Halobacteria-Based Biofertilizers: A Promising Alternative for Enhancing Soil Fertility and Crop Productivity under Biotic and Abiotic Stresses—A Review
Abstract
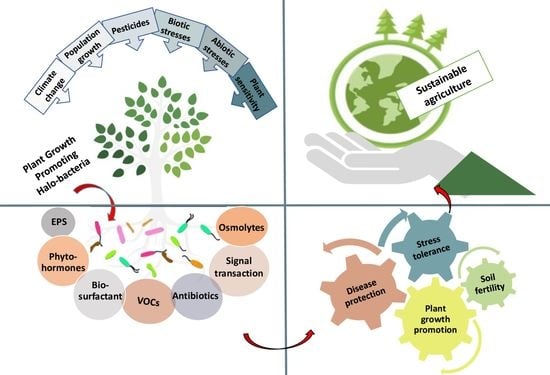
:1. Introduction
2. Challenges Limiting the Sustainability of Conventional Agriculture
2.1. Soil Salinization
2.2. Phytopathogenic Disease
2.3. Plant Sensitivity
3. Increasing the Adaptability of Plants to Stressors Using PGPH
3.1. Mechanisms of Bacteria to Promote Plant Growth
3.2. Utilization of PGPH for Sustainable Agriculture
3.3. The Role of Halobacteria in Promoting Plant Growth Facing Biotic and Abiotic Stress
- (i)
- Supporting the production of non-enzymatic antioxidants such as ascorbate (ASC), glutathione (GSH), tocopherols (TCP), carotenoids (Car), and polyphenols, as well as enzymatic antioxidants such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) by plant antioxidant defense mechanisms (PPs) [66,67].
- (ii)
- (iii)
- Maintaining increased stomatal conductance, boosting photosynthetic processes, and controlling ion transporter activity to improve plant selectivity, maintain the balance of the K+/Na+ ratio, prevent salt and chloride buildup, and promote nutrient uptake of both macro- and micronutrients.
- (iv)
- Generating EPS that plays a crucial part in creating a physical barrier surrounding the roots by binding Na+ cations and inhibiting their accumulation and transfer to higher plant organs in addition to protecting the bacterial cell from stressful situations [11,68,69]. EPS also promotes soil aggregation and enhances soil structure which subsequently improves water retention and plant nutrient availability [59,70].
- (v)
- Producing 1-aminocyclopropane-1-carboxylate (ACC) deaminase, the enzyme responsible for the depletion of plant ethylene levels which are increased in vegetable crops exposed to limiting conditions or pathogen attacks [71].
- (vi)
- Increasing the synthesis of phytohormones such as cytokinins, gibberellins, and auxin (primarily indole-3-acetic acid (IAA)), which affect root architecture and morphology as well as hydraulic conductivity. These root modifications provide the plant with more nutrients and greater flexibility so it can absorb the most soil water possible [72,73].
- (vii)
- (viii)
- Mediating the expression of numerous stress tolerance genes, including up-regulating genes encoding ion-transporter proteins such as malate transporter and ROS-responsive calcium channel proteins involved in cell division, ion homeostasis, and energy metabolism [15,76]. Additionally, it up-regulates the expression of genes responsible for the production of aquaporins, which induce water absorption. It also modifies the expression patterns of certain genes involved in ion homeostasis, including down-regulating the high-affinity K+ transporter (HKT1) and increasing sodium–hydrogen exchanger 2 (NHX2) in order to expel excess amounts of Na+ from cells and improve K+ uptake, thereby enhancing the K+/Na+ ratio when plants are exposed to salt-affected conditions [15,69,77].
- (ix)
- Protecting crops efficiently from disease attack via indirect stimulation, which is related to biocontrol. PGPH produce antimicrobial compounds, chelate the available iron in the rhizosphere to starve phytopathogens, synthesize various extracellular enzymes responsible for the hydrolysis of the fungal cell wall, efficiently colonize the niches within the rhizosphere to exclude pathogens by competing for nutrients and sites on roots, and improve “induced systemic resistance” (ISR) [78].
3.4. Effect of PGPH on Soil Fertility
4. Interaction between PGPH and Soil Microbiota
5. Limitations of Using PGPH in Agriculture
6. Recommendations for Future Research
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef]
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Keesstra, S.; Mol, G.; De Leeuw, J.; Okx, J.; Molenaar, C.; De Cleen, M.; Visser, S. Soil-Related Sustainable Development Goals: Four Concepts to Make Land Degradation Neutrality and Restoration Work. Land 2018, 7, 133. [Google Scholar] [CrossRef]
- Keesstra, S.; Sannigrahi, S.; López-Vicente, M.; Pulido, M.; Novara, A.; Visser, S.; Kalantari, Z. The Role of Soils in Regulation and Provision of Blue and Green Water. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200175. [Google Scholar] [CrossRef]
- Chaparro-Encinas, L.A.; Santoyo, G.; Peña-Cabriales, J.J.; Castro-Espinoza, L.; Parra-Cota, F.I.; Santos-Villalobos, S.d.l. Transcriptional Regulation of Metabolic and Cellular Processes in Durum Wheat (Triticum Turgidum subsp. Durum) in the Face of Temperature Increasing. Plants 2021, 10, 2792. [Google Scholar] [CrossRef]
- Chouhan, G.K.; Verma, J.P.; Jaiswal, D.K.; Mukherjee, A.; Singh, S.; de Araujo Pereira, A.P.; Liu, H.; Abd_Allah, E.F.; Singh, B.K. Phytomicrobiome for Promoting Sustainable Agriculture and Food Security: Opportunities, Challenges, and Solutions. Microbiol. Res. 2021, 248, 126763. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Martínez, A.C.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Beneficial Microorganisms in Sustainable Agriculture: Harnessing Microbes’ Potential to Help Feed the World. Plants 2022, 11, 372. [Google Scholar] [CrossRef]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, Z.; Cerdà, A. The Superior Effect of Nature Based Solutions in Land Management for Enhancing Ecosystem Services. Sci. Total Environ. 2018, 610–611, 997–1009. [Google Scholar] [CrossRef]
- Masmoudi, F.; Abdelmalek, N.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Abiotic Stress Resistance, Plant Growth Promotion and Antifungal Potential of Halotolerant Bacteria from a Tunisian Solar Saltern. Microbiol. Res. 2019, 229, 126331. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Rabhi, N.E.H.; Cherif-Silini, H.; Silini, A.; Alenezi, F.N.; Chenari Bouket, A.; Oszako, T.; Belbahri, L. Alleviation of Salt Stress via Habitat-Adapted Symbiosis. Forests 2022, 13, 586. [Google Scholar] [CrossRef]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An Overview on Improvement of Crop Productivity in Saline Soils by Halotolerant and Halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef]
- Hernández-Canseco, J.; Bautista-Cruz, A.; Sánchez-Mendoza, S.; Aquino-Bolaños, T.; Sánchez-Medina, P.S. Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils. Agronomy 2022, 12, 804. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-Tolerant Plant Growth Promoting Rhizobacteria for Improving Productivity and Remediation of Saline Soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A. Developments in Soil Salinity Assessment, Modeling, Mapping, and Monitoring from Regional to Submicroscopic Scales. In Developments in Soil Salinity Assessment and Reclamation: Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Shahid, S.A., Abdelfattah, M.A., Taha, F.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 3–43. ISBN 978-94-007-5684-7. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; Food & Agriculture Organization: Rome, Italy, 1988; ISBN 978-92-5-102686-1. [Google Scholar]
- Yadav, S.; Irfan, M.; Ahmad, A.; Hayat, S. Causes of Salinity and Plant Manifestations to Salt Stress: A Review. J. Environ. Biol. 2011, 32, 667–685. [Google Scholar] [PubMed]
- Pessarakli, M. Formation of Saline and Sodic Soils and Their Reclamation. J. Environ. Sci Health A 1991, 26, 1303–1320. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.; Cruz, C. Current Advances in Plant Growth Promoting Bacteria Alleviating Salt Stress for Sustainable Agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- FAO. Status of the World’s Soil Resources: Main Report; FAO; ITPS: Rome, Italy, 2015; ISBN 978-92-5-109004-6. [Google Scholar]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water Resources: Agricultural and Environmental Issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Poss, J.A.; Grattan, S.R.; Grieve, C.M.; Suarez, D. Bacterial Diversity in Cucumber (Cucumis sativus) Rhizosphere in Response to Salinity, Soil PH, and Boron. Soil Biol. Biochem. 2010, 42, 567–575. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in Phytopathogenic Bacteria: Do We Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef]
- John, E.; Singh, K.B.; Oliver, R.P.; Tan, K.-C. Transcription Factor Control of Virulence in Phytopathogenic Fungi. Mol. Plant Pathol. 2021, 22, 858–881. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The Use of Plants to Protect Plants and Food against Fungal Pathogens: A Review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and How to Kill a Plant Cell: Infection Strategies of Plant Pathogenic Fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Randhawa, M.A.; Saeed, F.; Waqas, K. Aflatoxins: Biosynthesis, Occurrence, Toxicity, and Remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qin, T.; Liu, H.; Wu, M.; Li, J.; Shi, Y.; Gao, Y.; Ren, A. Endophytic Fungi Activated Similar Defense Strategies of Achnatherum sibiricum Host to Different Trophic Types of Pathogens. Front. Microbiol. 2020, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Coque, J.J.R.; Álvarez-Pérez, J.M.; Cobos, R.; González-García, S.; Ibáñez, A.M.; Diez Galán, A.; Calvo-Peña, C. Chapter Four—Advances in the Control of Phytopathogenic Fungi That Infect Crops through Their Root System. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volumn 111, pp. 123–170. [Google Scholar]
- De Silva, N.I.; Lumyong, S.; Hyde, K.D.; Bulgakov, T.; Phillips, A.J.L.; Yan, J.Y. Mycosphere Essays 9: Defining Biotrophs and Hemibiotrophs. Mycosphere 2016, 7, 545–559. [Google Scholar] [CrossRef]
- Dyakov, Y.T.; Zinovyeva, S.V. Chapter 1—Plant Parasite Microorganisms. In Comprehensive and Molecular Phytopathology; Studies in Plant Science; Dyakov, Y.T., Dzhavakhiya, V.G., Korpela, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 19–47. [Google Scholar]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas Putida UW4 Facilitates the Growth of Canola in the Presence of Salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant. Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S.; Cervone, F.; Okun, E. Extracellular DAMPs in Plants and Mammals: Immunity, Tissue Damage and Repair. Trends Immunol. 2018, 39, 937–950. [Google Scholar] [CrossRef]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane Dynamics during Individual and Combined Abiotic Stresses in Plants and Tools to Study the Same. Physiol. Plant 2021, 171, 653–676. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Tippmann, H.F.; Schlüter, U.; Collinge, D.B. Floriculture, ornamental and plant biotechnology. In Common Themes in Biotic and Abiotic Stress Signalling in Plants; Teixeira da Silva, J.A., Ed.; Global Science Books: Middlesex, UK; Kagawa, Japan, 2006; pp. 52–67. ISBN 4903313069. [Google Scholar]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.F.; van der Linden, G. Plant Behaviour under Combined Stress: Tomato Responses to Combined Salinity and Pathogen Stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef]
- Fan, F.; Hamada, M.S.; Li, N.; Li, G.Q.; Luo, C.X. Multiple Fungicide Resistance in Botrytis cinerea from Greenhouse Strawberries in Hubei Province, China. Plant. Dis. 2017, 101, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Yoon, M.-Y.; Cha, B.; Kim, J.-C. Recent Trends in Studies on Botanical Fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, e963401. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Masmoudi, F.; Ben Khedher, S.; Kamoun, A.; Zouari, N.; Tounsi, S.; Trigui, M. Combinatorial Effect of Mutagenesis and Medium Component Optimization on Bacillus amyloliquefaciens Antifungal Activity and Efficacy in Eradicating Botrytis cinerea. Microbiol. Res. 2017, 197, 29–38. [Google Scholar] [CrossRef]
- Zouari, I.; Masmoudi, F.; Medhioub, K.; Tounsi, S.; Trigui, M. Biocontrol and Plant Growth-Promoting Potentiality of Bacteria Isolated from Compost Extract. Antonie Van Leeuwenhoek 2020, 113, 2107–2122. [Google Scholar] [CrossRef]
- Timmusk, S.; Paalme, V.; Pavlicek, T.; Bergquist, J.; Vangala, A.; Danilas, T.; Nevo, E. Bacterial Distribution in the Rhizosphere of Wild Barley under Contrasting Microclimates. PLoS ONE 2011, 6, e17968. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Larsen, H. Halophilic and Halotolerant Microorganisms—An Overview and Historical Perspective. FEMS Microbiol. Rev. 1986, 2, 3–7. [Google Scholar] [CrossRef]
- Ruppel, S.; Franken, P.; Witzel, K.; Ruppel, S.; Franken, P.; Witzel, K. Properties of the Halophyte Microbiome and Their Implications for Plant Salt Tolerance. Funct. Plant Biol. 2013, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Endophytic Halotolerant Bacillus Velezensis FMH2 Alleviates Salt Stress on Tomato Plants by Improving Plant Growth and Altering Physiological and Antioxidant Responses. Plant. Physiol. Biochem. 2021, 165, 217–227. [Google Scholar] [CrossRef]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Halotolerant Bacillus Spizizenii FMH45 Promoting Growth, Physiological, and Antioxidant Parameters of Tomato Plants Exposed to Salt Stress. Plant Cell Rep. 2021, 40, 1199–1213. [Google Scholar] [CrossRef]
- Litchfield, C.D. Survival Strategies for Microorganisms in Hypersaline Environments and Their Relevance to Life on Early Mars. Meteorit. Planet. Sci. 1998, 33, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary Metabolites From Halotolerant Plant Growth Promoting Rhizobacteria for Ameliorating Salinity Stress in Plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Złoch, M.; Ruppel, S.; Hrynkiewicz, K. Metabolic Potential and Community Structure of Endophytic and Rhizosphere Bacteria Associated with the Roots of the Halophyte Aster tripolium L. Microbiol. Res. 2016, 182, 68–79. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial Amelioration of Crop Salinity Stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-Based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Cheng, C.; Shang-Guan, W.; He, L.; Sheng, X. Effect of Exopolysaccharide-Producing Bacteria on Water-Stable Macro-Aggregate Formation in Soil. Geomicrobiol. J. 2020, 37, 738–745. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant. Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Nabti, E.; Schmid, M.; Hartmann, A. Application of Halotolerant Bacteria to Restore Plant Growth Under Salt Stress. In Halophiles: Biodiversity and Sustainable Exploitation; Sustainable Development and, Biodiversity; Maheshwari, D.K., Saraf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–259. ISBN 978-3-319-14595-2. [Google Scholar]
- Farag, M.A.; Zhang, H.; Ryu, C.-M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Arora, S. Halotolerant Microbes for Amelioration of Salt-Affected Soils for Sustainable Agriculture. In Phyto-Microbiome in Stress Regulation; Environmental and Microbial, Biotechnology; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 323–343. ISBN 9789811525766. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive Oxygen Species-Mediated Signaling during Abiotic Stress. Plant. Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Nadeem, S.M.; Khan, M.Y.; Binyamin, R.; Waqas, M.R. Role of Halotolerant Microbes in Plant Growth Promotion Under Salt Stress Conditions. In Saline Soil-based Agriculture by Halotolerant Microorganisms; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Singapore, 2019; pp. 209–253. ISBN 9789811383359. [Google Scholar]
- Ul Hassan, T.; Bano, A. Construction of IAA-Deficient Mutants of Pseudomonas Moraviensis and Their Comparative Effects with Wild Type Strains as Bio-Inoculant on Wheat in Saline Sodic Soil. Geomicrobiol. J. 2019, 36, 376–384. [Google Scholar] [CrossRef]
- Parak, H.-G.; Jeong, M.-H.; Ahn, Y.-S. Inoculation with Bacillus Licheniformis MH48 to Improve Camellia Japonicaseedling Development in Coastal Lands. Turk. J. Agric. 2017, 41, 381–388. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Wang, W.; Tian, S.; Jiang, P.; Qi, Q.; Li, F.; Li, H.; Wang, Q.; Li, H.; et al. Potential Biodegradation of Phenanthrene by Isolated Halotolerant Bacterial Strains from Petroleum Oil Polluted Soil in Yellow River Delta. Sci. Total Environ. 2019, 664, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Feizi, R.; Jorfi, S.; Takdastan, A. Bioremediation of Phenanthrene-Polluted Soil Using Bacillus Kochii AHV-KH14 as a Halo-Tolerant Strain Isolated from Compost. Environ. Eng. Manag. J. 2020, 7, 23–30. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z. The Production of Exopolysaccharide by Pseudomonas Putida GAP-P45 under Various Abiotic Stress Conditions and Its Role in Soil Aggregation. Microbiology 2015, 84, 512–519. [Google Scholar] [CrossRef]
- Anees, M.; Qayyum, A.; Jamil, M.; Rehman, F.U.; Abid, M.; Malik, M.S.; Yunas, M.; Ullah, K. Role of Halotolerant and Chitinolytic Bacteria in Phytoremediation of Saline Soil Using Spinach Plant. Inter. J. Phytoremediat. 2020, 22, 653–661. [Google Scholar] [CrossRef]
- Orji, O.U.; Awoke, J.N.; Aja, P.M.; Aloke, C.; Obasi, O.D.; Alum, E.U.; Udu-Ibiam, O.E.; Oka, G.O. Halotolerant and Metalotolerant Bacteria Strains with Heavy Metals Biorestoration Possibilities Isolated from Uburu Salt Lake, Southeastern, Nigeria. Heliyon 2021, 7, e07512. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Konnova, S.A.; Sigida, E.N.; Lyubun, E.V.; Muratova, A.Y.; Fedonenko, Y.P.; Elbanna, K. Bioremediation Potential of a Halophilic Halobacillus sp. Strain, EG1HP4QL: Exopolysaccharide Production, Crude Oil Degradation, and Heavy Metal Tolerance. Extremophiles 2020, 24, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.R.; Rathore, A.P.; Sharma, S. Effect of Halotolerant Plant Growth Promoting Rhizobacteria Inoculation on Soil Microbial Community Structure and Nutrients. Appl. Soil Ecol. 2020, 150, 103461. [Google Scholar] [CrossRef]
- Mukhtar, S.; Zareen, M.; Khaliq, Z.; Mehnaz, S.; Malik, K.A. Phylogenetic Analysis of Halophyte-associated Rhizobacteria and Effect of Halotolerant and Halophilic Phosphate-solubilizing Biofertilizers on Maize Growth under Salinity Stress Conditions. J. Appl. Microbiol. 2020, 128, 556–573. [Google Scholar] [CrossRef]
- Won, S.J.; Kwon, J.H.; Kim, D.H.; Ahn, Y.S. The Effect of Bacillus Licheniformis MH48 on Control of Foliar Fungal Diseases and Growth Promotion of Camellia Oleifera Seedlings in the Coastal Reclaimed Land of Korea. Pathogens 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Al-Enazy, A.-A.; Al-Barakah, F.; Al-Oud, S.; Usman, A. Effect of Phosphogypsum Application and Bacteria Co-Inoculation on Biochemical Properties and Nutrient Availability to Maize Plants in a Saline Soil. Arch. Agron. Soil Sci. 2018, 64, 1394–1406. [Google Scholar] [CrossRef]
- UI Hassan, T.; Bano, A.; Naz, I. Halophyte Root Powder: An Alternative Biofertilizer and Carrier for Saline Land. Soil Sci. Plant. Nutr. 2018, 64, 653–661. [Google Scholar] [CrossRef]
- Pankaj, U.; Singh, D.N.; Mishra, P.; Gaur, P.; Babu, C.S.V.; Shanker, K.; Verma, R.K. Autochthonous Halotolerant Plant Growth-Promoting Rhizobacteria Promote Bacoside A Yield of Bacopa monnieri (L.) Nash and Phytoextraction of Salt-Affected Soil. Pedosphere 2020, 30, 671–683. [Google Scholar] [CrossRef]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’ Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Lee, S.-J.; Kim, S.-H.; Park, I.-H.; Lee, Y.-S.; Chung, S.-Y.; Choi, Y.-L. Characterization of New Biosurfactant Produced by Klebsiella Sp. Y6-1 Isolated from Waste Soybean Oil. Bioresour. Technol. 2008, 99, 2288–2292. [Google Scholar] [CrossRef]
- Nikolopoulou, M.; Pasadakis, N.; Norf, H.; Kalogerakis, N. Enhanced Ex Situ Bioremediation of Crude Oil Contaminated Beach Sand by Supplementation with Nutrients and Rhamnolipids. Mar. Pollut. Bull. 2013, 77, 37–44. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, Z.; Bai, Z.; Zhuang, G.; Shim, H. Progress in Decontamination by Halophilic Microorganisms in Saline Wastewater and Soil. Environ. Pollut. 2010, 158, 1119–1126. [Google Scholar] [CrossRef]
- Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review. Appl. Sci. 2018, 8, 1336. [Google Scholar] [CrossRef]
- Rahman, Z. An Overview on Heavy Metal Resistant Microorganisms for Simultaneous Treatment of Multiple Chemical Pollutants at Co-Contaminated Sites, and Their Multipurpose Application. J. Hazard. Mater. 2020, 396, 122682. [Google Scholar] [CrossRef]
- Bachate, S.P.; Nandre, V.S.; Ghatpande, N.S.; Kodam, K.M. Simultaneous Reduction of Cr(VI) and Oxidation of As(III) by Bacillus Firmus TE7 Isolated from Tannery Effluent. Chemosphere 2013, 90, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, B.; Li, S.; Yang, M.; Yin, C. Simultaneous Microbial Reduction of Vanadium (V) and Chromium (VI) by Shewanella Loihica PV-4. Bioresour. Technol. 2017, 227, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Tirry, N.; Kouchou, A.; Laghmari, G.; Lemjereb, M.; Hnadi, H.; Amrani, K.; Bahafid, W.; El Ghachtouli, N. Improved Salinity Tolerance of Medicago Sativa and Soil Enzyme Activities by PGPR. Biocatal. Agric. Biotechnol. 2021, 31, 101914. [Google Scholar] [CrossRef]
- Di Salvo, L.P.; Cellucci, G.C.; Carlino, M.E.; García de Salamone, I.E. Plant Growth-Promoting Rhizobacteria Inoculation and Nitrogen Fertilization Increase Maize (Zea Mays L.) Grain Yield and Modified Rhizosphere Microbial Communities. Appl. Soil Ecol. 2018, 126, 113–120. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of Bio-Fertilizer Containing Beneficial Fungi and Rhizospheric Bacteria on Health Promoting Compounds and Antioxidant Activity of Spinacia Oleracea L. Bot. Stud. 2017, 58, 35. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, M. Biosynthesis of Amino Acid Functionalized Silver Nanoparticles for Potential Catalytic and Oxygen Sensing Applications. Inorg. Chem. Front. 2018, 5, 233–257. [Google Scholar] [CrossRef]
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Oszako, T.; Sikora, K.; Szmidla, H.; Hilszczańska, D. The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus Sylvatica and Quercus Robur Seedlings in a Bare-Root Nursery Experiment. Forests 2018, 9, 597. [Google Scholar] [CrossRef]
- Deng, S.; Wipf, H.M.-L.; Pierroz, G.; Raab, T.K.; Khanna, R.; Coleman-Derr, D. A Plant Growth-Promoting Microbial Soil Amendment Dynamically Alters the Strawberry Root Bacterial Microbiome. Sci. Rep. 2019, 9, 17677. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of Phosphate Solubilizing Microorganisms in Sustainable Agriculture—A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 551–570. ISBN 978-90-481-2666-8. [Google Scholar]


| PGPH | Role in Soil Bioremediation | Type of Affected Soil | Bioremediation Mechanisms | References |
|---|---|---|---|---|
| B. cereus P. moraviensis S. maltophilia | Improvement in P, NO3−, N, and K contents. | Saline sodic soils | Phosphate solubilization Atmospheric N2 fixation | [80] |
| B. licheniformis MH48 | Increase in P contents | Saline soils | Phosphate solubilization | [81] |
| Delftia sp. Achromobacter sp. B. kochii AHV-KH14 | Phenanthrene degradation | Oil-contaminated and saline soils | Biodegradation | [82] [83] |
| P. putida GAP-P45 | Soil aggregation. Aggregate stability | Dry and saline soils | EPS production | [84] |
| Pseudomonas sp. Thalassobacillus sp. Terribacillus sp. | Decline in Na contents. Increase in Ca2+, Mg2+ and organic matter levels. | Saline soils | Salt leaching | [85] |
| K. pneumoniae USL2S P. putida USL4W P. putida USL5W | Decrease in Hg, Pb, Cd, Ni, Cu, and Zn contents. | Acidic, heavy-metal-, and salt-contaminated soils | Bioremoval capacity | [86] |
| Halobacillus sp. EG1HP4QL | Removal of paraffins, naphthenes, mono- and bicyclic aromatic hydrocarbons, polycyclic aromatic hydrocarbons, and alcohol–benzene resins. | Oil-contaminated soils | Enzymatic activity | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masmoudi, F.; Alsafran, M.; Jabri, H.A.; Hosseini, H.; Trigui, M.; Sayadi, S.; Tounsi, S.; Saadaoui, I. Halobacteria-Based Biofertilizers: A Promising Alternative for Enhancing Soil Fertility and Crop Productivity under Biotic and Abiotic Stresses—A Review. Microorganisms 2023, 11, 1248. https://doi.org/10.3390/microorganisms11051248
Masmoudi F, Alsafran M, Jabri HA, Hosseini H, Trigui M, Sayadi S, Tounsi S, Saadaoui I. Halobacteria-Based Biofertilizers: A Promising Alternative for Enhancing Soil Fertility and Crop Productivity under Biotic and Abiotic Stresses—A Review. Microorganisms. 2023; 11(5):1248. https://doi.org/10.3390/microorganisms11051248
Chicago/Turabian StyleMasmoudi, Fatma, Mohammed Alsafran, Hareb AL Jabri, Hoda Hosseini, Mohammed Trigui, Sami Sayadi, Slim Tounsi, and Imen Saadaoui. 2023. "Halobacteria-Based Biofertilizers: A Promising Alternative for Enhancing Soil Fertility and Crop Productivity under Biotic and Abiotic Stresses—A Review" Microorganisms 11, no. 5: 1248. https://doi.org/10.3390/microorganisms11051248