Risk Factors and the Impact of Multidrug-Resistant Bacteria on Community-Acquired Urinary Sepsis
Abstract
:1. Introduction
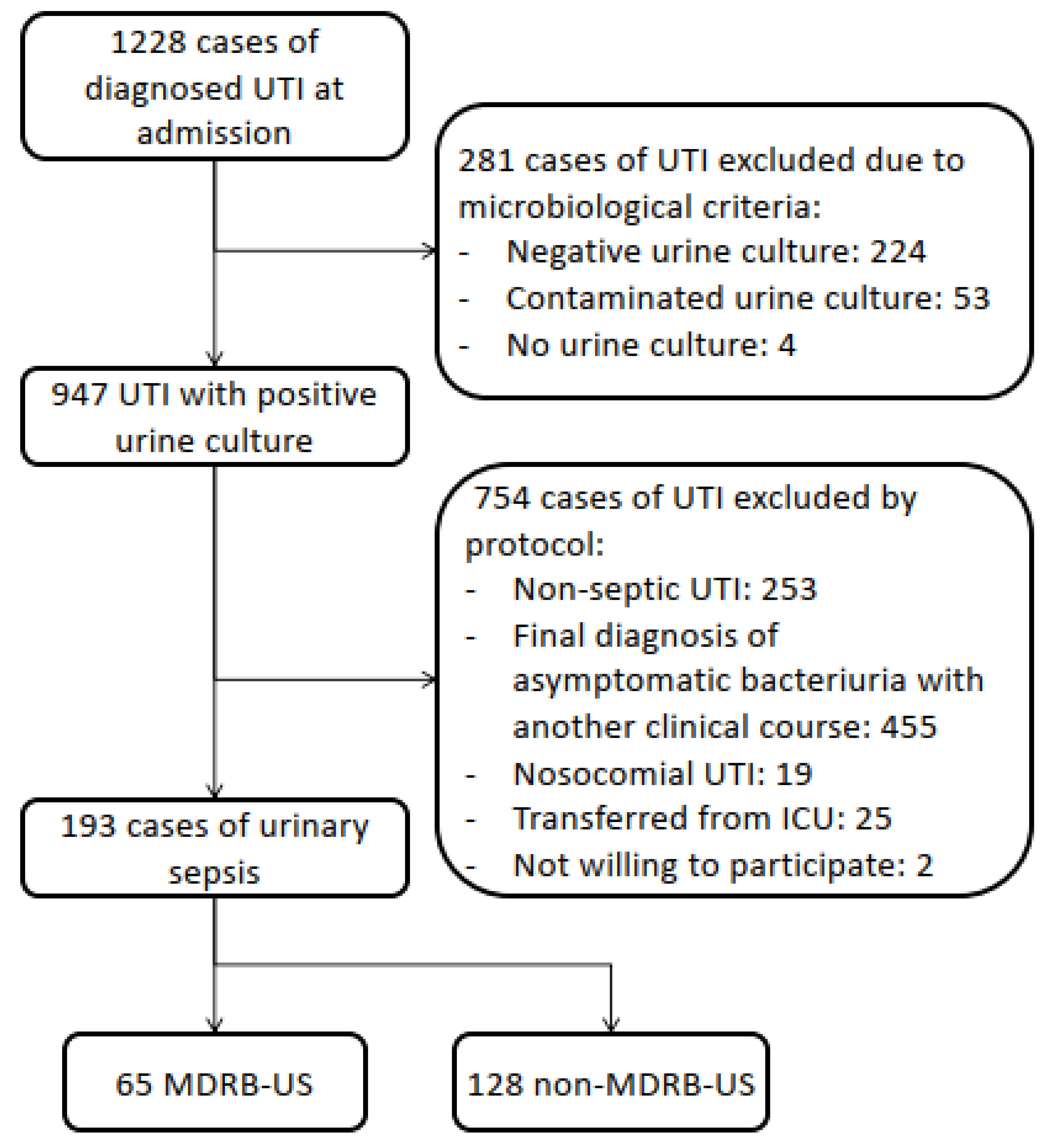
2. Material and Methods
2.1. Study Design and Patients
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kadri, S.S.; Lai, Y.L.; Warner, S.; Strich, J.R.; Babiker, A.; Ricotta, E.E.; Demirkale, C.; Dekker, J.; Palmore, T.; Rhee, C.; et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: A retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect. Dis. 2021, 21, 241–251. [Google Scholar] [CrossRef]
- Tabak, Y.P.; Sung, A.H.; Ye, G.; Vankeepuram, L.; Gupta, V.; McCann, E. Attributable clinical and economic burden of carbapenem-non-susceptible Gram-negative infections in patients hospitalized with complicated urinary tract infections. J. Hosp. Infect. 2019, 102, 37–44. [Google Scholar] [CrossRef]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. N. Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am 2020, 34, 709–722. [Google Scholar] [CrossRef]
- From the Federal Task Force on Combating Antibiotic-Resistant Bacteria 2 National Action Plan for Combating Antibiotic-Resistant Bacteria. 2020. Available online: https://www.hhs.gov/sites/default/files/carb-national-action-plan-2020-2025.pdf (accessed on 1 May 2023).
- Palacios-ceña, D.; Florencio, L.L.; Hernández-barrera, V.; Fernandez-de-las-peñas, C.; de Miguel-Diez, J.; Martínez-hernández, D.; Carabantes-Alarcón, D.; Jimenez-García, R.; Lopez-de-Andres, A.; Lopez-Herranz, M. Trends in incidence and outcomes of hospitalizations for urinary tract infection among older people in spain (2001–2018). J. Clin. Med. 2021, 10, 2332. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Haberling, D.L.; Huang, C.C.; Lessa, F.C.; Lucero, D.E.; Daskalakis, D.C.; Vora, N. Infectious Disease Hospitalizations: United States, 2001 to 2014. Chest 2019, 156, 255–268. [Google Scholar] [CrossRef]
- Clifford, K.M.; Grelle, J.L.; Tawwater, J.C.; Ramanathan, M.; Duncan, N. Management of Extended-Spectrum Beta-Lactamase Urinary Tract Infections: Diagnostic and Treatment Considerations for the Older Adult. Sr. Care Pharm. 2019, 34, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, 14. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.; Walter, T.; Gerigk, M.; Ebert, M.; Vogelmann, R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect. Dis. 2018, 18, 56. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Multiple antimicrobial resistance and outcomes among hospitalized patients with complicated urinary tract infections in the US, 2013–2018: A retrospective cohort study. BMC Infect. Dis. 2021, 21, 159. [Google Scholar] [CrossRef]
- Madrazo, M.; Esparcia, A.; López-Cruz, I.; Alberola, J.; Piles, L.; Viana, A.; Eiros, J.M.; Artero, A. Clinical impact of multidrug-resistant bacteria in older hospitalized patients with community-acquired urinary tract infection. BMC Infect. Dis. 2021, 21, 1232. [Google Scholar] [CrossRef]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, L.; Vaux, S.; Maugat, S.; Blake, A.; Barlier, R.; Heym, B.; Strat, Y.; Blanchon, T.; Hanslik, T.; Coignard, B. Incidence of urinary tract infections and antibiotic resistance in the outpatient setting: A cross-sectional study. Infection 2017, 45, 33–40. [Google Scholar] [CrossRef]
- Naziri, Z.; Derakhshandeh, A.; Borchaloee, A.S.; Poormaleknia, M.; Azimzadeh, N. Treatment failure in urinary tract infections: A warning witness for virulent multi-drug resistant ESBL-producing Escherichia coli. Infect Drug Resist. 2020, 13, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.; Sievert, D. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Mantengoli, E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2005, 11, 17–32. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Annual Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007 for the National Healthcare Safety Network Team and Participating National Healthcare Safety Network Facilities. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [PubMed]
- Gomila, A.; Shaw, E.; Carratalà, J.; Leibovici, L.; Tebé, C.; Wiegand, I.; Vallejo-Torres, L.; Vigo, J.; Morris, S.; Stoddart, M.; et al. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections. Antimicrob. Resist. Infect. Control. 2018, 7, 111. [Google Scholar] [CrossRef]
- Capsoni, N.; Bellone, P.; Aliberti, S.; Sotgiu, G.; Pavanello, D.; Visintin, B.; Callisto, E.; Saderi, L.; Soldini, D.; Lardera, L.; et al. Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip. Respir. Med. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, K.; Saito, T.; Iwamoto, T. Risk factors for nursing- and healthcare-associated urinary tract infection. Geriatr. Gerontol. Int. 2018, 18, 1183–1188. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Clsi. M100-S24 Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement An informational Supplement for Global Application Developed through the Clinical and Laboratory Standards Institute Consensus Process [Internet]. 2014. Available online: www.clsi.org (accessed on 1 May 2023).
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA-J. Am. Med. Assoc. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA-J. Am. Med. Assoc. 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; Mcgarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health Care-Associated Bloodstream Infections in Adults: A Reason To Change the Accepted Definition of Community-Acquired Infections Background: Bloodstream Infections Occurring in Persons Resid [Internet]. 2002. Available online: https://annals.org (accessed on 1 May 2023).
- Artero, A.; Esparcia, A.; Eiros, J.M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Effect of Bacteremia in Elderly Patients With Urinary Tract Infection. Am. J. Med. Sci. 2016, 352, 267–271. [Google Scholar] [CrossRef]
- Holmbom, M.; Andersson, M.; Grabe, M.; Peeker, R.; Saudi, A.; Styrke, J.; Aljabery, F. Community-onset urosepsis: Incidence and risk factors for 30-day mortality—A retrospective cohort study. Scand. J. Urol. 2022, 56, 414–420. [Google Scholar] [CrossRef]
- Busani, S.; Serafini, G.; Mantovani, E.; Venturelli, C.; Giannella, M.; Viale, P.; Mussini, C.; Cossarizza, A.; Girardis, M. Mortality in Patients With Septic Shock by Multidrug Resistant Bacteria: Risk Factors and Impact of Sepsis Treatments. J. Intensive Care Med. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Lambregts, M.M.C.; Wijnakker, R.; Bernards, A.T.; Visser, L.G.; Le Cessie, S.; de Boer, M.G.J. Mortality after delay of adequate empiric antimicrobial treatment of bloodstream infection. J. Clin. Med. 2020, 9, 1378. [Google Scholar] [CrossRef]
- Esparcia, A.; Artero, A.; Eiros, J.M.; Balaguer, M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. Eur. J. Intern. Med. 2014, 25, 523–527. [Google Scholar] [CrossRef]
- Chin, B.S.; Kim, M.S.; Han, S.H.; Shin, S.Y.; Choi, H.K.; Chae, Y.T.; Jin, S.J.; Baek, J.-H.; Choi, J.Y.; Song, Y.G.; et al. Risk factors of all-cause in-hospital mortality among Korean elderly bacteremic urinary tract infection (UTI) patients. Arch. Gerontol. Geriatr. 2011, 52, e50–e55. [Google Scholar] [CrossRef]
- Ryan, J.; McLornan, L.; O’Neill, E. The impact of increasing antimicrobial resistance in the treatment of urosepsis. Ir. J. Med. Sci. 2020, 189, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Gomila, A.; Carratalà, J.; Eliakim-Raz, N.; Shaw, E.; Tebé, C.; Wolkewitz, M.; Wiegand, I.; Grier, S.; Vank, C.; Cuperus, N.; et al. Clinical outcomes of hospitalised patients with catheter-associated urinary tract infection in countries with a high rate of multidrug-resistance: The COMBACTE-MAGNET RESCUING study. Antimicrob. Resist. Infect. Control. 2019, 8, 198. [Google Scholar] [CrossRef]
- Fasugba, O.; Das, A.; Mnatzaganian, G.; Mitchell, B.G.; Collignon, P.; Gardner, A. Incidence of single-drug resistant, multidrug-resistant and extensively drug-resistant Escherichia coli urinary tract infections: An Australian laboratory-based retrospective study. J. Glob. Antimicrob. Resist. 2019, 16, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Faine, B.A.; Harland, K.K.; Porter, B.; Liang, S.Y.; Mohr, N. A Clinical Decision Rule Identifies Risk Factors Associated with Antimicrobial-Resistant Urinary Pathogens in the Emergency Department: A Retrospective Validation Study. Ann. Pharmacother. 2015, 49, 649–655. [Google Scholar] [CrossRef]
- Karve, S.; Ryan, K.; Peeters, P.; Baelen, E.; Rojas-Farreras, S.; Potter, D.; Rodríguez-Baño, J. The impact of initial antibiotic treatment failure: Real-world insights in patients with complicated urinary tract infection. J. Infect. 2018, 76, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, W.; Fletcher, E.; Kavanagh, K.; Deshpande, A.; Wiuff, C.; Marwick, C.; Bennie, M. Risk factors for resistance and MDR in community urine isolates: Population-level analysis using the NHS Scotland Infection Intelligence Platform. J. Antimicrob. Chemother. 2018, 73, 223–230. [Google Scholar] [CrossRef]
- Smithson, A.; Ramos, J.; Niño, E.; Culla, A.; Pertierra, U.; Friscia, M.; Bastida, M.T. Characteristics of febrile urinary tract infections in older male adults. BMC Geriatr. 2019, 19, 334. [Google Scholar] [CrossRef]
- Faine, B.A.; Mohr, N.; Vakkalanka, P.; Gao, A.S.; Liang, S.Y. Validation of a Clinical Decision Rule to Identify Risk Factors Associated With Multidrug-Resistant Urinary Pathogens in the Emergency Department. Ann. Pharmacother. 2019, 53, 56–60. [Google Scholar] [CrossRef]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Li, J.T.C.; Fung, K.H. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Peghin, M.; Losito, A.R.; Chirico, L.; Giuliano, G.; Spanu, T.; Sartor, A.; Fiori, B.; Bassetti, M. Characterisation and risk factor profiling of Pseudomonas aeruginosa urinary tract infections: Pinpointing those likely to be caused by multidrug-resistant strains. Int. J. Antimicrob. Agents 2020, 55, 105900. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Hsiao, C.Y.; Hung, M.C.; Hung, S.C.; Wang, H.P.; Huang, Y.J.; Wang, J.-T. Bacteremic Urinary Tract Infection Caused by Multidrug-Resistant Enterobacteriaceae Are Associated with Severe Sepsis at Admission. Medicine 2016, 95, e3694. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Shaw, E.; Padilla, B.; Pintado, V.; Calbo, E.; Benito, N.; Gamallo, R.; Gozalo, M.; Rodríguez-Baño, J. ITUBRAS group; Grupo de Estudio de Infección Hospitalaria (GEIH); Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC)Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: A prospective multicentre cohort study in the era of antimicrobial resistance. Clin. Microbiol. Infect. 2013, 19, 962–968. [Google Scholar]
- Stefaniuk, E.; Suchocka, U.; Bosacka, K.; Hryniewicz, W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Venier, A.G.; Gruson, D.; Lavigne, T.; Jarno, P.; L’Hériteau, F.; Coignard, B.; Savey, A.; Rogues, A.M.; REA-RAISIN group. Identifying new risk factors for Pseudomonas aeruginosa pneumonia in intensive care units: Experience of the French national surveillance, REA-RAISIN. J. Hosp. Infect. 2011, 79, 44–48. [Google Scholar] [CrossRef]
- Vitkauskienė, A.; Skrodenienė, E.; Dambrauskienė, A.; Macas, A.; Sakalauskas, R. Correspondence to Pseudomonas aeruginosa bacteremia: Resistance to antibiotics, risk factors, and patient mortality. Medicina 2010, 46, 490. [Google Scholar] [CrossRef]
- Esparcia, A.; Madrazo, M.; Alberola, J.; López-Cruz, I.; Eiros, J.M.; Nogueira, J.M.; Artero, A. Community-onset Pseudomonas aeruginosa urinary sepsis in elderly people: Predictive factors, adequacy of empirical therapy and outcomes. Int. J. Clin. Pract. 2019, 1, 73. [Google Scholar] [CrossRef]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Mower, W.R.; Pallin, D.J.; Garg, M.; Femling, J.; Rothman, R.E.; Moore, J.C.; Jones, A.E.; et al. Emergence of Extended-Spectrum β-Lactamase Urinary Tract Infections Among Hospitalized Emergency Department Patients in the United States. Ann. Emerg. Med. 2021, 77, 32–43. [Google Scholar] [CrossRef]
- Ahn, S.T.; Kim, S.W.; Kim, J.W.; Park, H.S.; Moon, D.G.; Oh, M.M. Does urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli show same antibiotic resistance when it recurs? J. Infect. Chemother. 2019, 25, 498–502. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30852104 (accessed on 1 May 2023). [CrossRef]
- Paumier, A.; Asquier-Khati, A.; Thibaut, S.; Coeffic, T.; Lemenand, O.; Larramendy, S.; Leclère, B.; Caillon, J.; Boutoille, D.; Birgand, G.; et al. Assessment of Factors Associated with Community-Acquired Extended-Spectrum β-Lactamase-Producing Escherichia coli Urinary Tract Infections in France. JAMA Netw. Open 2022, 5, E2232679. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Costa, E.; Freitas, A.; Almeida, A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.E.; Wagenlehner, F.M.E.; Mulgirigama, A.; Twynholm, M. Escherichia coli resistance to fluoroquinolones in community-acquired uncomplicated urinary tract infection in women: A systematic review. Antimicrob. Agents Chemother. 2020, 64, e00862-20. [Google Scholar] [CrossRef] [PubMed]
- Kahlmeter, G.; Åhman, J.; Matuschek, E. Antimicrobial Resistance of Escherichia coli Causing Uncomplicated Urinary Tract Infections: A European Update for 2014 and Comparison with 2000 and 2008. Infect. Dis. Ther. 2015, 4, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, Y.; Geffen, Y.; Andreassen, S.; Leibovici, L.; Paul, M. Predicting antibiotic resistance in urinary tract infection patients with prior urine cultures. Antimicrob. Agents Chemother. 2016, 60, 4717–4721. [Google Scholar] [CrossRef]
- Korkmaz, P.; Kurtaran, B.; Armagan, S.O.; Özden, H.T.; Kaçar, F.; Ateş, S.; Durmus, G.; Bayindir, F.; Uygun, Y.; Hamidi, A.; et al. Factors affecting inadequate empirical antimicrobial therapy and the clinical course of upper urinary tract infections in elderly patients: A multicenter study. Mediterr. J. Infect. Microbes Antimicrob. 2020, 9. [Google Scholar] [CrossRef]
- Sujatha, S.; Praharaj, I. Glycopeptide resistance in gram-positive Cocci: A review. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 781679. [Google Scholar] [CrossRef]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J. Glob. Antimicrob. Resist. 2019, 16, 36–47. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes. Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Mendes, R.E.; Castanheira, M.; Farrell, D.J.; Flamm, R.K.; Sader, H.S.; Jones, R.N. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J. Antimicrob. Chemother. 2016, 71, 3453–3458. [Google Scholar] [CrossRef]
- Farman, M.; Yasir, M.; Al-Hindi, R.R.; Farraj, S.A.; Jiman-Fatani, A.A.; Alawi, M.; Azhar, E.I. Genomic analysis of multidrug-resistant clinical Enterococcus faecalis isolates for antimicrobial resistance genes and virulence factors from the western region of Saudi Arabia. Antimicrob. Resist. Infect. Control 2019, 8, 55. [Google Scholar] [CrossRef]
- Esmail, M.A.M.; Abdulghany, H.M.; Khairy, R.M. Prevalence of Multidrug-Resistant Enterococcus faecalis in Hospital-Acquired Surgical Wound Infections and Bacteremia: Concomitant Analysis of Antimicrobial Resistance Genes. Infect. Dis. Res. Treat. 2019, 12, 117863371988292. [Google Scholar] [CrossRef]
- Prest, J.; Nguyen, T.; Rajah, T.; Prest, A.B.; Sathananthan, M.; Jeganathan, N. Sepsis-Related Mortality Rates and Trends Based on Site of Infection. Crit. Care Explor. 2022, 4, E0775. [Google Scholar] [CrossRef]
- Tocut, M.; Zohar, I.; Schwartz, O.; Yossepowitch, O.; Maor, Y. Short- and long-term mortality in patients with urosepsis caused by Escherichia coli susceptible and resistant to 3rd generation cephalosporins. BMC Infect. Dis. 2022, 22, 571. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; Isberg, R.R. Interplay between antibiotic resistance and virulence during Disease promoted by multidrug-resistant bacteria. J. Infect. Dis. 2017, 215, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Milucky, J.; Pondo, T.; Gregory, C.J.; Iuliano, D.; Chaves, S.S.; McCracken, J.; Mansour, A.; Zhang, Y.; Aleem, M.; Wolff, B.; et al. The epidemiology and estimated etiology of pathogens detected from the upper respiratory tract of adults with severe acute respiratory infections in multiple countries, 2014–2015. PLoS ONE 2020, 15, e0240309. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.B.; Kim, J.H.; Kang, S.; Durey, A. Risk factors of urinary tract infection caused by extended spectrum β-lactamase-producing Escherichia coli in emergency department. Am. J. Emerg. Med. 2018, 36, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Anesi, J.A.; Lautenbach, E.; Nachamkin, I.; Garrigan, C.; Bilker, W.B.; Omorogbe, J.; Dankwa, L.; Wheeler, M.; Tolomeo, P.; Han, J.H.; et al. The role of extended-spectrum cephalosporin-resistance in recurrent community-onset Enterobacteriaceae urinary tract infections: A retrospective cohort study. BMC Infect. Dis. 2019, 19, 163. [Google Scholar] [CrossRef]
| Total N 193 | MDRB-US N 65 (33.7%) | Non-MDRB US N 128 (66.3%) | p | |
|---|---|---|---|---|
| Female sex, n (%) | 98 (50.8) | 31 (47.7) | 67 (52.3) | 0.541 |
| Age (years), median (IQR) | 82 (76–88) | 84 (77–89) | 82 (76–88) | 0.305 |
| Charlson ≥ 3, n (%) | 184 (95.3) | 62 (95.4) | 122 (95.3) | 0.982 |
| Barthel < 40, n (%) | 85 (44) | 38 (58.5) | 47 (36.7) | 0.004 |
| Comorbidities | ||||
| Dementia, n (%) | 65 (33.7) | 25 (38.5) | 40 (31.3) | 0.208 |
| Diabetes mellitus, n (%) | 73 (37.8) | 29 (44.6) | 44 (34.4) | 0.166 |
| COPD, n (%) | 24 (12.4) | 7 (10.8) | 17 (13.4) | 0.604 |
| CKD, n (%) | 69 (35.8) | 25 (38.5) | 44 (34.6) | 0.602 |
| Cancer, n (%) | 40 (20.7) | 13 (20) | 27 (21.1) | 0.859 |
| Indwelling urinary catheter, n (%) | 36 (18.7) | 16 (24.6) | 20 (15.6) | 0.130 |
| HCA-UTI, n (%) | 110 (57) | 50 (76.9) | 60 (46.9) | <0.001 |
| Previous hospitalization, n (%) | 66 (34.2) | 31 (47.7) | 35 (27.3) | 0.005 |
| Previous antimicrobial therapy, n (%) | 89 (46.1) | 37 (56.9) | 52 (40.6) | 0.032 |
| Nursing home residence, n (%) | 17 (8.8) | 13 (20) | 4 (3.1) | <0.001 |
| Clinical characteristics | ||||
| APACHE II, median (IQR) | 15 (11–20) | 16 (13–21) | 15 (11–19) | 0.117 |
| APN, n (%) | 109 (56.5) | 38 (58.5) | 71 (55.5) | 0.692 |
| Altered mental status, n (%) | 120 (62.2) | 49 (75.4) | 71 (55.9) | 0.008 |
| RR ≥ 22 bpm, n (%) | 76 (39.4) | 23 (35.4) | 53 (41.7) | 0.395 |
| SBP < 100 mmHg, n (%) | 66 (34.2) | 25 (38.5) | 41 (32.3) | 0.394 |
| Fever, n (%) | 142 (73.6) | 52 (80) | 90 (70.3) | 0.149 |
| qSOFA ≥ 2, n (%) | 104 (53.9) | 40 (61.5) | 64 (50) | 0.129 |
| Septic shock-3, n (%) | 39 (20.2) | 15 (23.1) | 24 (18.8) | 0.479 |
| Lactate ≥ 2 mg/dl, n (%) | 106 (54.9) | 34 (52.3) | 72 (56.3) | 0.603 |
| Leukocytosis, median (IQR) | 13,900 (10,050–19,050) | 13,900 (10,700–20,500) | 13,700 (9650–18,750) | 0.281 |
| Blood cultures positive/BC taken (%) | 55/125 (44) | 17/40 (42.5) | 38/85 (44.7) | 0.632 |
| Univariate Analysis p | Multivariate Analysis p | OR (95% CI) | |
|---|---|---|---|
| Barthel < 40 | 0.004 | 0.180 | 1.6 (0.8–3.1) |
| Healthcare-associated urinary sepsis | <0.001 | 0.001 | 3.1 (1.6–6.2) |
| Altered mental status | 0.008 | 0.121 | 1.7 (0.9–3.7) |
| Total N 215 | MDRB N 76 (35.3) | Non-MDRB N 139 (64.7) | p | |
|---|---|---|---|---|
| Gram-negative bacteria, n (%) | ||||
| Escherichia coli | 113 (52.6) | 36 (47.4) | 77 (55.4) | 0.525 |
| Klebsiella pneumoniae | 30 (13.9) | 12 (15.8) | 18 (12.9) | 0.425 |
| Klebsiella oxytoca | 6 (2.7) | 4 (5.3) | 2 (1.4) | 0.082 |
| Proteus mirabilis | 12 (5.6) | 7 (9.2) | 5 (3.6) | 0.062 |
| Other Enterobacteriaceae | 12 (5.6) | 9 (11.8) | 3 (2.1) | 0.059 |
| ESBL-EB * | 19 (11) | 17 (25) | 2 (1.9) | <0.001 |
| Pseudomonas aeruginosa | 17 (7.9) | 3 (3.9) | 14 (10.1) | 0.143 |
| Acinetobacter baumanii | 1 (0.5) | 1 (1.3) | 0 | - |
| Gram-positive bacteria, n (%) | ||||
| Enterococcus faecalis | 14 (6.5) | 2 (2.6) | 12 (8.6) | 0.111 |
| Enterococcus faecium | 2 (0.9) | 0 | 2 (1.4) | - |
| Enterococcus gallinarum | 2 (0.9) | 1 (1.3) | 1 (0.7) | - |
| Streptococcus agalactiae | 2 (0.9) | 0 | 2 (1.4) | - |
| Staphylococcus aureus | 1 (0.5) | 0 | 1 (0.7) | - |
| Fungi, n (%) | ||||
| Candida spp. | 3 (1.4) | 1 (1.3) | 2 (1.4) | - |
| Polymicrobial US, n (%) | 20 (10.4) | 9 (13.8) | 11 (8.6) | 0.258 |
| Total N 193 | MDRB-US N 65 (33.7%) | Non-MDRB US N 128 (66.3%) | p | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 34 (17.6) | 13 (20) | 21 (16.4) | 0.536 |
| 30-day mortality, n (%) | 45 (23.3) | 19 (29.2) | 26 (20.3) | 0.166 |
| Length of hospital stay (days), median (IQR) | 5 (4–8) | 6 (4–10) | 5 (4–8) | 0.051 |
| Univariate Analysis p | Multivariate Analysis p | OR (95% CI) | |
|---|---|---|---|
| Age ≥ 75 years | 0.482 | - | |
| Charlson ≥ 3 | 0.090 | - | |
| Barthel ≤ 40 | <0.001 | <0.001 | 4.1 (1.8–8.9) |
| HCA-US | 0.004 | 0.074 | 2.1 (0.9–4.9) |
| Septic shock | 0.001 | 0.006 | 3.2 (1.4–7.1) |
| IEAT | 0.611 | - | |
| MDR-US | 0.166 | 0.917 | 0.9 (0.4–2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrazo, M.; López-Cruz, I.; Piles, L.; Viñola, S.; Alberola, J.; Eiros, J.M.; Artero, A. Risk Factors and the Impact of Multidrug-Resistant Bacteria on Community-Acquired Urinary Sepsis. Microorganisms 2023, 11, 1278. https://doi.org/10.3390/microorganisms11051278
Madrazo M, López-Cruz I, Piles L, Viñola S, Alberola J, Eiros JM, Artero A. Risk Factors and the Impact of Multidrug-Resistant Bacteria on Community-Acquired Urinary Sepsis. Microorganisms. 2023; 11(5):1278. https://doi.org/10.3390/microorganisms11051278
Chicago/Turabian StyleMadrazo, Manuel, Ian López-Cruz, Laura Piles, Sofía Viñola, Juan Alberola, José María Eiros, and Arturo Artero. 2023. "Risk Factors and the Impact of Multidrug-Resistant Bacteria on Community-Acquired Urinary Sepsis" Microorganisms 11, no. 5: 1278. https://doi.org/10.3390/microorganisms11051278





