RNase Y Autoregulates Its Synthesis in Bacillus subtilis
Abstract
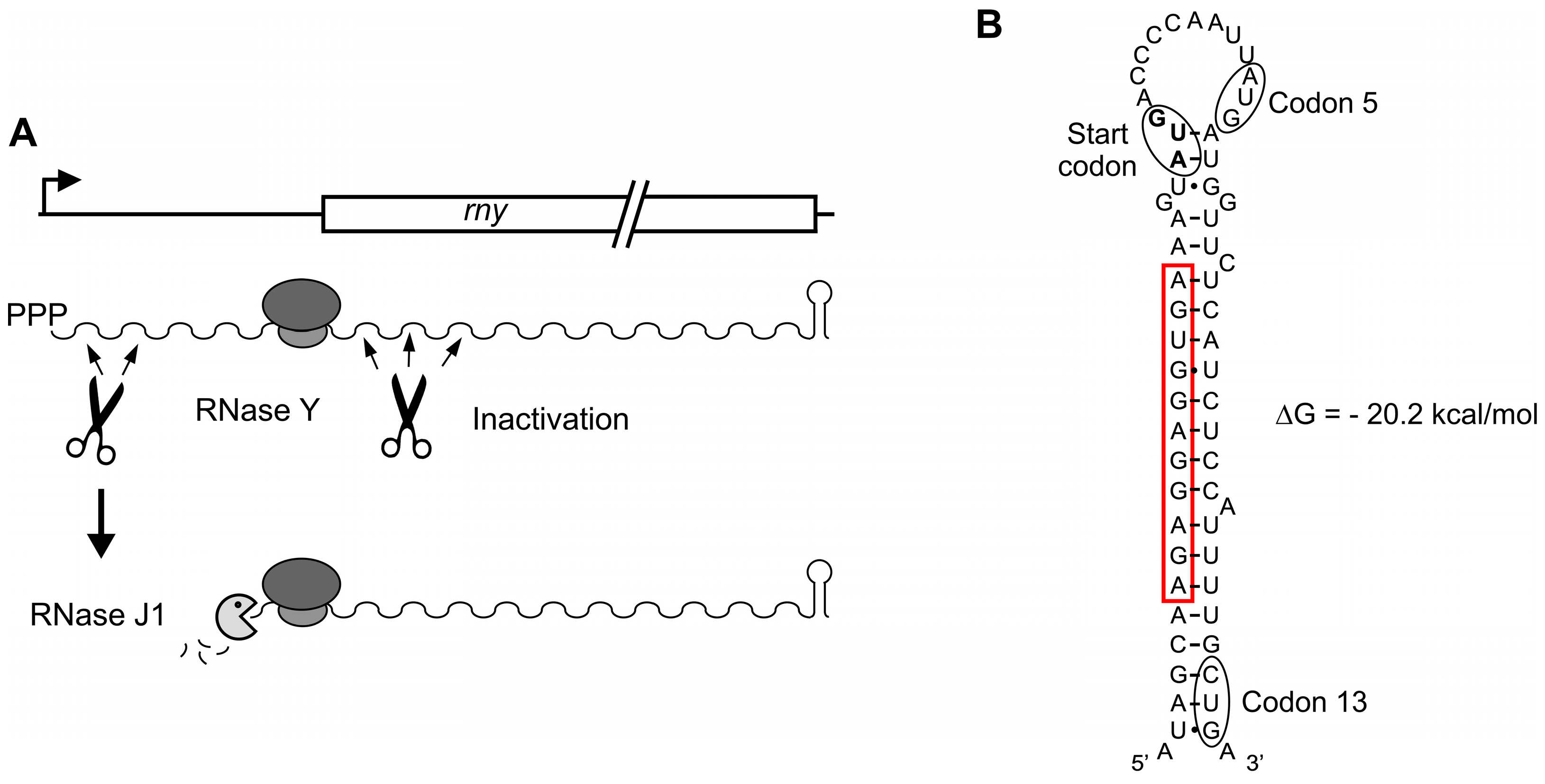
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Plasmid Constructs
2.3. Western Blot
2.4. Northern Blot
2.5. Half-Life Measurements
2.6. Primer Extension Analysis
2.7. β-Galactosidase Measurements
3. Results
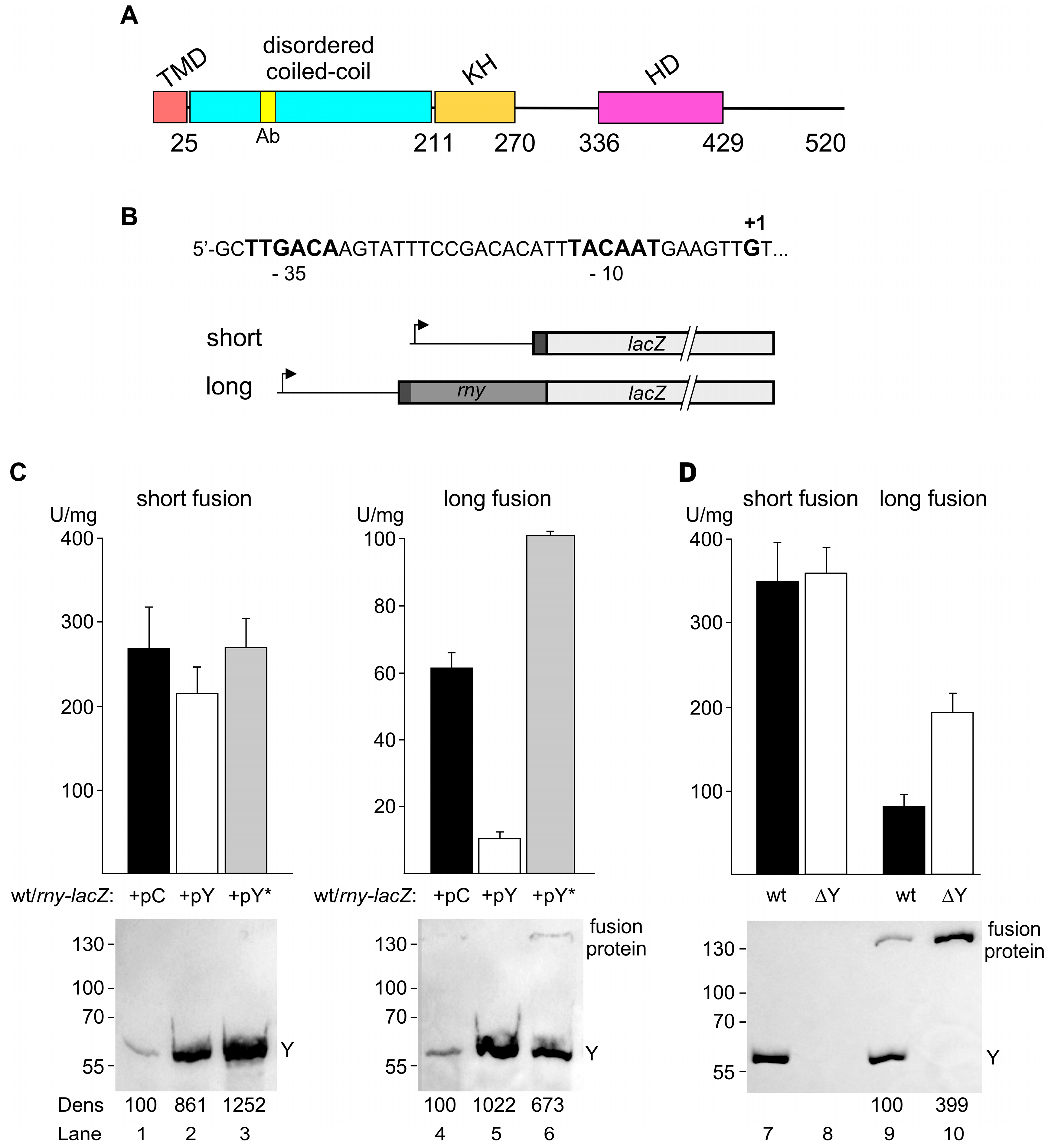
3.1. Rny-lacZ Expression Is Responsive to RNase Y Levels In Vivo
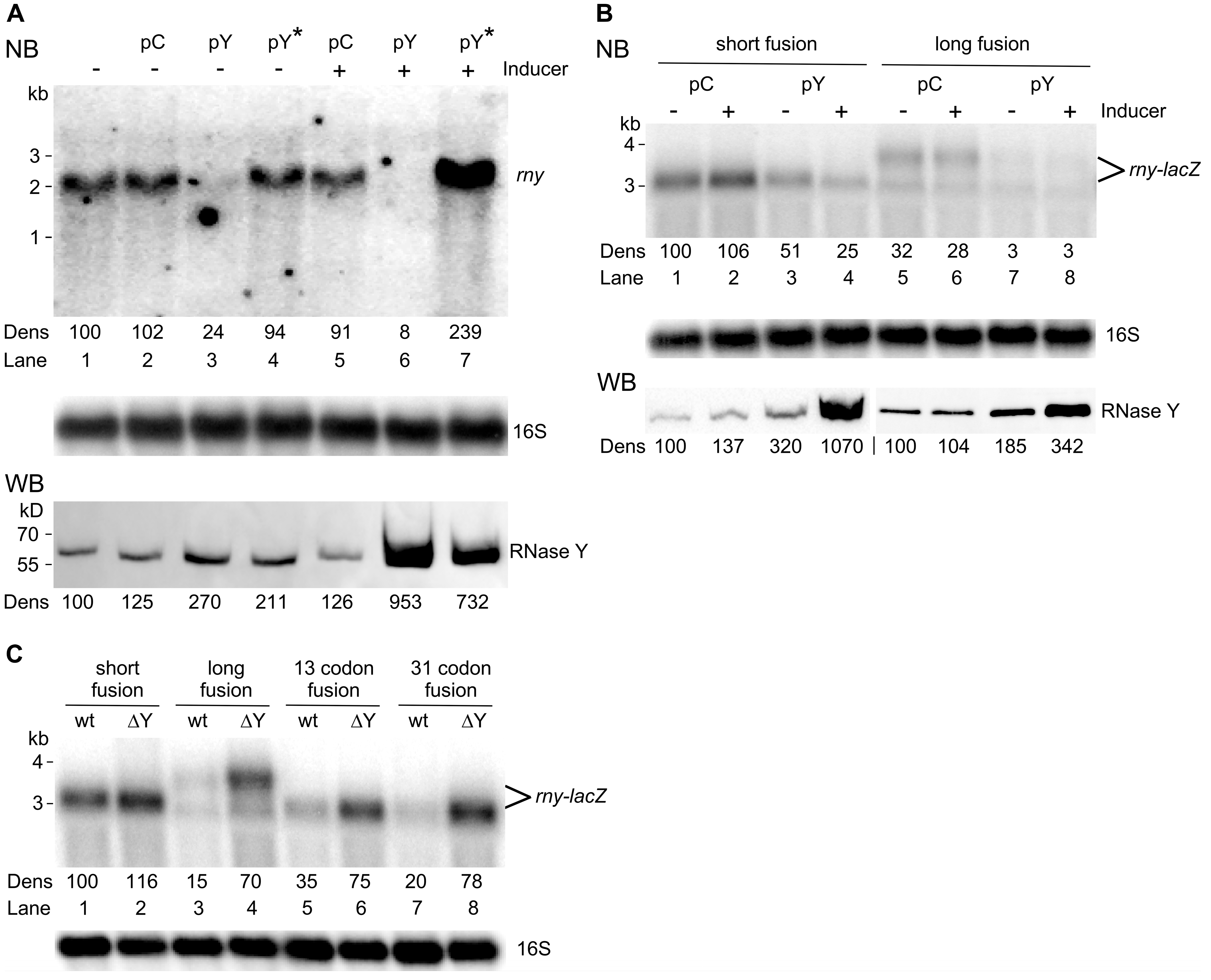
3.2. RNase Y Affects rny and rny-lacZ Transcript Levels Alike
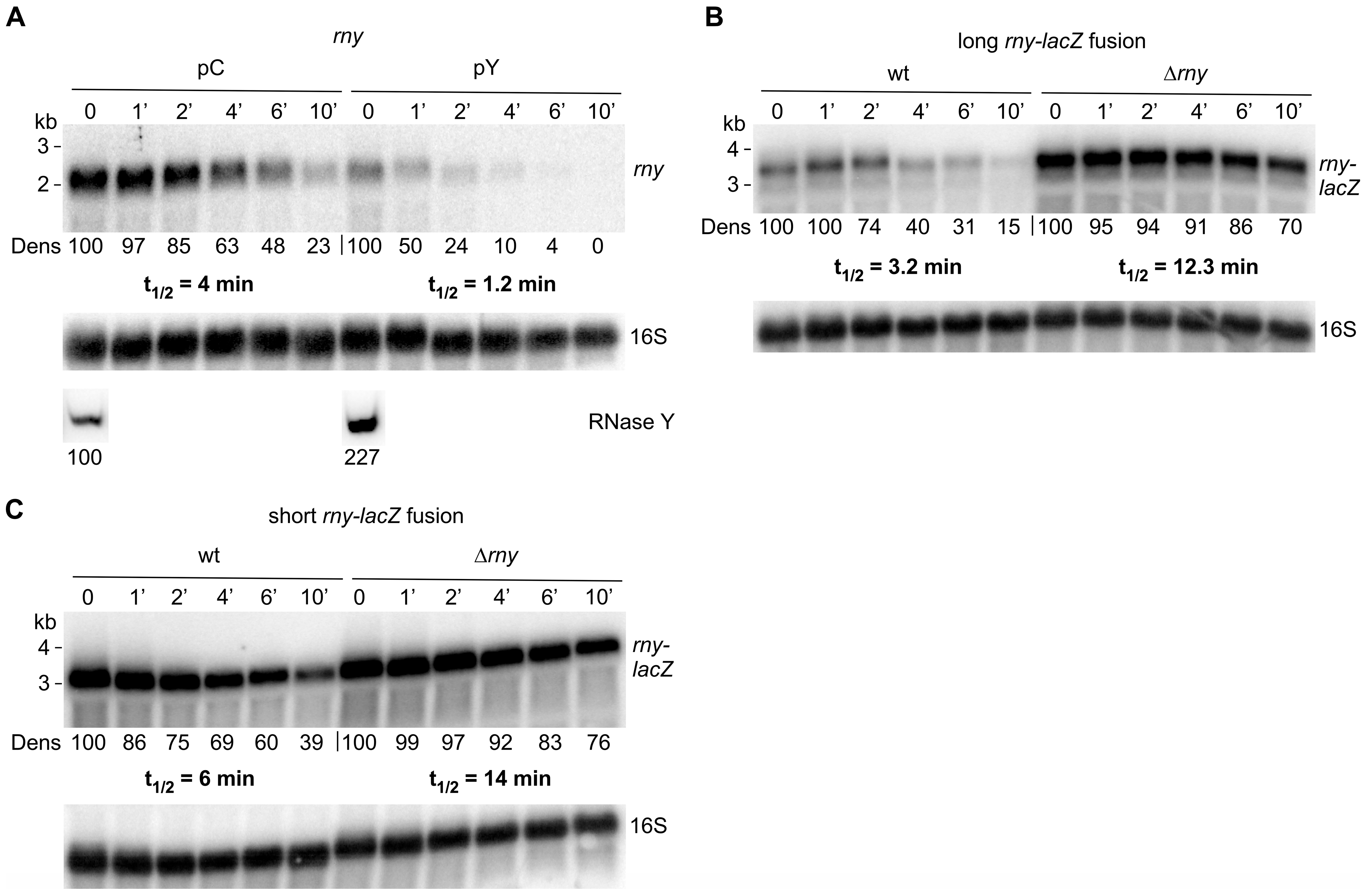
3.3. RNase Y Affects the Stability of Its Own mRNA
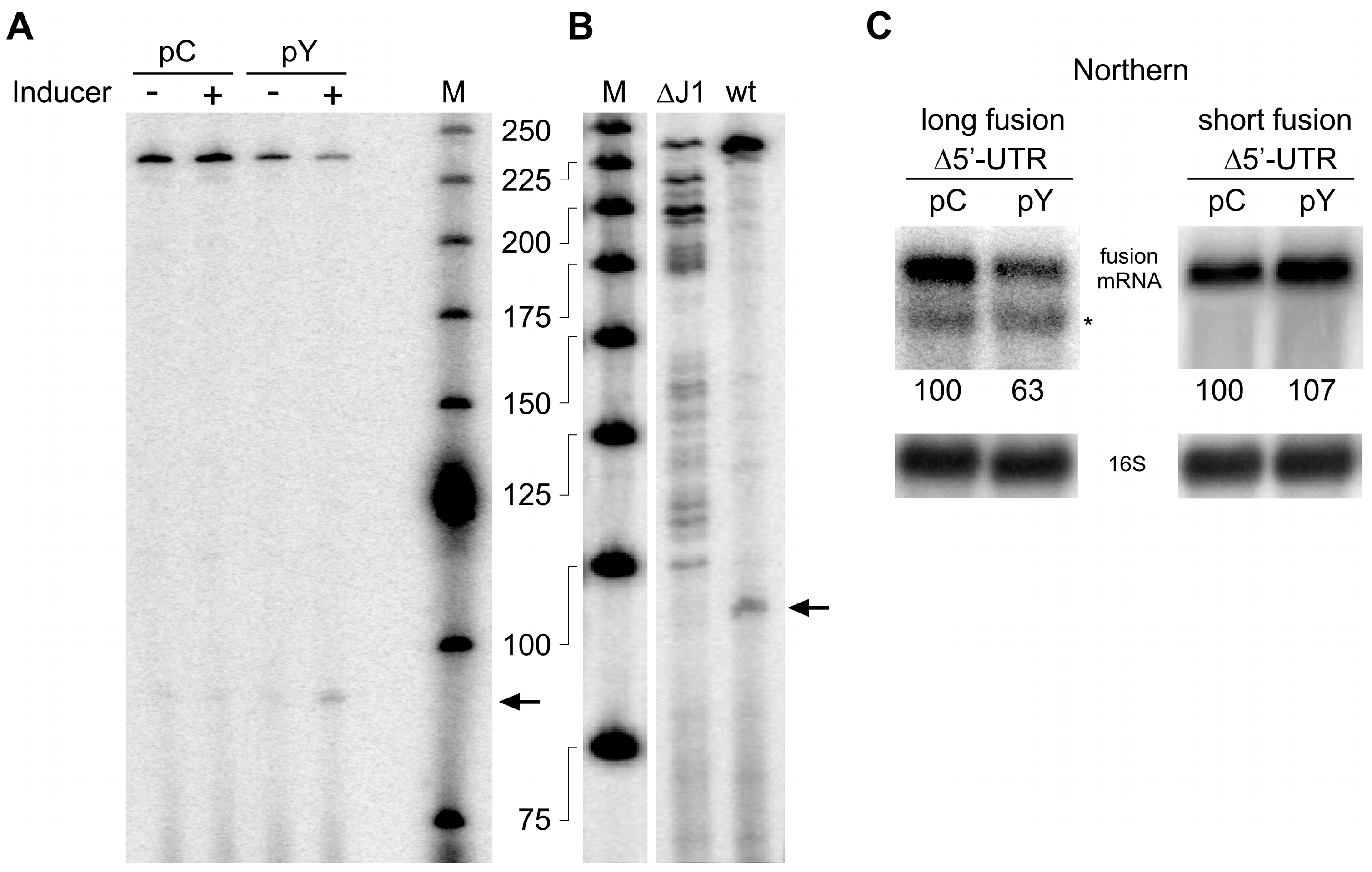
3.4. RNase Y Can Cleave Efficiently in the 5′ UTR of its mRNA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laalami, S.; Zig, L.; Putzer, H. Initiation of mRNA decay in bacteria. Cell. Mol. Life Sci. 2014, 71, 1799–1828. [Google Scholar] [CrossRef] [PubMed]
- Shahbabian, K.; Jamalli, A.; Zig, L.; Putzer, H. RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J. 2009, 28, 3523–3533. [Google Scholar] [CrossRef]
- Lehnik-Habrink, M.; Schaffer, M.; Mader, U.; Diethmaier, C.; Herzberg, C.; Stulke, J. RNA processing in Bacillus subtilis: Identification of targets of the essential RNase Y. Mol. Microbiol. 2011, 81, 1459–1473. [Google Scholar] [CrossRef]
- Durand, S.; Gilet, L.; Bessieres, P.; Nicolas, P.; Condon, C. Three essential ribonucleases-RNase Y, J1, and III-control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet. 2012, 8, e1002520. [Google Scholar] [CrossRef] [PubMed]
- Laalami, S.; Bessieres, P.; Rocca, A.; Zig, L.; Nicolas, P.; Putzer, H. Bacillus subtilis RNase Y activity in vivo analysed by tiling microarrays. PLoS ONE 2013, 8, e54062. [Google Scholar] [CrossRef]
- Richards, J.; Liu, Q.; Pellegrini, O.; Celesnik, H.; Yao, S.; Bechhofer, D.H.; Condon, C.; Belasco, J.G. An RNA pyrophosphohydrolase triggers 5′-exonucleolytic degradation of mRNA in Bacillus subtilis. Mol. Cell 2011, 43, 940–949. [Google Scholar] [CrossRef]
- Even, S.; Pellegrini, O.; Zig, L.; Labas, V.; Vinh, J.; Brechemmier-Baey, D.; Putzer, H. Ribonucleases J1 and J2: Two novel endoribonucleases in B. subtilis with functional homology to E. coli RNase E. Nucleic Acids Res. 2005, 33, 2141–2152. [Google Scholar] [CrossRef]
- Li de la Sierra-Gallay, I.; Zig, L.; Jamalli, A.; Putzer, H. Structural insights into the dual activity of RNase J. Nat. Struct. Mol. Biol. 2008, 15, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mathy, N.; Benard, L.; Pellegrini, O.; Daou, R.; Wen, T.; Condon, C. 5′-to-3′ Exoribonuclease Activity in Bacteria: Role of RNase J1 in rRNA Maturation and 5′ Stability of mRNA. Cell 2007, 129, 681–692. [Google Scholar] [CrossRef]
- Mathy, N.; Hebert, A.; Mervelet, P.; Benard, L.; Dorleans, A.; Li de la Sierra-Gallay, I.; Noirot, P.; Putzer, H.; Condon, C. Bacillus subtilis ribonucleases J1 and J2 form a complex with altered enzyme behaviour. Mol. Microbiol. 2010, 75, 489–498. [Google Scholar] [CrossRef]
- Khemici, V.; Prados, J.; Linder, P.; Redder, P. Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation. PLoS Genet. 2015, 11, e1005577. [Google Scholar] [CrossRef]
- Marincola, G.; Wolz, C. Downstream element determines RNase Y cleavage of the saePQRS operon in Staphylococcus aureus. Nucleic Acids Res. 2017, 45, 5980–5994. [Google Scholar] [CrossRef]
- DeLoughery, A.; Lalanne, J.B.; Losick, R.; Li, G.W. Maturation of polycistronic mRNAs by the endoribonuclease RNase Y and its associated Y-complex in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2018, 115, E5585–E5594. [Google Scholar] [CrossRef] [PubMed]
- Broglia, L.; Lecrivain, A.L.; Renault, T.T.; Hahnke, K.; Ahmed-Begrich, R.; Le Rhun, A.; Charpentier, E. An RNA-seq based comparative approach reveals the transcriptome-wide interplay between 3′-to-5′ exoRNases and RNase Y. Nat. Commun. 2020, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Ehretsmann, C.; Carpousis, A.J.; Krisch, H.M. Specificity of Escherichia coli endoribonuclease RNAse E: In vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992, 6, 149–159. [Google Scholar] [CrossRef]
- Mackie, G.A. Secondary structure of the mRNA for ribosomal protein S20. Implications for cleavage by ribonuclease E. J. Biol. Chem. 1992, 267, 1054–1061. [Google Scholar] [CrossRef]
- McDowall, K.J.; Lin-Chao, S.; Cohen, S.N. A + U content rather than a particular nucleotide order determines the specificity of RNase E cleavage. J. Biol. Chem. 1994, 269, 10790–10796. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Li, L.; Girodat, D.; Forstner, K.U.; Said, N.; Corcoran, C.; Smiga, M.; Papenfort, K.; Reinhardt, R.; Wieden, H.J.; et al. In Vivo Cleavage Map Illuminates the Central Role of RNase E in Coding and Non-coding RNA Pathways. Mol. Cell 2017, 65, 39–51. [Google Scholar] [CrossRef]
- Bandyra, K.J.; Luisi, B.F. RNase E and the High-Fidelity Orchestration of RNA Metabolism. Microbiol. Spectr. 2018, 6, 23. [Google Scholar] [CrossRef]
- Del Campo, C.; Bartholomaus, A.; Fedyunin, I.; Ignatova, Z. Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function. PLoS Genet. 2015, 11, e1005613. [Google Scholar] [CrossRef]
- Laalami, S.; Cavaiuolo, M.; Roque, S.; Chagneau, C.; Putzer, H. Escherichia coli RNase E can efficiently replace RNase Y in Bacillus subtilis. Nucleic Acids Res. 2021, 49, 4643–4654. [Google Scholar] [CrossRef]
- Khemici, V.; Poljak, L.; Luisi, B.F.; Carpousis, A.J. The RNase E of Escherichia coli is a membrane-binding protein. Mol. Microbiol. 2008, 70, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Turlan, C.; Khalid, S.; Bond, P.J.; Kebalo, J.M.; Peyron, P.; Poljak, L.; Bouvier, M.; Hamoen, L.; Luisi, B.F.; et al. Membrane recognition and dynamics of the RNA degradosome. PLoS Genet. 2015, 11, e1004961. [Google Scholar] [CrossRef]
- Hunt, A.; Rawlins, J.P.; Thomaides, H.B.; Errington, J. Functional analysis of 11 putative essential genes in Bacillus subtilis. Microbiology 2006, 152, 2895–2907. [Google Scholar] [CrossRef]
- Lewis, P.J.; Thaker, S.D.; Errington, J. Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J. 2000, 19, 710–718. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Weber, M.H.; Graumann, P.L. Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep. 2001, 2, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Hamouche, L.; Billaudeau, C.; Rocca, A.; Chastanet, A.; Ngo, S.; Laalami, S.; Putzer, H. Dynamic Membrane Localization of RNase Y in Bacillus subtilis. mBio 2020, 11, e03337-19. [Google Scholar] [CrossRef]
- Commichau, F.M.; Rothe, F.M.; Herzberg, C.; Wagner, E.; Hellwig, D.; Lehnik-Habrink, M.; Hammer, E.; Völker, U.; Stülke, J. Novel activities of glycolytic enzymes in Bacillus subtilis: Interactions with essential proteins involved in mRNA processing. Mol. Cell Proteom. 2009, 8, 1350–1360. [Google Scholar] [CrossRef]
- Lehnik-Habrink, M.; Pfortner, H.; Rempeters, L.; Pietack, N.; Herzberg, C.; Stülke, J. The RNA degradosome in Bacillus subtilis: Identification of CshA as the major RNA helicase in the multiprotein complex. Mol. Microbiol. 2010, 77, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Carpousis, A.J.; Van Houwe, G.; Ehretsmann, C.; Krisch, H.M. Copurification of E. coli RNAse E and PNPase: Evidence for a specific association between two enzymes important in mRNA processing and degradaion. Cell 1994, 76, 889–900. [Google Scholar] [CrossRef]
- Gao, J.; Lee, K.; Zhao, M.; Qiu, J.; Zhan, X.; Saxena, A.; Moore, C.J.; Cohen, S.N.; Georgiou, G. Differential modulation of E. coli mRNA abundance by inhibitory proteins that alter the composition of the degradosome. Mol. Microbiol. 2006, 61, 394–406. [Google Scholar] [CrossRef]
- Cascante-Estepa, N.; Gunka, K.; Stulke, J. Localization of Components of the RNA-Degrading Machine in Bacillus subtilis. Front. Microbiol. 2016, 7, 1492. [Google Scholar] [CrossRef]
- Newman, J.A.; Hewitt, L.; Rodrigues, C.; Solovyova, A.S.; Harwood, C.R.; Lewis, R.J. Dissection of the network of interactions that links RNA processing with glycolysis in the Bacillus subtilis degradosome. J. Mol. Biol. 2012, 416, 121–136. [Google Scholar] [CrossRef]
- Redder, P. Molecular and genetic interactions of the RNA degradation machineries in Firmicute bacteria. Wiley Interdiscip. Rev. RNA 2018, 9, e1460. [Google Scholar] [CrossRef]
- Adusei-Danso, F.; Khaja, F.T.; DeSantis, M.; Jeffrey, P.D.; Dubnau, E.; Demeler, B.; Neiditch, M.B.; Dubnau, D. Structure-Function Studies of the Bacillus subtilis Ric Proteins Identify the Fe-S Cluster-Ligating Residues and Their Roles in Development and RNA Processing. mBio 2019, 10, e01841-19. [Google Scholar] [CrossRef]
- Carabetta, V.J.; Tanner, A.W.; Greco, T.M.; Defrancesco, M.; Cristea, I.M.; Dubnau, D. A complex of YlbF, YmcA and YaaT regulates sporulation, competence and biofilm formation by accelerating the phosphorylation of Spo0A. Mol. Microbiol. 2013, 88, 283–300. [Google Scholar] [CrossRef]
- DeLoughery, A.; Dengler, V.; Chai, Y.; Losick, R. Biofilm formation by Bacillus subtilis requires an endoribonuclease-containing multisubunit complex that controls mRNA levels for the matrix gene repressor SinR. Mol. Microbiol. 2016, 99, 425–437. [Google Scholar] [CrossRef]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Messing, J. New M13 vectors for cloning genes. In Methods in Enzymology; Wu, R., Grossman, L., Moldave, K., Eds.; Academic Press: New York, NY, USA, 1983; Volume 101, Part C, pp. 20–89. [Google Scholar]
- Jamalli, A.; Hebert, A.; Zig, L.; Putzer, H. Control of expression of the RNases J1 and J2 in Bacillus subtilis. J. Bacteriol. 2014, 196, 318–324. [Google Scholar] [CrossRef]
- Guerout-Fleury, A.M.; Shazand, K.; Frandsen, N.; Stragier, P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 1995, 167, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Heusterspreute, M.; Thi, V.H. Vectors with restriction site banks. IV. pJRD184, a 3793-bp plasmid vector having 43 unique cloning sites. Gene 1985, 39, 299–304. [Google Scholar] [CrossRef]
- Geissendorfer, M.; Hillen, W. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl. Microbiol. Biotechnol. 1990, 33, 657–663. [Google Scholar] [CrossRef]
- Guerout-Fleury, A.M.; Frandsen, N.; Stragier, P. Plasmids for ectopic integration in Bacillus subtilis. Gene 1996, 180, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Putzer, H.; Brackhage, A.A.; Grunberg-Manago, M. Independent genes for two threonyl-tRNA synthetases in Bacillus subtilis. J. Bacteriol. 1990, 172, 4593–4602. [Google Scholar] [CrossRef]
- Putzer, H.; Gendron, N.; Grunberg-Manago, M. Co-ordinate expression of the two threonyl-tRNA synthetase genes in Bacillus subtilis: Control by transcriptional antitermination involving a conserved regulatory sequence. EMBO J. 1992, 11, 3117–3127. [Google Scholar] [CrossRef]
- Irnov, I.; Sharma, C.M.; Vogel, J.; Winkler, W.C. Identification of regulatory RNAs in Bacillus subtilis. Nucleic Acids Res. 2010, 38, 6637–6651. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 1998, 23, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Lehnik-Habrink, M.; Newman, J.; Rothe, F.M.; Solovyova, A.S.; Rodrigues, C.; Herzberg, C.; Commichau, F.M.; Lewis, R.J.; Stulke, J. RNase Y in Bacillus subtilis: A Natively disordered protein that is the functional equivalent of RNase E from Escherichia coli. J. Bacteriol. 2011, 193, 5431–5441. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, P.; Velours, C.; Bou-Nader, C.; Assrir, N.; Laalami, S.; Putzer, H.; Durand, D.; Golinelli-Pimpaneau, B. Dissociation of the Dimer of the Intrinsically Disordered Domain of RNase Y upon Antibody Binding. Biophys. J. 2018, 115, 2102–2113. [Google Scholar] [CrossRef]
- Yusupova, G.Z.; Yusupov, M.M.; Cate, J.H.; Noller, H.F. The path of messenger RNA through the ribosome. Cell 2001, 106, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hue, K.K.; Cohen, S.D.; Bechhofer, D.H. A polypurine sequence that acts as a 5′ mRNA stabilizer in Bacillus subtilis. J. Bacteriol. 1995, 177, 3465–3471. [Google Scholar] [CrossRef]
- Hambraeus, G.; Karhumaa, K.; Rutberg, B. A 5′ stem-loop and ribosome binding but not translation are important for the stability of Bacillus subtilis aprE leader mRNA. Microbiology 2002, 148, 1795–1803. [Google Scholar] [CrossRef]
- Schuck, A.; Diwa, A.; Belasco, J.G. RNase E autoregulates its synthesis in Escherichia coli by binding directly to a stem-loop in the rne 5′ untranslated region. Mol. Microbiol. 2009, 72, 470–478. [Google Scholar] [CrossRef]
- Vienna RNA Webservers. Available online: http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on 10 May 2023).
- Wei, Y.; Silke, J.R.; Xia, X. Elucidating the 16S rRNA 3′ boundaries and defining optimal SD/aSD pairing in Escherichia coli and Bacillus subtilis using RNA-Seq data. Sci. Rep. 2017, 7, 17639. [Google Scholar] [CrossRef]
| B. subtilis Strain | Relevant Genotype | Reference |
|---|---|---|
| SSB1002 | Wild-type strain | Lab stock |
| SSB525 | amyE::rny-lacZ (short, 5 aa) | This work |
| SSB530 | amyE::rny-lacZ (long, 210 aa) | This work |
| SSB593 | amyE::rny-lacZ (short, 5 aa), ∆rny::spc | This work |
| SSB594 | amyE::rny-lacZ (long, 210 aa), ∆rny::spc | This work |
| SSB599 | amyE::rny-lacZ (13 aa) | This work |
| SSB600 | amyE::rny-lacZ (31 aa) | This work |
| SSB602 | amyE::rny-lacZ (13 aa), ∆rny::spc | This work |
| SSB603 | amyE::rny-lacZ (31 aa), ∆rny::spc | This work |
| SSB614 | SSB1002, pWH353m | This work |
| SSB615 | SSB1002, pHMD22 | This work |
| SSB616 | SSB1002, pHMD33 | This work |
| SSB630 | amyE::rny-lacZ (short, 5 aa, ∆5′ UTR), pWH353 | This work |
| SSB631 | amyE::rny-lacZ (short, 5 aa, ∆5′ UTR), pHMD22 | This work |
| SSB638 | amyE::rny-lacZ (long, 210 aa, ∆5′ UTR), pWH353 | This work |
| SSB639 | amyE::rny-lacZ (long, 210 aa, ∆5′ UTR), pHMD22 | This work |
| SSB1082 | amyE::rny-lacZ (short, 5 aa), pWH353m | This work |
| SSB1083 | amyE::rny-lacZ (short, 5 aa), pHMD22 | This work |
| SSB1084 | amyE::rny-lacZ (long, 210 aa), pWH353m | This work |
| SSB1085 | amyE::rny-lacZ (long, 210 aa), pHMD22 | This work |
| SSB1086 | amyE::rny-lacZ (short, 5 aa), ∆rnjA::tet | This work |
| Oligonucleotide | Sequence 5′–3′ |
|---|---|
| HP899 | ACTGAGAATTCACGGTTTTTCTCGTACTTTCCGGT |
| HP905 | GATTAAGTTGGGTAACGCCAGGGT |
| HP1024 | ATTACCTGCAGACCGCTCATAACTCCAATAC |
| HP1039 | ACTATCTCGAGTCCTGCCGATCATTATGGAGGT |
| HP1040 | TAAGTTCTAGATGACGATGGTAAAAGGGCAGAC |
| HP1714 | ATCGGAATTCTGATTGGCGATCTTCTTTTGG |
| HP1715 | TATCGGATCCATAATTGGGGTCATACTTTCAC |
| HP1801 | TATCGGATCCGTTGTTTCGGCAACGTGGTC |
| HP1832 | ATCCGCATGCACCAAGTTCATAGCAAG |
| HP1833 | ATACTGTCGACTAAACAAAAAACCCAGCTCATTAAGC |
| TGGGTTTGCGCATCACTTTATTTTGCATACTCTACGGCT | |
| CGAGTC | |
| HP2078 | ACTGAGAATTCCGTAAACACTACATTCAAAATAATCC |
| HP2079 | CATAGCAAGAGGAGGTGAAAGTATGAATACATACGAA |
| CAAATTAATAAAG | |
| HP2080 | CTTTATTAATTTGTTCGTATGTATTCATACTTTCACCTCCT |
| CTTGCTATG | |
| HP2083 | TAAGTTCTAGAGGATAAGTGAGTGTTCATTAGAAC |
| HP2104 | AATTCCTTGCCGTCAAAAAAATAAAGTGATGCTTAGCGC |
| ATCACTTTATAATTTTTTTAATCTGTTATTTAAATAGTTTA | |
| TAG | |
| HP2104rev | CTATAAACTATTTAAATAACAGATTAAAAAAATTATAAA |
| GTGATGCGCTAAGCATCACTTTATTTTTTTGACGGCAAGG | |
| AATT | |
| HP2113 | CAGCGTTCGTCCTGAGC |
| HP2164 | ATCGGAATTCTGATTGGCGATCTTCTTTTGGATGAATTGC |
| HP2165 | TATCGGATCCAGCAAAATGGAGATGAGAACCATCATAA |
| TTGG | |
| HP2227 | GGGTCTTCTTGCCGCCATCGGGAAAGC |
| HP2228 | GCTTTCCCGATGGCGGCAAGAAGACCC |
| HP2166 | TATCGGATCCGCTTCGGCAATGGTTTTAC |
| HP2238 | GTAACATTTGTTTTTTTAACATGATTG |
| HP2239 | TTCAATTAATACGACTCACTATAGGTTGTTGCTTAAA |
| AAGTGTC | |
| HP2248 | CTCCAGTCTTCACATCGGTTTG |
| HP2249 | GGTTTTGGCCGACGCTGGATCTC |
| HP2268 | TATCGGATCCATAATTGGGGTCATACTTTCACCTCCTCTT GCTATGTTACAACTTCATTGTAAATGTGTCGG |
| HP2269 | GGGTCATACTTTCACCTCCTCTTGCTATGTTACAACTTCA |
| TTGTAAATGTGTCGGAAATACTTG | |
| HP2270 | CAGTTGCGCAGCCTGAATGG |
| HP2271 | TAATACGACTCACTATAGGGAACAAACGGCGGATTGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobeinikova, A.; Laalami, S.; Berthy, C.; Putzer, H. RNase Y Autoregulates Its Synthesis in Bacillus subtilis. Microorganisms 2023, 11, 1374. https://doi.org/10.3390/microorganisms11061374
Korobeinikova A, Laalami S, Berthy C, Putzer H. RNase Y Autoregulates Its Synthesis in Bacillus subtilis. Microorganisms. 2023; 11(6):1374. https://doi.org/10.3390/microorganisms11061374
Chicago/Turabian StyleKorobeinikova, Anna, Soumaya Laalami, Clément Berthy, and Harald Putzer. 2023. "RNase Y Autoregulates Its Synthesis in Bacillus subtilis" Microorganisms 11, no. 6: 1374. https://doi.org/10.3390/microorganisms11061374





