Research Progress and Prospects on Microbial Response and Gas Potential in the Coal Gasification Process
Abstract
:1. Introduction
2. Characteristics of Microbial Community Structure in CBM Reservoirs
2.1. Abundance and Diversity of Microbial Community in CBM Reservoirs In Situ
2.2. Characteristics of Microbial Community in Laboratory Conditions
3. Microbial Means Apply for Microbial Communities and CBM Production Potential
3.1. Response of Microbial Communities and CBM Potential to Microbial Stimulations
3.2. Microbial Enhancement Accelerating the Process of Coal Microbial Gasification
4. Coal Pretreated by Physicochemical Means and the Response of Microbial Communities
5. Improve Environmental Conditions and the Response of Microbial Communities
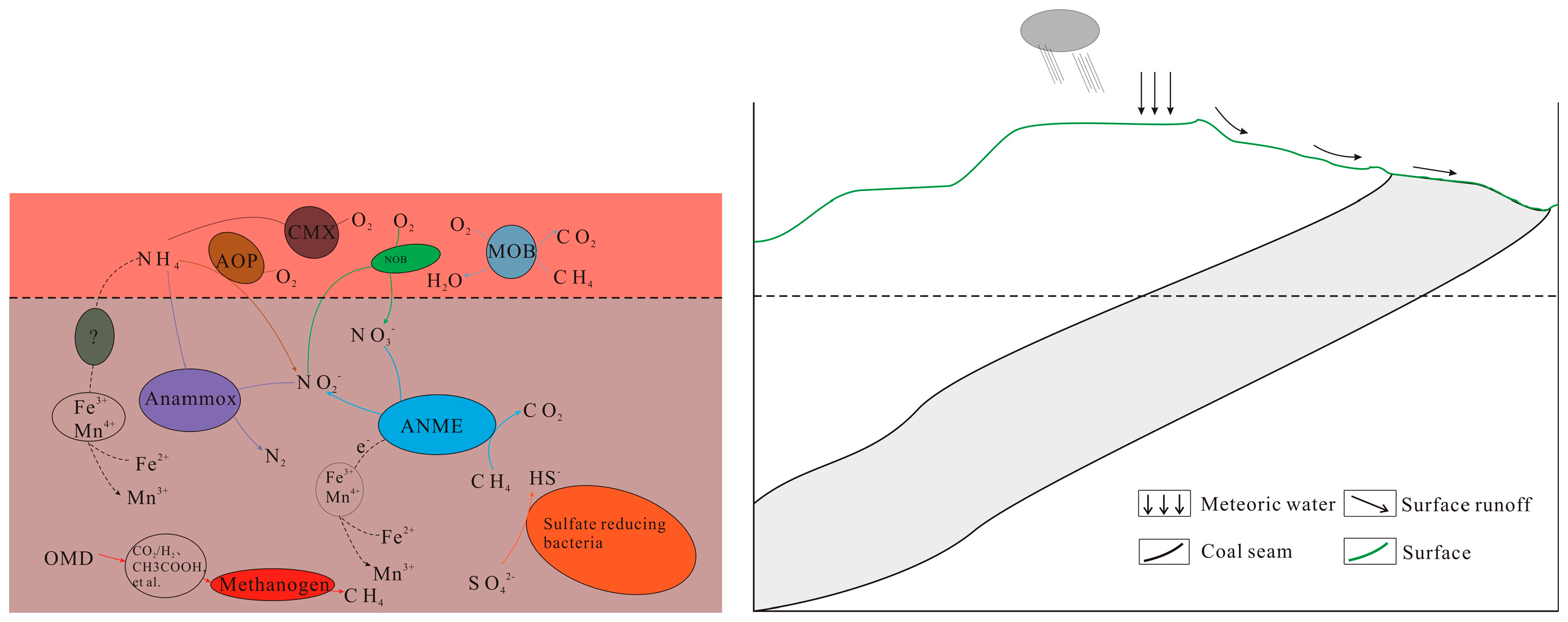
6. Microbial-Driven Biogeochemical Cycle
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, J.K.; Gerlach, R. Transition of biogenic coal-to-methane conversion from the laboratory to the field: A review of important parameters and studies. Int. J. Coal Geol. 2018, 185, 33–43. [Google Scholar] [CrossRef]
- Li, Y.; Shi, W.; Tang, S.H. Microbial geochemical characteristics of the coalbed methane in the Shizhuangnan block of Qinshui Basin, north China and their geological implications. Acta Geol. Sin.-Engl. 2019, 93, 660–674. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Wang, S.; Schobert, H.H. Clean coal geology in China: Research advance and its future. Int. J. Coal Sci. Technol. 2020, 7, 299–310. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Vinson, D.S.; Blair, N.E.; Ritter, D.J.; Martini, A.M.; McIntosh, J.C. Carbon Mass Balance, Isotopic Tracers of Biogenic Methane, and the Role of Acetate in Coal Beds: Powder River Basin (USA). Chem. Geol. 2019, 530, 119329. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Z.S.; Zhang, H.X. Microbial diversity and abundance in a representative small-production coal mine of central China. Energy Fuels 2013, 27, 3821–3829. [Google Scholar] [CrossRef]
- Olawale, J.T.; Edeki, O.G.; Cowan, A.K. Bacterial degradation of coal discard and geologically weathered coal. Int. J. Coal Sci. Technol. 2020, 7, 405–416. [Google Scholar] [CrossRef]
- Keboletse, K.P.; Ntuli, F.; Oladijo, O.P. Influence of coal properties on coal conversion processes-coal carbonization, carbon fiber production, gasification and liquefaction technologies: A review. Int. J. Coal Sci. Technol. 2021, 8, 817–843. [Google Scholar] [CrossRef]
- Kirk, M.F.; Wilson, B.H.; Marquart, K.A. Solute concentrations influence microbial methanogenesis in coal-bearing strata of the Cherokee Basin, USA. Front. Microbiol. 2015, 6, 1287. [Google Scholar] [CrossRef]
- Zhang, D.; He, H.; Ren, Y.; Haider, R.; Urynowicz, M.; Fallgren, P.H.; Jin, S.; Ali, M.I.; Jamal, A.; Sabar, M.A.; et al. A mini review on biotransformation of coal to methane by enhancement of chemical pretreatment. Fuel 2022, 308, 121961. [Google Scholar] [CrossRef]
- Barnhart, E.P.; Weeks, E.P.; Jones, E.J.P.; Ritter, D.J.; McIntosh, J.C.; Clark, A.C.; Ruppert, L.F.; Cunningham, A.B.; Vinson, D.S.; Orem, W.; et al. Hydrogeochemistry and coal-associated bacterial populations from a methanogenic coal bed. Int. J. Coal Geol. 2016, 162, 14–26. [Google Scholar] [CrossRef]
- Guo, H.G.; Zhang, Y.W.; Zhang, J.L.; Huang, Z.X.; Urynowicz, M.A.; Liang, W.G.; Han, Z.Y.; Liu, J. Characterization of anthracite-degrading methanogenic microflora enriched from Qinshui Basin in China. Energy Fuels 2019, 33, 6380–6389. [Google Scholar] [CrossRef]
- Yang, X.Q.; Chen, Y.M.; Wu, R.W.; Nie, Z.Q.; Han, Z.Y.; Tan, K.L.; Chen, L.Y. Potential of biogenic methane for pilot-scale fermentation ex situ with lump anthracite and the changes of methanogenic consortia. J. Ind. Microbiol. Biot. 2018, 45, 229–237. [Google Scholar] [CrossRef]
- Guo, H.G.; Zhang, Y.X.; Huang, Z.X.; Liang, W.G.; Urynowicz, M.; Ali, M.I. High potential of methane production from coal by fungi and hydrogenotrophic methanogens from produced water. Energy Fuels 2020, 34, 10958–10967. [Google Scholar] [CrossRef]
- Chen, F.; He, H.; Zhao, S.M.; Yao, J.H.; Sun, Q.; Huang, G.H.; Xiao, D.; Tang, L.F.; Leng, Y.W.; Tao, X.X. Analysis of microbial community succession during methane production from Baiyinhua lignite. Energy Fuels 2018, 32, 10311–10320. [Google Scholar] [CrossRef]
- He, H.; Zhan, D.; Chen, F.; Huang, Z.X.; Huang, H.Z.; Wang, A.K.; Huang, G.H.; Muhammad, I.A.; Tao, X.X. Microbial community succession between coal matrix and culture solution in a simulated methanogenic system with lignite. Fuel 2020, 264, 116905. [Google Scholar] [CrossRef]
- Wang, B.B.; Yu, Z.S.; Zhang, Y.M.; Zhang, H.X. Microbial communities from the Huaibei Coalfield alter the physicochemical properties of coal in methanogenic bioconversion. Int. J. Coal Geol. 2019, 202, 85–94. [Google Scholar] [CrossRef]
- Susilawati, R.; Evans, P.N.; Esterle, J.S. Temporal changes in microbial community composition during culture enrichment experiments with Indonesian coals. Int. J. Coal Geol. 2015, 137, 66–76. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Rahim, M.U. On comparison between fungal and bacterial pretreatment of coal for enhanced biogenic methane generation. Geomicrobiol. J. 2018, 35, 432–437. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, H.P.; He, D.S.; Ju, Y.W.; Qi, Y. Microbial enhancing coal-bed methane generation potential, constraints and mechanism-a mini-review. J. Nat. Gas Sci. Eng. 2016, 35, 68–78. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.H.; Zhang, S.H.; Xi, Z.D. In situ analysis of methanogenic pathways and biogeochemical features of CBM co-produced water from the Shizhuangnan Block in the Southern Qinshui Basin, China. Energy Fuels 2020, 34, 5466–5475. [Google Scholar] [CrossRef]
- Wang, A.; Shao, P.; Wang, Q. Biogenic gas generation effects on anthracite molecular structure and pore structure. Front. Earth Sci. 2021, 15, 272–282. [Google Scholar] [CrossRef]
- Ma, J.; Wei, H.; Su, Y.; Gu, W.; Wang, B.; Xie, B. Powdered activated carbon facilitates methane productivity of anaerobic co-digestion via acidification alleviating: Microbial and metabolic insights. Bioresour. Technol. 2020, 313, 123706. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.; Fang, X.Y.; Tu, B. Methanogenic potential and community structure of coalbed-methane production water microbiome. Acta Microbiol. Sin. 2020, 60, 727–738. [Google Scholar]
- Li, Y.; Chen, J.; Tang, S.H.; Zhang, S.H.; Xi, Z.D. Biogeochemical assessment of the coalbed methane source, migration, and fate: A case study of the Shizhuangnan Block, Southern Qinshui Basin. ACS Omega 2022, 7, 7715–7724. [Google Scholar] [CrossRef]
- Lyles, C.N.; Parisi, V.A.; Beasley, W.H.; Van Nostrand, J.D.; Zhou, J.Z.; Suflita, J.M. Elucidation of the methanogenic potential from coalbed microbial communities amended with volatile fatty acids. FEMS Microbiol. Ecol. 2017, 93, 4. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Y.; Feng, L.; Zhou, Q. Metagenomic analysis reveals nonylphenolshaped acidification and methanogenesis during sludge anaerobic digestion. Water Res. 2021, 196, 117004. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, E.P.; Davis, K.J.; Varonk, M.; Orem, W.; Cunningham, A.B.; Ramsay, B.D.; Fields, M.W. Enhanced coal-dependent methanogenesis coupled with algal biofuels: Potential water recycle and carbon capture. Int. J. Coal Geol. 2017, 171, 69–75. [Google Scholar] [CrossRef]
- Davis, K.J.; Lu, S.P.; Barnhart, E.P.; Parker, A.E.; Fields, M.W.; Gerlach, R. Type and amount of organic amendments affect enhanced biogenic methane production from coal and microbial community structure. Fuel 2018, 211, 600–608. [Google Scholar] [CrossRef]
- Xia, D.P.; Huang, S.; Yan, X.T. Influencing mechanism of Fe2+ on biomethane production from coal. J. Nat. Gas Sci. Eng. 2021, 91, 103959. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Ding, L.; Zhou, J.; Song, W.; Li, Y.; Lin, R. Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: The effect of magnetite nanoparticles on microbial electron transfer and syntrophism. Chem. Eng. J. 2020, 397, 125394. [Google Scholar] [CrossRef]
- Wen, L.; Huang, L.; Wang, Y.; Yuan, Y.; Zhou, L. Facet-engineered hematite boosts microbial electrogenesis by synergy of promoting electroactive biofilm formation and extracellular electron transfer. Sci. Total Environ. 2022, 819, 153154. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Liang, Q.; Han, Z.Y. Effect of Ethanol on the structure and gas production pathways of coal geological microorganisms in biogas production process. J. Shanxi Univ. 2019, 42, 941–950. [Google Scholar]
- Yang, X.Q.; Liang, Q.; Chen, Y.M.; Wang, B.Y. Alteration of methanogenic archaeon by ethanol contribute to the enhancement of biogenic methane production of lignite. Front. Microbiol. 2019, 10, 2323. [Google Scholar] [CrossRef]
- Elsayed, M.; Abomohra, A.-E.-F.; Ai, P.; Jin, K.; Fan, Q.; Zhang, Y. Acetogenesis and methanogenesis liquid digestates for pretreatment of rice straw: A holistic approach for efficient biomethane production and nutrient recycling. Energy Convers. Manag. 2019, 195, 447–456. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, M.L.; Dong, Z.W.; Wang, Q.; Xia, D.P.; Lv, J.H.; Yu, H.F. The mechanisms of biogenic methane metabolism by synergistic biodegradation of coal and corn straw. Bioresour. Technol. 2020, 298, 122577. [Google Scholar] [CrossRef]
- Su, X.B.; Zhao, W.Z.; Xia, D.P. The diversity of hydrogen-producing bacteria and methanogens within an in situ coal seam. Biotechnol. Biofuels 2018, 11, 245. [Google Scholar] [CrossRef]
- Liu, S.; Sang, S.; Wang, T.; Du, Y.; Jia, J.; Fang, H. The effects of CO2 on organic groups in bituminous coal and high-rank coal via Fourier transform infrared spectroscopy. Energy Explor. Exploit. 2018, 36, 1566–1592. [Google Scholar] [CrossRef]
- Fuertez, J.; Cordoba, G.; Mclennan, J.D.; Adams, D.J.; Sparks, T.D. Potential application of developed methanogenic microbial consortia for coal biogasification. Int. J. Coal Geol. 2018, 188, 165–180. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Su, X.B.; Xia, D.P.; Li, D.; Guo, H.Y. Contribution of microbial acclimation to lignite biomethanization. Energy Fuels 2020, 34, 3223–3238. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, D.; Cai, Y.; Fang, X.; Li, L. Petrophysics characteristics of coalbed methane reservoir: A comprehensive review. Front. Earth Sci. 2020, 15, 202–223. [Google Scholar] [CrossRef]
- McLeish, A.G.; Vick, S.H.W.; Grigore, M.; Pinetown, K.L.; Midgley, D.J.; Paulsen, I.T. Adherent microbes in coal seam environments prefer mineral-rich and crackassociated microhabitats. Int. J. Coal Geol. 2021, 234, 103652. [Google Scholar] [CrossRef]
- Robbins, S.J.; Evans, P.N.; Parrks, D.H.; Golding, S.D.; Tyson, G.W. Genome-centric analysis of microbial populations enriched by hydraulic fracture fluid additives in a coal bed methane production well. Front. Microbiol. 2016, 7, 731. [Google Scholar] [CrossRef]
- Raudsepp, M.J.; Gagen, E.J.; Evans, P.; Tyson, G.W.; Golding, S.D.; Southam, G. The influence of hydrogeological disturbance and mining on coal seam microbial communities. Geobiology 2016, 14, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Oreggioni, G.D.; Luberti, M.; Tassou, S.A. Agricultural greenhouse CO2 utilization inanaerobic-digestion-based biomethane production plants: A techno-economic and environmental assessment and comparison with CO2 geological storage. Appl. Energy 2019, 242, 1753–1766. [Google Scholar] [CrossRef]
- Huang, Z.; Liers, C.; Ullrich, R.; Hofrichter, M.; Urynowicz, M.A. Depolymerization and solubilization of chemically pretreated powder river basin subbituminous coal by manganese peroxidase (MnP) from Bjerkandera adusta. Fuel 2013, 112, 295–301. [Google Scholar] [CrossRef]
- Wang, Q.R.; Guo, H.G.; Wang, H.J.; Urynowicz, M.A.; Hu, A.Y.; Yu, C.P.; Fallgren, P.; Jin, S.; Zheng, H.; Zeng, R.J.; et al. Enhanced production of secondary biogenic coalbed natural gas from a subbituminous coal treated by hydrogen peroxide and its geochemical and microbiological analyses. Fuel 2019, 236, 1345–1355. [Google Scholar] [CrossRef]
- Xia, D.P.; Zhang, H.W.; Su, X.B.; Chem, H.; Li, D. Adsorption and heat characteristics of coal–microorganisms during the cogeneration of H2 and CH4 following pretreatment with white rot fungi. J. Clean. Prod. 2020, 255, 120242. [Google Scholar] [CrossRef]
- Orr, F.M. Onshore geologic storage of CO2. Science 2009, 325, 1656–1658. [Google Scholar] [CrossRef]
- Fan, C.; Elsworth, D.; Li, S.; Zhou, L.; Yang, Z.; Song, Y. Thermo-hydro-mechanicalchemical couplings controlling CH4 production and CO2 sequestration in enhanced coalbed methane recovery. Energy 2019, 173, 1054–1077. [Google Scholar] [CrossRef]
- Guo, H.Y.; Gao, Z.X.; Xia, D.P.; Yin, X.J.; Jia, J.B.; Dou, Y.L. Biological methanation of coal in various atmospheres containing CO2. Fuel 2019, 242, 334–342. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, J.; Li, J.; Zhou, X.; Dong, W.; Cheng, Z. Experimental study on oil recovery mechanism of CO2 associated enhancing oil recovery methods in low permeability reservoirs. J. Petrol. Sci. Eng. 2021, 197, 108047. [Google Scholar] [CrossRef]
- Su, X.B.; Wang, L.F.; Zhao, W.Z. Physical simulation of in-situ microbial methanation in coal reservoirs with the participation of supercritical CO2. Coal Geol. Explor. 2022, 50, 119–126. [Google Scholar]
- Zhang, Y.; Gong, L.; Jiang, Q.; Cui, M.H.; Liu, H. In-situ CO2 sequestration and nutrients removal in an anaerobic digestion-microbial electrolysis cell by silicates application: Effect of dosage and biogas circulation. Chem. Eng. J. 2020, 399, 125680. [Google Scholar] [CrossRef]
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a carbon fiber brush improves anaerobic digestion compared to external voltage application. Water Res. 2021, 188, 116575. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Su, X.B.; Zhang, Y.F.; Xia, D.P.; Hou, S.H.; Zhou, Y.X.; Fu, H.J.; Wang, L.F.; Yin, X.J. Microbial electrolysis enhanced bioconversion of coal to methane compared with anaerobic digestion: Insights into differences in metabolic pathways. Energy Convers. Manag. 2022, 259, 115553. [Google Scholar] [CrossRef]
- Guo, H.G.; Chen, C.; Liang, W.G.; Zhang, Y.X.; Duan, K.X.; Zhang, P.P. Enhanced biomethane production from anthracite by application of an electric field. Int. J. Coal Geol. 2020, 219, 103393. [Google Scholar] [CrossRef]
- Mukherjee, M.; Misra, S. A review of experimental research on enhanced coal bed methane (ECBM) recovery via CO2 sequestration. Earth–Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Fan, C.J.; Elsworth, D.; Li, S.; Chen, Z.W.; Luo, M.K.; Song, Y.; Zhang, H.H. Modelling and optimization of enhanced coalbed methane recovery using CO2/N2 mixtures. Fuel 2019, 253, 1114–1129. [Google Scholar] [CrossRef]
- Sampath, K.H.S.M.; Sin, I.; Perera, M.S.A.; Matthai, S.K.; Ranjith, P.G.; Dong-yin, L. Effect of supercritical-CO2 interaction time on the alterations in coal pore structure. J. Nat. Gas Sci. Eng. 2020, 76, 103214. [Google Scholar] [CrossRef]
- Feng, G.; Kang, Y.; Sun, Z.; Wang, X.; Hu, Y. Effects of supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 2019, 173, 870–882. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Wen, G.; Han, L.; Liu, H. A dynamic evaluation technique for assessing gas output from coal seams during commingling production within a coalbed methane well: A case study from the Qinshui Basin. Int. J. Coal Sci. Technol. 2020, 7, 122–132. [Google Scholar] [CrossRef]
- Gong, K.; Zhang, Y.; Guo, H.; Huang, Z.; Urynowicz, M.; Ali, M.I. Enhancing biomethane production from lignite by an anaerobic polycyclic aromatic hydrocarbon degrading fungal flora enriched from produced water. Front. Microbiol. 2022, 13, 899863. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.U. Revival of archaeal methane microbiology. Msystems 2018, 3, e00181-17. [Google Scholar] [CrossRef]
- Lyimo, T.J.; Pol, A.; Op Den Camp, H.J.; Harhangi, H.R.; Vogels, G.D. Methanosarcina semesiae sp. nov., a dimethylsulfide-utilizing methanogen from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2000, 50, 171–178. [Google Scholar] [CrossRef]
- Huser, B.A.; Wuhrmann, K.; Zehnder, A.J.B. Methanothrix soehngenii gen. nov. sp. nov., a new acetotrophic non-hydrogenoxidizing methane bacterium. Arch. Microbiol. 1982, 132, 1–9. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, T.L.; Yin, X.B.; Wu, X.L.; Hu, G.Q.; Deng, Y.; Zhang, H. Methermicoccus shengliensis gen. nov., sp. nov., a thermophilic, methylotrophic methanogen isolated from oil-production water, and proposal of Methermicoccaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2964–2969. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Makarova, K.S.; Abbas, B.; Ferrer, M.; Golyshin, P.N.; Galinski, E.A.; Ciordia, S.; Mena, M.C.; Merkel, A.Y.; Wolf, Y.I.; et al. Discovery of extremely halophilic, methyl-reducing euryarchaea provides insights into the evolutionary origin of methanogenesis. Nat. Microbiol. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Nobu, M.K.; Narihiro, T.; Kuroda, K.; Mei, R.; Liu, W.T. Chasing the elusive Euryarchaeota class WSA2: Genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J. 2016, 10, 2478–2487. [Google Scholar] [CrossRef]
- Pratscher, J.; Vollmers, J.; Wiegand, S.; Dumont, M.G.; Kaster, A.K. Unravelling the identity, metabolic potential and global biogeography of the atmospheric methane-oxidizing upland soil cluster α. Environ. Microbiol. 2018, 20, 1016–1025. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Berestovskaya, Y.Y.; Vasylieva, L.V.; Belova, S.E.; Khmelenina, V.N.; Suzina, N.E.; Trotsenko, Y.A.; Liesack, W.Z.G.A. Methylocella tundrae sp. nov., a novel methanotrophic bacterium from acidic tundra peatlands. Int. J. Syst. Evol. Microbiol. 2004, 54, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Luke, C.; Jetten, M.S.M. Enrichment of anaerobic nitrate-dependent methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Pol, A.; van de Pas-Schoonen, K.T.; Smolders, A.J.P.; Ettwig, K.F.; Rijpstra, W.I.C.; Schouten, S.; Damste, J.S.S.; Op den Camp, H.J.M.; Jetten, M.S.M.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Leu, A.O.; Xie, G.J.; Guo, J.H.; Feng, Y.X.; Zhao, J.X.; Tyson, G.W.; Yuan, Z.G.; Hu, S.H. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 94, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Ettwig, K.F.; Zhu, B.; Speth, D.; Keltjens, J.T.; Jetten, M.S.M.; Kartal, B. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl. Acad. Sci. USA 2016, 113, 12792–12796. [Google Scholar] [CrossRef]
- Nauhaus, K.; Treude, T.; Boetius, A.; Kruger, M. Environmental regulation of the anaerobic oxidation of methane: A comparison of ANME-I and ANME-II communities. Environ. Microbiol. 2005, 7, 98–106. [Google Scholar] [CrossRef]
- Jian, K.; Chen, G.; Guo, C.; Ma, G.S.; Ru, Z.L. Biogenic gas simulation of low-rank coal and its structure evolution. J. Petrol. Sci. Eng. 2019, 173, 1284–1288. [Google Scholar] [CrossRef]
- Aramaki, N.; Tamamura, S.; Ueno, A.; Badrul, A.A.K.M.; Murakami, T.; Tamazawa, S.; Yamaguchi, S.; Aoyama, H.; Kaneko, K. Experimental investigation on the feasibility of industrial methane production in the subsurface environment via microbial activities in northern Hokkaido, Japan—A process involving subsurface cultivation and gasification. Energy Convers. Manag. 2017, 153, 566–575. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.I.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial electrolysis followed by chemical precipitation for effective nutrients recovery from digested sludge centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265. [Google Scholar] [CrossRef]
- Fang, H.T.; Oberoi, A.S.; He, Z.Q.; Khanal, S.K.; Lu, H. Ciprofloxacin−degrading Paraclostridium sp. isolated from sulfate−reducing bacteria−enriched sludge: Optimization and mechanism. Water Res. 2021, 191, 116808. [Google Scholar] [CrossRef]
| Electron Acceptor | Formula | Microorganism | ∆G0′ (kJ/mol) | Reference |
|---|---|---|---|---|
| CH3OH | 4 CH3OH → CO2 + 3 CH4 + 2 H2O | Methanosarcina semesiae | −103 | [64] |
| CH3-R | (CH3)2SH + H2O → 0.5 CO2 + 1.5 CH4 + H2S | Methanomethylovorans hollandica | −56 | [65] |
| CH3COOH | CH3COOH → CO2 + CH4 | Methanothrix soehngenii | −36 | [66] |
| CH3O-R | 4 CH3O-R + 2 H2O→4 R-OH + CO2 + 3 CH4 | Methermicoccus shengliensis | −106 | [67] |
| CH3OH | CH3OH + H2 → CH4 + H2O | Methanomassiliicoccus luminyiensis | −113 | [68,69] |
| Candidatus Methanonatronarchaeia | ||||
| Candidatus Methanofastidiosa | ||||
| O2/H2O | CH4 + 2 O2 → CO2 + 2 H2O | Methane-oxidizing bacteria (MOB) | −801 | [70,71] |
| Alphaproteobacteria | ||||
| Methylocella palustris | ||||
| Methylocella tundra | ||||
| Gammaproteobacteria | ||||
| Verrucomicrobia | ||||
| NO3−/NO2− | CH4 + 4 NO3− → CO2 + 4 NO2− + 2 H2O | Candidatus Methanoperedens nitroreducens | −503 | [72] |
| NO2−/N2 | 3 CH4 + 8 NO2− + 8 H+ → 3 CO2 + 4 N2 + 10 H2O | Candidatus Methylomirabilis oxyfera | −928 | [73] |
| Fe3+/Fe2+ | CH4 + 8 Fe3+ + 2 H2O → CO2 + 8 Fe2+ + 8 H+ | Candidatus Methanoperedens nitroreducens, ANME-2C | −454 | [74,75] |
| SO42−/H2S | CH4 + SO42− → HCO3− + H2S + H2O | Anaerobic methanotrophic archaea (ANME) | −21 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tang, S.; Chen, J.; Xi, Z. Research Progress and Prospects on Microbial Response and Gas Potential in the Coal Gasification Process. Microorganisms 2023, 11, 1293. https://doi.org/10.3390/microorganisms11051293
Li Y, Tang S, Chen J, Xi Z. Research Progress and Prospects on Microbial Response and Gas Potential in the Coal Gasification Process. Microorganisms. 2023; 11(5):1293. https://doi.org/10.3390/microorganisms11051293
Chicago/Turabian StyleLi, Yang, Shuheng Tang, Jian Chen, and Zhaodong Xi. 2023. "Research Progress and Prospects on Microbial Response and Gas Potential in the Coal Gasification Process" Microorganisms 11, no. 5: 1293. https://doi.org/10.3390/microorganisms11051293





