive the Bacterial Diversity Metadatabase. Nucleic Acids Res. 2014, 42, D592–D599. [Google Scholar] [CrossRef] [Green Version]
- Gunkova, P.I.; Buchilina, A.S.; Maksimiuk, N.N.; Bazarnova, Y.G.; Girel, K.S. Carbohydrate Fermentation Test of Lactic Acid Starter Cultures. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012035. [Google Scholar] [CrossRef]
- Axelsson, L. Lactic Acid Bacteria: Classification and Physiology. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Salmine, S., Wright, A.V., Ouwehand, A., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 1–67. [Google Scholar]
- Singer, J.R.; Blosser, E.G.; Zindl, C.L.; Silberger, D.J.; Conlan, S.; Laufer, V.A.; DiToro, D.; Deming, C.; Kumar, R.; Morrow, C.D.; et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 2019, 25, 1772–1782. [Google Scholar] [CrossRef]
- Perelmuter, K.; Fraga, M.; Zunino, P. In vitro activity of potential probiotic Lactobacillus murinus isolated from the dog. J. Appl. Microbiol. 2008, 104, 1718–1725. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Jimenez-Trigos, E.; Toquet, M.; Barba, M.; Gómez-Martín, Á.; Quereda, J.J.; Bataller, E. Search of antimicrobial lactic acid bacteria from Salmonella-negative dogs. BMC Veter. Res. 2022, 18, 12. [Google Scholar] [CrossRef]
- Park, S.; Itoh, K. Species-specific oligonucleotide probes for the detection and identification of Lactobacillus isolated from mouse faeces. J. Appl. Microbiol. 2005, 99, 51–57. [Google Scholar] [CrossRef]
- Dubois, D.R.; Sabrina, V.; Isabelle, D.; Christopher, M.; Andre, T.; Philippe, T. In Vitro Antagonistic Activity Evaluation of Lactic Acid Bacteria (LAB) Combined with Cellulase Enzyme Against Campylobacter jejuni Growth in Co-Culture. J. Microbiol. Biotechnol. 2011, 21, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, S.; Kergourlay, G.; Rossero, A.; Ferchichi, M.; Prevost, H.; Drider, D.; Manai, M.; Dousset, X. Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. Int. Microbiol. 2011, 14, 103–110. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Martens, E.; Steen, P.E.V.D.; Opdenakker, G. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kumar, S.; Dhakan, D.B.; Sharma, V.K. Prediction of peptidoglycan hydrolases—A new class of antibacterial proteins. BMC Genom. 2016, 17, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, S.; Dellarole, M.; Baxter, N.J.; Rouget, J.-B.; Dimitrov, J.D.; Wang, N.; Fujimoto, Y.; Hounslow, A.M.; Lacroix-Desmazes, S.; Fukase, K.; et al. Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat. Commun. 2014, 5, 4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.U.; de Château, M.; Wikström, M.; Forsén, S.; Drakenberg, T.; Björck, L. Solution structure of the albumin-binding GA module: A versatile bacterial protein domain. J. Mol. Biol. 1997, 266, 859–865. [Google Scholar] [CrossRef]
- Lopez-Arvizu, A.; Rocha-Mendoza, D.; Ponce-Alquicira, E.; García-Cano, I. Characterization of antibacterial activity of a N-acetylmuramoyl-l-alanine amidase produced by Latilactobacillus sakei isolated from salami. World J. Microbiol. Biotechnol. 2021, 37, 65. [Google Scholar] [CrossRef]
- Jayakumar, J.; Kumar, V.; Biswas, L.; Biswas, R. Therapeutic applications of lysostaphin against Staphylococcus aureus. J. Appl. Microbiol. 2021, 131, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- WHO. Campylobacter. 2018. Available online: https://www.who.int/es/news-room/factsheets/detail/campylobacter (accessed on 10 March 2022).
- Farfán-García, A.E.; Ariza-Rojas, S.C.; Vargas-Cárdenas, F.A.; Vargas-Remolina, L.V. Mecanismos de virulencia de Escherichia coli enteropatógena. Rev. Chil. Infectol. 2016, 33, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, R.C.; Foley, S.L.; Harvey, R.B. Editorial for the Special Issue: Foodborne Pathogen Distribution, Ecology, Inactivation, and Methods of Differentiation. Microorganisms 2019, 7, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| LAB Name | PrimerID | Sequence (5′→3′) | Amplicon Size (bp) | Annealing Temp. (°C) |
|---|---|---|---|---|
| Lactobacillus intestinalis | Lbinte-F | GTACAACGAGAAGCGAGCCT | ||
| Lbinte-R | CACATAAGTGGTTAGGCCACC | 213 | 58 | |
| Ligilactobacillus animalis | Lbanim-F | GAGTAACACGTGGGCAACCT | ||
| Lbanim-R | TGTCTCAGTCCCAATGTGGC | 224 | 57 | |
| Limosilactibacillus reuteri | Lbreute-F | AGTCACGGCTAACTACGTGC | ||
| Lbreute-R | TTCGGTTAAGCCGAGTTTCCA | 127 | 57 | |
| Lactobacillus delbrueckii spp. bulgaricus | Lbdelb1-F | CCAAGGCAATGATGCGTAGC | ||
| Lbdelb1-R | TTGCTCCATCAGACTTGCGT | 127 | 56 | |
| Lactobacillus acidophilus | Lbacid-F | ACGTCAAGTCATCATGCCCC | ||
| Lbacid-R | TTAGACGGCTCCTTCCCGAGT | 277 | 59 | |
| Limosilactobacillus fermentum | Lbferm-F | TCTTGCGCCAACCCTAGAGA | ||
| Lbferm-R | GACTCGGTGTTTGGGTGTTACAAAC | 446 | 63 | |
| Lactococcus lactis spp. lactis | Lclact1-F | GAGCGCTGAAGGTTGGTACT | ||
| Lclact1-R | TGTCTCAGTCCCAATGTGGC | 272 | 57 | |
| Lactococcus lactis spp. cremoris | Lclact2-F | GGCGTGCCTAATACATGCAA | ||
| Lclact2-R | CCGTTCGCTGCTCTTCAAAT | 70 | 55 | |
| Lactobacillus gasseri | Lbgass-F | GAGCGAGCTTGCCTAGATGA | ||
| Lbgass-R | CTCTAGACATGCGTCTAGTGTT | 163 | 58 | |
| Ligilactobacillus murinus | Lbmuri-F | AAGAGTTGAGCTGAGCGAACG | ||
| Lbmuri-R | CGTAGAAGTTTGGGCCGTGTTT | 268 | 60 | |
| Lactobacillus crispatus | Lbcris-F | GTACCAAGCCAAAGCAAGAC | ||
| Lbcris-R | GTTTGAAGCCTTTACGTAAGTC | 383 | 55 | |
| Lactiplantibacillus plantarum | Lbplan-F | CCGTTTATGCGGAACACCTA | ||
| Lbplan-R | TCGGGATTACCAAACATCAC | 318 | 55 |
| I1 | H3 | H2 | H1 | G1 | F3 | F2 | F1 | E5 | E4 | E3 | E2 | E1 | D1 | C1 | B1 | A2 | A1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. murinus A1 | 96.0 | 99.0 | 89.8 | 97.5 | 94.2 | 94.0 | 98.0 | 97.0 | 93.2 | 71.4 | 97.0 | 96.5 | 97.5 | 96.5 | 97.5 | 97.0 | 97.5 | 100 |
| L. murinus A2 | 94.6 | 97.0 | 87.9 | 96.0 | 92.7 | 93.5 | 96.0 | 96.5 | 93.1 | 71.0 | 95.1 | 96.0 | 96.0 | 96.0 | 96.0 | 96.5 | 100 | |
| L. murinus B1 | 96.5 | 97.5 | 89.8 | 98.0 | 94.6 | 92.1 | 97.5 | 98.5 | 92.2 | 72.4 | 97.0 | 99.5 | 98.0 | 99.5 | 98.0 | 100 | ||
| L. murinus C1 | 98.0 | 97.5 | 90.7 | 98.0 | 96.0 | 92.1 | 97.5 | 98.5 | 92.7 | 72.0 | 99.0 | 97.5 | 100 | 97.5 | 100 | |||
| L. murinus D1 | 96.0 | 97.0 | 89.8 | 98.5 | 94.6 | 91.6 | 97.0 | 99.0 | 92.2 | 72.4 | 96.5 | 100 | 97.5 | 100 | ||||
| L. murinus E1 | 98.0 | 97.5 | 90.7 | 98.0 | 96.0 | 92.1 | 97.5 | 98.5 | 92.7 | 72.0 | 99.0 | 97.5 | 100 | |||||
| L. murinus E2 | 96.0 | 97.0 | 89.8 | 98.5 | 94.6 | 91.6 | 97.0 | 99.0 | 92.2 | 72.4 | 96.5 | 100 | ||||||
| L. murinus E3 | 99.0 | 97.0 | 91.2 | 97.5 | 97.0 | 91.7 | 97.0 | 97.5 | 92.2 | 71.2 | 100 | |||||||
| S. salivarius E4 | 71.1 | 71.9 | 67.4 | 71.9 | 70.3 | 69.6 | 71.9 | 72.4 | 68.9 | 100 | ||||||||
| L. murinus E5 | 92.2 | 94.1 | 86.6 | 93.6 | 91.3 | 90.2 | 93.2 | 93.1 | 100 | |||||||||
| L. murinus F1 | 97.0 | 97.5 | 90.2 | 99.5 | 95.6 | 92.1 | 97.5 | 100 | ||||||||||
| L. murinus F2 | 98.0 | 99.0 | 90.7 | 98.0 | 95.1 | 93.6 | 100 | |||||||||||
| L. murinus F3 | 91.7 | 94.5 | 87.8 | 92.6 | 89.9 | 100 | ||||||||||||
| L. murinus G1 | 97.0 | 95.1 | 91.2 | 95.6 | 100 | |||||||||||||
| L. murinus H1 | 97.0 | 98.0 | 90.7 | 100 | ||||||||||||||
| L. animalis H2 | 91.2 | 90.7 | 100 | |||||||||||||||
| L. murinus H3 | 97.0 | 100 | ||||||||||||||||
| L. murinus I1 | 100 |
| Isolated LAB | Specific Growth Rate (μ) h−1 | Generation Time (τ) h |
|---|---|---|
| 1. L. murinus A1 | 0.82 ± 0.04 cd | 0.84 ± 0.04 cd |
| 2. L. murinus A2 | 0.91 ± 0.11 bc | 0.77 ± 0.06 cd |
| 3. L. murinus B1 | 0.60 ± 0.02 def | 1.11 ± 0.15 bcd |
| 4. L. murinus C1 | 0.74 ± 0.03 cde | 0.93 ± 0.04 bcd |
| 5. L. murinus D1 | 0.51 ± 0.06 ef | 1.43 ± 0.32 b |
| 6. L. murinus E1 | 0.56 ± 0.09 def | 1.19 ± 0.18 bc |
| 7. L. murinus E2 | 0.78 ± 0.04 cde | 0.88 ± 0.05 bcd |
| 8. L. murinus E3 | 1.25 ± 0.27 a | 0.59 ± 0.14 d |
| 9. S. salivarius E4 | 1.13 ± 0.18 ab | 0.62 ± 0.13 d |
| 10. L. murinus E5 | 0.50 ± 0.06 ef | 1.26 ± 0.23 bc |
| 11. L. murinus F1 | 0.60 ± 0.04 def | 1.15 ± 0.09 bcd |
| 12. L. murinus F2 | 0.79 ± 0.01 cde | 0.91 ± 0.06 bcd |
| 13. L. murinus F3 | 0.54 ± 0.06 def | 1.41 ± 0.14 b |
| 14. L. murinus G1 | 0.72 ± 0.02 cde | 0.93 ± 0.06 bcd |
| 15. L. murinus H1 | 0.50 ± 0.06 ef | 1.43 ± 0.26 b |
| 16. L. animalis H2 | 0.54 ± 0.04 def | 1.28 ± 0.18 bc |
| 17. L. murinus H3 | 0.39 ± 0.05 f | 2.12 ± 0.36 a |
| 18. L. murinus I1 | 0.56 ± 0.06 def | 1.29 ± 0.28 bc |
| Inhibition Diameter (mm) | |||
|---|---|---|---|
| Isolated LAB | L. monocytogenes | E. coli | C. jejuni |
| 1. L. murinus A1 | 15 ± 0.5 ab | 21 ± 1.2 abc | 36 ± 3.7 b |
| 2. L. murinus A2 | 15 ± 0.1 ab | 19 ± 1.6 bc | 42 ± 1.0 ab |
| 3. L. murinus B1 | 15 ± 0.6 ab | 20 ± 1.0 bc | 39 ± 2.6 ab |
| 4. L. murinus C1 | 15 ± 0.6 ab | 20 ± 0.5 bc | 42 ± 2.5 ab |
| 5. L. murinus D1 | 14 ± 0.6 ab | 19 ± 1.5 bc | 41 ± 1.1 ab |
| 6. L. murinus E1 | 14 ± 0.6 ab | 19 ± 1.0 c | 38 ± 2.0 ab |
| 7. L. murinus E2 | 15 ± 1.1 ab | 19 ± 1.5 c | 41 ± 2.3 ab |
| 8. L. murinus E3 | 14 ± 1.1 ab | 18 ± 1.1 c | 41 ± 3.7 ab |
| 9. S. salivarius E4 | 14 ± 0.6 ab | 18 ± 1.1 c | 38 ± 1.5 ab |
| 10. L. murinus E5 | 14 ± 0.0 ab | 18 ± 1.5 c | 40 ± 2.6 ab |
| 11. L. murinus F1 | 15 ± 0.6 ab | 20 ± 1.8 bc | 41 ± 2.0 ab |
| 12. L. murinus F2 | 15 ± 0.6 ab | 20 ± 1.8 bc | 40 ± 2.0 ab |
| 13. L. murinus F3 | 15 ± 0.6 ab | 21 ± 0.5 abc | 41 ± 2.5 ab |
| 14. L. murinus G1 | 14 ± 1.0 ab | 23 ± 1.6 ab | 40 ± 1.5 ab |
| 15. L. murinus H1 | 14 ± 1.0 ab | 19 ± 1.1 bc | 41 ± 3.2 ab |
| 16. L. animalis H2 | 17 ± 0.6 a | 25 ± 1.5 a | 44 ± 1.7 a |
| 17. L. murinus H3 | 13 ± 0.1 b | 20 ± 1.2 bc | 36 ± 2.0 b |
| 18. L. murinus I1 | 14 ± 1.1 ab | 18 ± 1.1 c | 36 ± 2.0 b |
| Time | Sc | Sequence | Prec m/z | z | Prec MW | Theor MW | ΔMass |
|---|---|---|---|---|---|---|---|
| (a) Peptidoglycan hydrolase OS Ligilactobacillus murinus OX 1622: PLGS Score 6997; coverage, 29% | |||||||
| 69.95 | 7.49 | TYPGNVQTFLNNIAGPAQQVAQQR | 872.44 | 3 | 2615.32 | 2615.32 | −1.87 |
| 64.18 | 7.90 | VNNLSSDLIYVGQTLK | 882.47 | 2 | 1763.95 | 1763.95 | −1.33 |
| 42.18 | 7.78 | AGDSLWAIANSHK | 457.23 | 3 | 1369.68 | 1369.68 | −0.36 |
| 72.07 | 7.58 | SLNNLNSDLIFAGQVLK | 923.50 | 2 | 1846.00 | 1846.00 | −2.75 |
| 39.78 | 7.99 | YGVYGTYATAPDYADK | 877.89 | 2 | 1754.78 | 1754.78 | −1.11 |
| 34.11 | 7.58 | ANSANYAIAAQNLR | 738.88 | 2 | 1476.75 | 1476.75 | −0.36 |
| 62.06 | 7.61 | NLNNLSSNLIMPGQVLK | 928.00 | 2 | 1855.00 | 1855.00 | −1.08 |
| 62.06 | 7.63 | NLNNLSSNLIMPGQVLK | 928.00 | 2 | 1855.00 | 1855.00 | −1.08 |
| 28.11 | 7.65 | AGDSLWR | 402.70 | 2 | 804.39 | 804.39 | −1.26 |
| 53.61 | 6.96 | YSSYAESLNGYANVITTR | 1004.9 | 2 | 2008.95 | 2008.95 | −2.34 |
| 76.54 | 6.01 | GAVTTANKPNTQSNTNK | 873.46 | 2 | 1745.92 | 1745.92 | 24.50 |
| 56.39 | 8.52 | NLNNLSSNLIMPGQVLK | 936.00 | 2 | 1871.00 | 1871.00 | −0.09 |
| 49.63 | 7.67 | ALNNLTSDMIYVGQNLK | 955.48 | 2 | 1909.96 | 1909.96 | −1.43 |
| 69.20 | 0.00 | VQTFLNNIAGPAQQVAQQR | 689.36 | 3 | 2066.08 | 2066.08 | 3.36 |
| 28.11 | 0.00 | DSLWR | 676.34 | 1 | 676.34 | 676.34 | −0.85 |
| 28.11 | 0.00 | SLWR | 561.31 | 1 | 561.31 | 561.31 | 0.35 |
| 42.43 | 0.00 | AIANSHK | 370.70 | 2 | 740.40 | 740.40 | 3.84 |
| 28.11 | 0.00 | GDSLWR | 733.36 | 1 | 733.36 | 733.36 | −1.68 |
| 28.12 | 0.00 | AGDSLWR | 393.69 | 2 | 786.38 | 786.38 | −1.73 |
| 42.19 | 0.00 | AGDSLWAIANSHK | 451.22 | 3 | 1351.67 | 1351.67 | −2.99 |
| (b) GA domain-containing protein Fragment Ligilactobacillus salivarius OX 1624: PLGS Score 31.20; coverage, 12% | |||||||
| 49.06 | 3.56 | EPETPVNPSEPGK | 690.83 | 2 | 1380.66 | 1380.66 | 2.87 |
| 53.41 | 3.40 | EPETPVDPSEPEK | 727.34 | 2 | 1453.67 | 1453.67 | 4.37 |
| 53.81 | 4.27 | SPQELDQIFTGNNDTIDK | 678.98 | 3 | 2034.94 | 2034.94 | −6.90 |
| 48.88 | 3.85 | EPETPVDPSESGKEPETPVDPSEPGK | 684.32 | 4 | 2734.29 | 2734.29 | 14.27 |
| 49.06 | 3.56 | EPETPVNPSEPGK | 690.83 | 2 | 1380.66 | 1380.66 | 2.87 |
| 56.78 | 3.77 | IDQMLELTVDQKDNFNK | 684.34 | 3 | 2051.00 | 2051.00 | −1.02 |
| 75.87 | 3.70 | EIYLTGHSLGGYLAEYFAATK | 768.74 | 3 | 2304.21 | 2304.21 | 25.52 |
| 51.72 | 3.92 | ISVEFDPQYEYYKK | 904.94 | 2 | 1808.87 | 1808.87 | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Mosqueda, I.L.; Llorente-Bousquets, A.; Soto, C.; Márquez, C.M.; Fadda, S.; Del Río García, J.C. Ligilactobacillus murinus Strains Isolated from Mice Intestinal Tract: Molecular Characterization and Antagonistic Activity against Food-Borne Pathogens. Microorganisms 2023, 11, 942. https://doi.org/10.3390/microorganisms11040942
Sandoval-Mosqueda IL, Llorente-Bousquets A, Soto C, Márquez CM, Fadda S, Del Río García JC. Ligilactobacillus murinus Strains Isolated from Mice Intestinal Tract: Molecular Characterization and Antagonistic Activity against Food-Borne Pathogens. Microorganisms. 2023; 11(4):942. https://doi.org/10.3390/microorganisms11040942
Chicago/Turabian StyleSandoval-Mosqueda, Ivonne Lizeth, Adriana Llorente-Bousquets, Carlos Soto, Crisóforo Mercado Márquez, Silvina Fadda, and Juan Carlos Del Río García. 2023. "Ligilactobacillus murinus Strains Isolated from Mice Intestinal Tract: Molecular Characterization and Antagonistic Activity against Food-Borne Pathogens" Microorganisms 11, no. 4: 942. https://doi.org/10.3390/microorganisms11040942








