Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia, through a Six-Year Infection Control Program in a Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Interventions
2.3. Data Collection and Outcomes
2.4. Detection of Bacteremia and Microbial Resistance
2.5. Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.I.; Prince, A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr. Opin. Pharmacol. 2007, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Rybtke, M.; Hultqvist, L.D.; Givskov, M.; Tolker-Nielsen, T. Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J. Mol. Biol. 2015, 427, 3628–3645. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.aidsdatahub.org/resource/who-global-priority-list-antibiotic-resistant-bacteria (accessed on 21 April 2023).
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S521–S528. [Google Scholar] [CrossRef]
- Karampatakis, T.; Antachopoulos, C.; Tsakris, A.; Roilides, E. Molecular epidemiology of carbapenem-resistant Pseudomonas aeruginosa in an endemic area: Comparison with global data. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1211–1220. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, H.Y.; Chuang, H.C.; Chen, B.L.; Wang, E.Y.; Tsao, L.H.; Ai, M.Y.; Lee, Y.J. Correlation between antibiotic consumption and resistance of Pseudomonas aeruginosa in a teaching hospital implementing an antimicrobial stewardship program: A longitudinal observational study. J. Microbiol. Immunol. Infect. 2023, 56, 337–343. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ko, W.C.; Hsueh, P.R. Geographic Patterns of Carbapenem-Resistant Pseudomonas aeruginosa in the Asia-Pacific Region: Results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) Program, 2015–2019. Antimicrob. Agents Chemother. 2022, 66, e0200021. [Google Scholar] [CrossRef]
- Chrysou, K.; Zarkotou, O.; Kalofolia, S.; Papagiannakopoulou, P.; Mamali, V.; Chrysos, G.; Themeli-Digalaki, K.; Sypsas, N.; Tsakris, A.; Pournaras, S. Impact of a 4-year antimicrobial stewardship program implemented in a Greek tertiary hospital. Eur. J. Clin. Microbiol. Infect. Dis 2022, 41, 127–132. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. 2017. Available online: https://apps.who.int/iris/handle/10665/259462 (accessed on 21 April 2023).
- Pierce, J.W.; Kirk, A.; Lee, K.B.; Markley, J.D.; Pakyz, A.; Bearman, G.; Doll, M.E.; Stevens, M.P. The impact of formulary restriction on the relative consumption of carbapenems in intensive care units at an academic medical center. Infect. Control. Hosp. Epidemiol. 2019, 40, 1056–1058. [Google Scholar] [CrossRef]
- Kirk, A.; Pierce, J.; Doll, M.; Lee, K.; Pakyz, A.; Kim, J.; Markley, D.; De la Cruz, O.; Bearman, G.; Stevens, M.P. Effect of carbapenem restriction on prescribing trends for immunocompromised wards at an academic medical center. Am. J. Infect. Control 2019, 47, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Papanikolopoulou, A.; Maltezou, H.C.; Kritikou, H.; Papadopoulos, T.; Kandalepas, G.; Pentzouris, A.; Kartsonakis, I.; Chronopoulou, G.; Gargalianos-Kakolyris, P.; Pantazis, N.; et al. Six-Year Time-Series Data on Multidrug-Resistant Bacteremia, Antibiotic Consumption, and Infection Control Interventions in a Hospital. Microb. Drug. Resist. 2022, 28, 806–818. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 21 April 2023).
- Papanikolopoulou, A.; Maltezou, H.C.; Gargalianos-Kakolyris, P.; Michou, I.; Kalofissoudis, Y.; Moussas, N.; Pantazis, N.; Kotteas, E.; Syrigos, K.N.; Pantos, C.; et al. Central-line-associated bloodstream infections, multi-drug-resistant bacteraemias and infection control interventions: A 6-year time-series analysis in a tertiary care hospital in Greece. J. Hosp. Infect. 2022, 123, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zay, Y.K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2023, 6, e2253806. [Google Scholar] [CrossRef] [PubMed]
- Donà, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control. 2020, 9, 3. [Google Scholar] [CrossRef]
- Rogers Van Katwyk, S.; Grimshaw, J.M.; Mendelson, M.; Taljaard, M.; Hoffman, S.J. Government policy interventions to reduce human antimicrobial use: Protocol for a systematic review and meta-analysis. Syst. Rev. 2017, 6, 256. [Google Scholar] [CrossRef]
- Hawkins, O.; Scott, A.M.; Montgomery, A.; Nicholas, B.; Mullan, J.; van Oijen, A.; Degeling, C. Comparing public attitudes, knowledge, beliefs and behaviours towards antibiotics and antimicrobial resistance in Australia, United Kingdom, and Sweden (2010–2021): A systematic review, meta-analysis, and comparative policy analysis. PLoS ONE 2022, 17, e0261917. [Google Scholar] [CrossRef]
- Diallo, O.O.; Baron, S.A.; Abat, C.; Colson, P.; Chaudet, H.; Rolain, J.M. Antibiotic resistance surveillance systems: A review. J. Glob. Antimicrob. Resist. 2020, 23, 430–438. [Google Scholar] [CrossRef]
- Schweitzer, V.A.; van Heijl, I.; van Werkhoven, C.H.; Islam, J.; Hendriks-Spoor, K.D.; Bielicki, J.; Bonten, M.J.M.; Walker, A.S.; Llewelyn, M.J. Consensus on Antimicrobial Stewardship Evaluations (CASE) study group. The quality of studies evaluating antimicrobial stewardship interventions: A systematic review. Clin. Microbiol. Infect. 2019, 25, 555–561. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, J.; Zhang, T.; Li, C.; Chen, J.; Fan, J.; Qu, L.; Su, X. Comparative genomics of the sequential Pseudomonas aeruginosa isolates obtained from the continuous imipenem stress evolution. Appl. Microbiol. Biotechnol. 2020, 104, 10655–10667. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti-Miganeh, C.; Redelberger, D.; Chambonnier, G.; Rechenmann, F.; Elsen, S.; Bordi, C.; Jeannot, K.; Attrée, I.; Plésiat, P.; de Bentzmann, S. Pseudomonas aeruginosa Genome Evolution in Patients and under the Hospital Environment. Pathogens 2014, 3, 309–340. [Google Scholar] [CrossRef] [PubMed]
- El Chakhtoura, N.G.; Saade, E.; Iovleva, A.; Yasmin, M.; Wilson, B.; Perez, F.; Bonomo, R.A. Therapies for multidrug resistant and extensively drug-resistant non-fermenting gram-negative bacteria causing nosocomial infections: A perilous journey toward ‘molecularly targeted’ therapy. Expert. Rev. Anti Infect. Ther. 2018, 16, 89–110. [Google Scholar] [CrossRef]
- Laborda, P.; Hernando-Amado, S.; Martínez, J.L.; Sanz-García, F. Antibiotic Resistance in Pseudomonas. Adv. Exp. Med. Biol. 2022, 1386, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.; Vrioni, G.; Saroglou, G.; Tsakris, A. The Impact of Antibiotic Stewardship Programs in Combating Quinolone Resistance: A Systematic Review and Recommendations for More Efficient Interventions. Adv. Ther. 2017, 34, 854–865. [Google Scholar] [CrossRef]
- Slain, D.; Sarwari, A.R.; Petros, K.O.; McKnight, R.L.; Sager, R.B.; Mullett, C.J.; Wilson, A.; Thomas, J.G.; Moffett, K.; Palmer, H.C.; et al. Impact of a Multimodal Antimicrobial Stewardship Program on Pseudomonas aeruginosa Susceptibility and Antimicrobial Use in the Intensive Care Unit Setting. Crit. Care Res. Pract. 2011, 2011, 416426. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Iosifidis, E.; Antachopoulos, C.; Karapanagiotou, A.; Karyoti, A.; Gritsi-Gerogianni, N.; Tsakris, A.; Roilides, E. Impact of active surveillance and infection control measures on carbapenem-resistant Gram-negative bacterial colonization and infections in intensive care. J. Hosp. Infect. 2018, 99, 396–404. [Google Scholar] [CrossRef]
- Frattari, A.; Savini, V.; Polilli, E.; Di Marco, G.; Lucisano, G.; Corridoni, S.; Spina, T.; Costantini, A.; Nicolucci, A.; Fazii, P.; et al. Control of Gram-negative multi-drug resistant microorganisms in an Italian ICU: Rapid decline as a result of a multifaceted intervention, including conservative use of antibiotics. Int. J. Infect. Dis. 2019, 84, 153–162. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
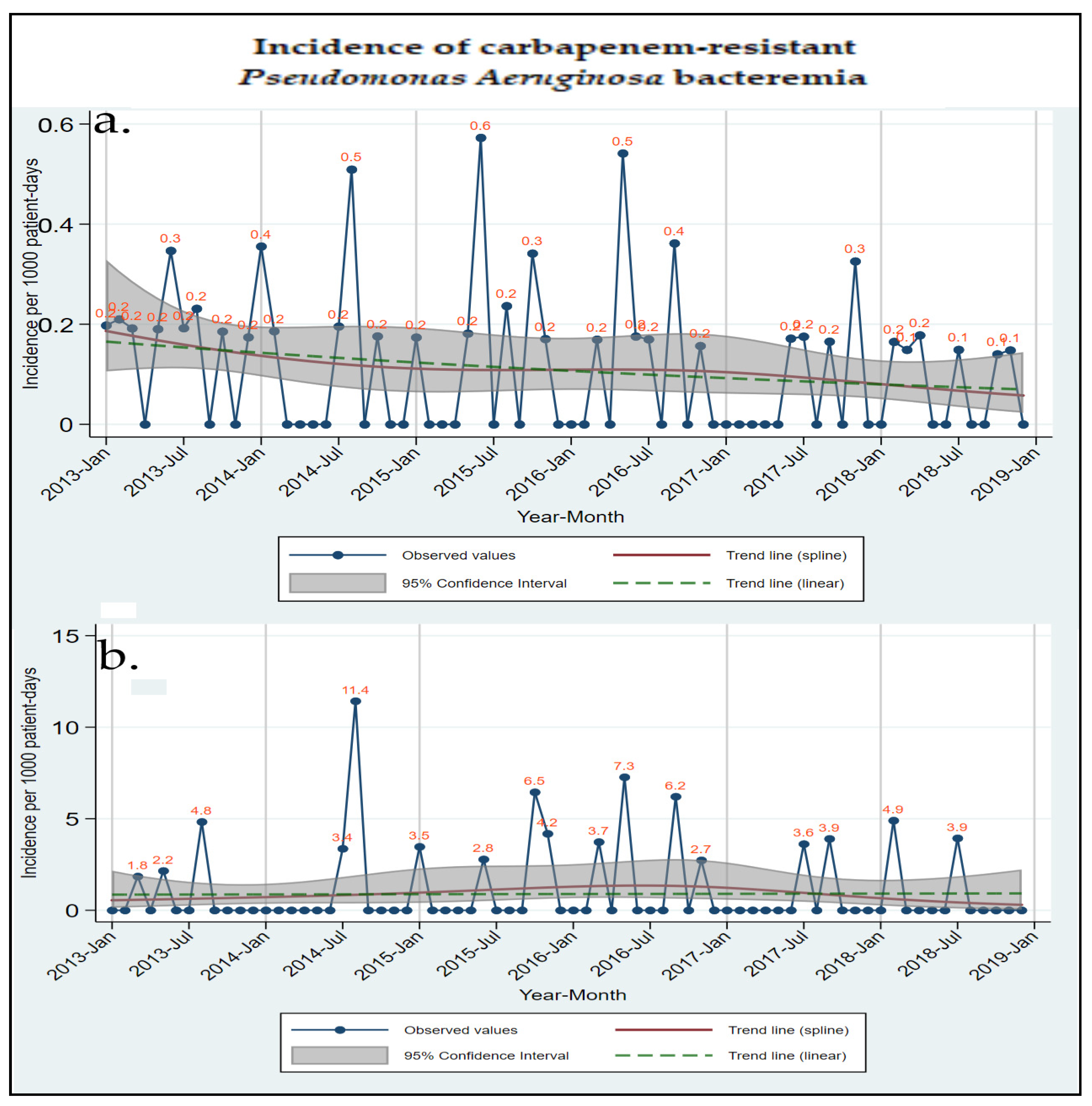
| Time Trend | |||||
|---|---|---|---|---|---|
| Incidence of Bacteremia/1000 Patient-Days | EVSP January 2013 (95% CI) | EVEP December 2018 (95% CI) | p-Value | % Relative Change/Year (95% CI) | p-Value |
| Total Hospital Clinics | |||||
| Total bacteremia | 3.4 (3.0 to 3.8) | 5.0 (4.5 to 5.5) | <0.001 | 2.35 (−2.15 to 7.05) up to 12/2016 | 0.311 |
| 15.87 (6.67 to 25.86) after 12/2016 | <0.001 | ||||
| Total CR Gram (−) bacteremia | 0.3 (0.2 to 0.5) | 0.2 (0.1 to 0.3) | 0.099 | −7.78 (−16.24 to 1.53) | 0.099 |
| Total CRPA bacteremia | 0.2 (0.1 to 0.2) | 0.1 (0.0 to 0.1) | 0.027 | −13.54 (−24.02 to −1.62) | 0.027 |
| Total Hospital Departments | |||||
| Total bacteremia | 2.7 (2.4 to 3.1) | 4.4 (3.5 to 5.5) | <0.001 | 2.37 (−1.72 to 6.63) up to 12/2017 | 0.260 |
| 43.24 (10.34 to 85.95) after 12/2017 | 0.007 | ||||
| Total CR Gram (−) bacteremia | 0.2 (0.1 to 0.3) | 0.1 (0.1 to 0.2) | 0.165 | −11.12 (−24.74 to 4.97) | 0.165 |
| Total CRPA bacteremia | 0.1 (0.1 to 0.2) | 0.0 (0.0 to 0.1) | 0.042 | −22.86 (−39.94 to 0.92) | 0.042 |
| Adults Clinic | |||||
| Total bacteremia | 4.8 (4.2 to 5.5) | 6.3 (5.6 to 7.2) | 0.001 | −0.57 (−5.41 to 4.51) up to 11/2016 | 0.821 |
| 15.50 (5.11 to 26.91) after 11/2016 | 0.003 | ||||
| Total CR Gram (−) bacteremia | 0.6 (0.4 to 0.8) | 0.4 (0.3 to 0.5) | 0.119 | −7.35 (−15.83 to 1.98) | 0.119 |
| Total CRPA bacteremia | 0.3 (0.2 to 0.4) | 0.1 (0.1 to 0.2) | 0.031 | −13.53 (−24.22 to −1.33) | 0.031 |
| Adults Clinic Departments | |||||
| Total bacteremia | 2.9 (2.6 to 3.4) | 4.1 (3.6 to 4.7) | 0.004 | 5.91 (1.85 to 10.14) | 0.004 |
| Total CR Gram (−) bacteremia | 0.3 (0.2 to 0.5) | 0.2 (0.1 to 0.3) | 0.205 | −10.01 (−23.56 to 5.93) | 0.205 |
| Total CRPA bacteremia | 0.2 (0.1 to 0.4) | 0.0 (0.0 to 0.1) | 0.051 | −21.77 (−38.86 to 0.10) | 0.051 |
| Adults ICU | |||||
| Total bacteremia | 18.2 (13.9 to 23.7) | 32.8 (27.5 to 39.2) | <0.001 | −3.81 (−15.16 to 9.07) up to 02/2016 | 0.545 |
| 28.57 (14.91 to 43.85) after 02/2016 | <0.001 | ||||
| Total CR Gram (−) bacteremia | 2.5 (1.7 to 3.5) | 3.3 (2.1 to 5.1) | 0.392 | 4.91 (−5.99 to 17.07) | 0.392 |
| Total CRPA bacteremia | 0.9 (0.4 to 1.8) | 0.9 (0.4 to 2.1) | 0.909 | 1.25 (−18.13 to 25.21) | 0.909 |
| Time Trend | |||||
|---|---|---|---|---|---|
| Antibiotic Consumption DDDs/100 Patient-Days | EVSP January 2013 (95% CI) | EVEP December 2018 (95% CI) | p-Value | % Relative Change/Year (95% CI) | p-Value |
| Total Hospital Clinics | |||||
| Fluoroquinolones | 10.2 (9.6 to 10.7) | 10.6 (10.2 to 11.0) | 0.176 | −2.96 (−3.72 to −2.20) up to 2/2014 | <0.001 |
| 0.69 (0.56 to 0.82) after 2/2014 | <0.001 | ||||
| Colistin | 2.7 (2.4 to 3.0) | 1.4 (1.2 to 1.6) | <0.001 | −0.92 (−1.14 to −0.70) up to 08/2014 | <0.001 |
| 0.04 (−0.04 to 0.11) after 08/2014 | 0.329 | ||||
| Aminoglycosides | 6.5 (6.3 to 6.7) | 4.3 (4.1 to 4.5) | <0.001 | −0.36 (−0.42 to −0.31) | <0.001 |
| 3rd generation cephalosporins | 16.1 (14.6 to 17.6) | 7.3 (6.9 to 7.7) | <0.001 | −12.42 (−14.11 to −10.73) up to 12/2013 | <0.001 |
| 0.51 (0.37 to 0.66) after 12/2013 | <0.001 | ||||
| Carbapenems | 9.3 (8.3 to 10.4) | 7.8 (7.3 to 8.2) | 0.008 | −2.30 (−3.06 to −1.53) up to 6/2014 | <0.001 |
| 0.37 (0.23 to 0.51) after 6/2014 | <0.001 | ||||
| Total Hospital Departments | |||||
| Fluoroquinolones | 11.0 (10.3 to 11.7) | 11.6 (11.1 to 12.0) | 0.159 | −3.13 (−4.08 to −2.18) up to 1/2014 | <0.001 |
| 0.76 (0.60 to 0.91) after 1/2014 | <0.001 | ||||
| Colistin | 1.6 (1.3 to 1.9) | 0.9 (0.8 to 1.1) | <0.001 | −0.41 (−0.63 to −0.19) up to 08/2014 | <0.001 |
| −0.01 (−0.07 to 0.06) after 08/2014 | 0.816 | ||||
| Aminoglycosides | 7.3 (6.8 to 7.7) | 4.5 (4.3 to 4.7) | <0.001 | −1.00 (−1.35 to −0.65) up to 7/2014 | <0.001 |
| −0.29 (−0.36 to −0.21) after 7/2014 | <0.001 | ||||
| 3rd generation cephalosporins | 18 (1.3 to 1.9) | 7.6 (0.8 to 1.1) | <0.001 | −14.05 (−15.87 to −12.22) up to 12/2013 | <0.001 |
| 0.49 (−0.34 to 0.64) after 12/2013 | <0.001 | ||||
| Carbapenems | 8.1 (7.2 to 9.0) | 7.6 (7.0 to 8.2) | 0.336 | −1.83 (−2.53 to −1.14) up to 7/2014 | <0.001 |
| 0.50 (0.32 to 0.68) after 7/2014 | <0.001 | ||||
| Adults Clinic | |||||
| Fluoroquinolones | 16.2 (14.9 to 17.4) | 17.8 (17.1 to 17.4) | 0.024 | −4.00 (−5.60 to −2.39) up to 1/2014 | <0.001 |
| 1.14 (0.90 to 1.38) after 1/2014 | <0.001 | ||||
| Colistin | 4.2 (3.7 to 4.8) | 2.4 (2.1 to 2.6) | <0.001 | −1.30 (−1.70 to −0.89) up to 08/2014 | <0.001 |
| 0.04 (−0.09 to 0.16) after 08/2014 | 0.565 | ||||
| Aminoglycosides | 9.4 (9.1 to 9.7) | 6.1 (5.9 to 6.4) | <0.001 | −0.55 (−0.63 to −0.47) | <0.001 |
| 3rd generation cephalosporins | 23.7 (21.6 to 25.9) | 8.1 (7.4 to 8.7) | <0.001 | −17.83 (−20.02 to −15.64) up to 1/2014 | <0.001 |
| 0.44 (0.21 to 0.67) after 1/2014 | <0.001 | ||||
| Carbapenems | 13.7 (11.7 to 15.7) | 12.1 (11.3 to 12.9) | 0.123 | −2.34 (−3.82 to −0.86) up to 7/2014 | 0.002 |
| 0.42 (0.17 to 0.67) after 7/2014 | 0.001 | ||||
| Adults Clinic Departments | |||||
| Fluoroquinolones | 17.1 (14.6 to 19.6) | 17.9 (17.2 to 18.6) | 0.539 | −5.08 (−7.87 to −2.29) up to 1/2014 | 0.001 |
| 1.19 (0.93 to 1.46) after 1/2014 | <0.001 | ||||
| Colistin | 2.2 (1.7 to 2.7) | 1.4 (1.2 to 1.6) | 0.004 | −0.46 (−0.84 to −0.08) up to 08/2014 | 0.018 |
| −0.02 (−0.12 to 0.08) after 08/2014 | 0.680 | ||||
| Aminoglycosides | 10.6 (10.3 to 10.9) | 6.2 (5.9 to 6.5) | <0.001 | −1.73 (−2.03 to −1.42) up to 5/2014 | <0.001 |
| −0.45 (−0.55 to −0.36) after 5/2014 | <0.001 | ||||
| 3rd generation cephalosporins | 26.2 (22.8 to 29.7) | 7.6 (6.9 to 8.2) | <0.001 | −20.34 (−23.82 to −16.86) up to 1/2014 | <0.001 |
| 0.33 (0.09 to 0.58) after 1/2014 | 0.008 | ||||
| Carbapenems | 11.2 (9.2 to 13.2) | 10.9 (10.0 to 11.7) | 0.776 | −1.82 (−3.25 to 0.40) up to 8/2014 | 0.013 |
| 0.60 (0.32 to 0.87) after 8/2014 | <0.001 | ||||
| Adults ICU | |||||
| Fluoroquinolones | 20.7 (17.0 to 24.4) | 18.6 (14.9 to 22.4) | 0.494 | −0.36 (−1.40 to 0.68) | 0.494 |
| Colistin | 11.6 (8.2 to 15.1) | 12.5 (9.5 to 15.5) | 0.681 | −1.63 (−3.20 to 0.06) up to 01/2017 | 0.042 |
| 7.27 (3.12 to 11.42) after 01/2017 | 0.001 | ||||
| Aminoglycosides | 9.3 (7.0 to 11.5) | 7.1 (4.6 to 9.6) | 0.289 | −0.36 (−1.04 to −0.31) | 0.289 |
| 3rd generation cephalosporins | 15.1 (10.3 to 19.9) | 21.3 (17.7 to 24.9) | 0.036 | −8.59 (−13.33 to −3.84) up to 4/2014 | 0.001 |
| 3.63 (2.44 to 4.81) after 4/2014 | <0.001 | ||||
| Carbapenems | 48.5 (41.0 to 55.9) | 65.2 (51.7 to 78.8) | 0.025 | −1.78 (−4.39 to 0.83) up to 3/2017 | 0.177 |
| 13.81 (4.08 to 23.55) after 3/2017 | 0.006 | ||||
| Total CRPA Bacteremia Correlation with Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotics (DDDs/100 Patient-Days) | Per (n)DDD | Month 0 | Month −1 | Month −2 | Month −3 | IRR | 95% CI | p-Value |
| Total Hospital Clinics | ||||||||
| Penicillin total | 1 | ◊ | 1.41 | (0.96, 2.08) | 0.076 | |||
| Monobactams | 0.1 | ◊ | 0.72 | (0.49, 1.05) | 0.084 | |||
| Total Hospital Departments | ||||||||
| Aminoglycosides | 1 | ◊ | 2.41 | (0.94, 6.21) | 0.068 | |||
| Colistin | 1 | ◊ | 2.42 | (0.85, 6.91) | 0.098 | |||
| Fosfomycin | 0.1 | ◊ | 1.39 | (1.05, 1.85) | 0.021 | |||
| Non-Advanced Antibiotics | 1 | ◊ | ◊ | 1.31 | (1.03, 1.65) | 0.025 | ||
| All Antibiotics | 10 | ◊ | 5.92 | (1.06, 32.96) | 0.042 | |||
| Adults Clinic | ||||||||
| Monobactams | 0.1 | ◊ | ◊ | 0.65 | (0.43, 0.98) | 0.041 | ||
| Adults Clinic Departments | ||||||||
| Monobactams | 0.1 | ◊ | 1.40 | (0.96, 2.04) | 0.078 | |||
| Monobactams | 0.1 | ◊ | 0.47 | (0.27, 0.82) | 0.008 | |||
| Monobactams | 0.1 | ◊ | 0.35 | (0.17, 0.70) | 0.003 | |||
| Aminoglycosides | 1 | ◊ | 3.63 | (1.67, 7.89) | 0.001 | |||
| Fosfomycin | 0.1 | ◊ | 1.24 | (1.03, 1.48) | 0.022 | |||
| Nonadvanced Antibiotics | 10 | ◊ | 2.33 | (1.18, 4.59) | 0.014 | |||
| All Antibiotics | 10 | ◊ | 1.82 | (1.12, 2.95) | 0.016 | |||
| Adults ICU | ||||||||
| Monobactams | 1 | ◊ | 1.61 | (1.10, 2.37) | 0.015 | |||
| Carbapenems | 10 | ◊ | 0.64 | (0.46, 0.90) | 0.011 | |||
| Aminoglycosides | 10 | ◊ | 0.25 | (0.10, 0.64) | 0.003 | |||
| Fosfomycin | 10 | ◊ | 0.18 | (0.05, 0.67) | 0.011 | |||
| Nonadvanced Antibiotics | 10 | ◊ | 1.22 | (1.02, 1.45) | 0.030 | |||
| Nonadvanced Antibiotics | 10 | ◊ | 0.83 | (0.70, 0.99) | 0.037 | |||
| Correlation of Bacteremias with Infection Control Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infection Control Interventions | Per (n) Unit | Month 0 | Month −1 | Month −2 | Month −3 | IRR | 95% CI | p-Value |
| 1. Total Bacteremia | ||||||||
| Total Hospital Clinics | n.s. | |||||||
| Total Hospital Departments | ||||||||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | 0.81 | (0.69, 0.95) | 0.011 | |
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | 0.94 | (0.90, 0.99) | 0.020 | |||
| Adults Clinic | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.04 | (1.02, 1.06) | 0.001 | |||
| Adults Clinic Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.06 | (1.03, 1.10) | <0.001 | |||
| Adults ICU | ||||||||
| % Isolations/Admissions | 10 | ◊ | 1.20 | (1.03, 1.39) | 0.020 | |||
| 2. Total CR Gram (−) Bacteremia | ||||||||
| Total Hospital Clinics | n.s. | |||||||
| Total Hospital Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.54 | (1.23, 1.93) | <0.001 | |||
| Adults Clinic | n.s. | |||||||
| Adults Clinic Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.25 | (1.06, 1.48) | 0.009 | |||
| Adults ICU | ||||||||
| % Isolations/Admissions | 10 | ◊ | 2.42 | (1.75, 3.35) | <0.001 | |||
| % Isolations/Admissions | 10 | ◊ | 0.35 | (0.18, 0.66) | 0.001 | |||
| 3. Total CRPA Bacteremia | ||||||||
| Total Hospital Clinics | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.19 | (0.97, 1.44) | 0.089 | |||
| Total Hospital Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | ◊ | 3.60 | (1.90, 6.82) | <0.001 | ||
| Adults Clinic | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.17 | (1.01, 1.35) | 0.033 | |||
| Adults Clinic Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 1.48 | (1.13, 1.94) | 0.004 | |||
| Adults ICU | ||||||||
| % Isolations/Admissions | 10 | ◊ | 3.70 | (1.75, 7.86) | 0.001 | |||
| % Isolations/Admissions | 10 | ◊ | 0.40 | (0.14, 1.16) | 0.092 | |||
| % Isolations/Admissions | 10 | ◊ | 0.20 | (0.05, 0.73) | 0.015 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | 0.90 | (0.79, 1.02) | 0.091 | |||
| Antibiotics Correlation with Infection Control Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infection Control Interventions | Per (n) Unit | Month 0 | Month −1 | Month −2 | Month −3 | β | 95% CI | p-Value |
| Advanced Antibiotics | ||||||||
| Total Hospital Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 0.53 | (0.27, 0.79) | <0.001 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | ◊ | −0.57 | (−1.05, −0.10) | 0.019 |
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | −0.75 | (−1.25, −0.25) | 0.004 | |||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | ◊ | −0.50 | (−0.82, −0.19) | 0.002 |
| Adults Clinic | ||||||||
| % Isolations/Admissions | 1 | ◊ | 0.47 | (0.08, 0.85) | 0.019 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | ◊ | −1.63 | (−2.40, −0.86) | <0.001 |
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | −0.98 | (−1.89, −0.07) | 0.035 | |||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | −1.06 | (−1.63, −0.49) | <0.001 | ||
| Adults Clinic Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 0.51 | (0.16, 0.87) | 0.005 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | −0.68 | (−1.35, −0.02) | 0.045 | |||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | −1.46 | (−2.26, −0.65) | 0.001 | |||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | −0.81 | (−1.30, −0.32) | 0.002 | |||
| Adults ICU | ||||||||
| % Isolations/Admissions | 10 | ◊ | 9.38 | (−1.60, 20.37) | 0.093 | |||
| Nonadvanced Antibiotics | ||||||||
| Total Hospital Clinics | ||||||||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | 2.99 | (1.76, 4.22) | <0.001 | |
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | 1.58 | (0.74, 2.42) | <0.001 | |
| Total Hospital Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | 0.62 | (−0.04, 1.28) | 0.064 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | 0.97 | (0.24, 1.70) | 0.010 | |||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | −3.07 | (−4.35, −1.79) | <0.001 | ||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | −0.72 | (−1.15, −0.29) | 0.001 | |||
| Adults Clinic Departments | ||||||||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | 1.87 | (0.42, 3.32) | 0.012 | ||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | −2.07 | (−3.92, −0.22) | 0.029 | |||
| All Antibiotics | ||||||||
| Total Hospital Clinics | ||||||||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | 2.97 | (1.29, 4.65) | 0.001 | |
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | 2.88 | (0.80, 4.97) | 0.007 | ||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | ◊ | 2.00 | (0.88, 3.12) | 0.001 |
| Total Hospital Departments | ||||||||
| % Isolations/Admissions | 1 | ◊ | ◊ | ◊ | ◊ | 2.18 | (0.83, 3.52) | 0.002 |
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | ◊ | −3.14 | (−5.23, −1.05) | 0.004 | |
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | −1.02 | (−1.70, −0.34) | 0.004 | ||
| Adults Clinic | ||||||||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | −1.89 | (−3.88, 0.10) | 0.062 | |||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | ◊ | −1.86 | (−3.04, −0.67) | 0.003 | ||
| Adults Clinic Departments | ||||||||
| % Isolations/Admissions | 10 | ◊ | 1.58 | (0.06, 3.10) | 0.042 | |||
| L of Alcohol Disinfectant sol/1000 patient-days | 10 | ◊ | −3.53 | (−6.07, −0.98) | 0.007 | |||
| L of All Hand Disinfectant sol/1000 patient-days | 10 | ◊ | −1.81 | (−3.35, −0.28) | 0.021 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolopoulou, A.; Gargalianos-Kakolyris, P.; Stoupis, A.; Moussas, N.; Pangalis, A.; Theodoridou, K.; Chronopoulou, G.; Pantazis, N.; Kantzanou, M.; Maltezou, H.C.; et al. Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia, through a Six-Year Infection Control Program in a Hospital. Microorganisms 2023, 11, 1315. https://doi.org/10.3390/microorganisms11051315
Papanikolopoulou A, Gargalianos-Kakolyris P, Stoupis A, Moussas N, Pangalis A, Theodoridou K, Chronopoulou G, Pantazis N, Kantzanou M, Maltezou HC, et al. Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia, through a Six-Year Infection Control Program in a Hospital. Microorganisms. 2023; 11(5):1315. https://doi.org/10.3390/microorganisms11051315
Chicago/Turabian StylePapanikolopoulou, Amalia, Panagiotis Gargalianos-Kakolyris, Athina Stoupis, Nikos Moussas, Anastasia Pangalis, Kalliopi Theodoridou, Genovefa Chronopoulou, Nikos Pantazis, Maria Kantzanou, Helena C. Maltezou, and et al. 2023. "Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia, through a Six-Year Infection Control Program in a Hospital" Microorganisms 11, no. 5: 1315. https://doi.org/10.3390/microorganisms11051315






