Culture-Dependent and Metabarcoding Characterization of the Sugar Beet (Beta vulgaris L.) Microbiome for High-Yield Isolation of Bacteria with Plant Growth-Promoting Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. DNA Isolation for 16S rRNA Metabarcoding
2.3. DNA Sequencing and Analysis
2.4. Isolation and Characterization of Bacteria from Plant Samples
| Medium | Composition | Reference |
|---|---|---|
| 0.1 TSA | TSB 3 g/L, agar 15 g/L | [28] |
| 0.01 TSA | TSB 0.3 g/L, agar 15 g/L | |
| WY | NaCl 5 g/L, yeast extract 0.05 g/L, agar 20 g/L | |
| PS | solution A: (NH4)2SO4 2.27 mM, MgSO4 0.2 mM, CaCl2 45 μM, agar 15 g/L solution B: KH2PO4 10 mM, Na2HPO4×12H2O 10 mM solution C: peptone 0.1 g/L, yeast extract 0.1 g/L, glucose 0.1 g/L | [11] |
| LEa | leaf extract 50 mL/L, agar 20 g/L | [4] |
| LEph | leaf extract 50 mL/L, phytagel 20 g/L | |
| REa | root extract 50 mL/L, agar 20 g/L | |
| REph | root extract 50 mL/L, phytagel 20 g/L | |
| SEa | glucose 1 g/L, peptone 1 g/L, yeast extract 1 g/L, K2HPO4 1 g/L, soil extract 400 mL/L, agar 15 g/L | [12] |
| SEph | glucose 1 g/L, peptone 1 g/L, yeast extract 1 g/L, K2HPO4 1 g/L, soil extract 400 mL/L, phytagel 20 g/L | |
| ISEM1 | I mineral salts: K2HPO4 0.23 g/L, MgSO4×7H2O 0.23 g/L, NH4NO3 0.33 g/L, NaHCO3 0.25 g/L, agar 15 g/L II amino acids: d-valine 5 mg, d-methionine 5 mg, d-leucine 5 mg, d-phenylalanine 5 mg, d-threonine 5 mg, and d-tryptophan 5 mg III vitamin B: 1 mL vitamin stock solution containing 50 mg each thiamine hydrochloride, riboflavin, niacin, pyridoxine HCl, inositol, calcium pantothenate, and β-aminobenzoic acid and 25 mg biotin in 100 mL distilled water III selenite–tungstate solution: 2 mL/L compositions in 1 L of distilled water: NaOH 0.5 g, Na2SeO3·5H2O 3 mg, Na2WO4×2H2O 4 mg IV trace elements: 2 mL/L HCl [25%, v/v] 10 mL, FeCl2·4H2O 1.5 g, ZnCl2 70 mg, MnCl2×4H2O 100 mg, H3BO3 6 mg, CoCl2×6H2O 190 mg, CuCl2×2H2O 2 mg, NiCl2×6H2O 24 mg, Na2MoO4×2H2O 36 mg in a final volume of 1 liter V methanol soil extract 200 mL/L | [5] |
| ISEM2 | All is the same as in ISEM1 but V is different where water soil extract 200 mL/L was used. | |
| MSEM | I mineral salts as in ISME with methanol soil extract 200 mL/L | This work |
| WSEM | I mineral salts as in ISME with water soil extract 200 mL/L |
2.5. Bacteria Identification to Species Level
2.6. Characterization of Plant-Beneficial Traits
3. Results
3.1. Seasonal Analysis of the Sugar Beet Rhizobiome and Phyllobiome by 16S rRNA Gene Metabarcoding
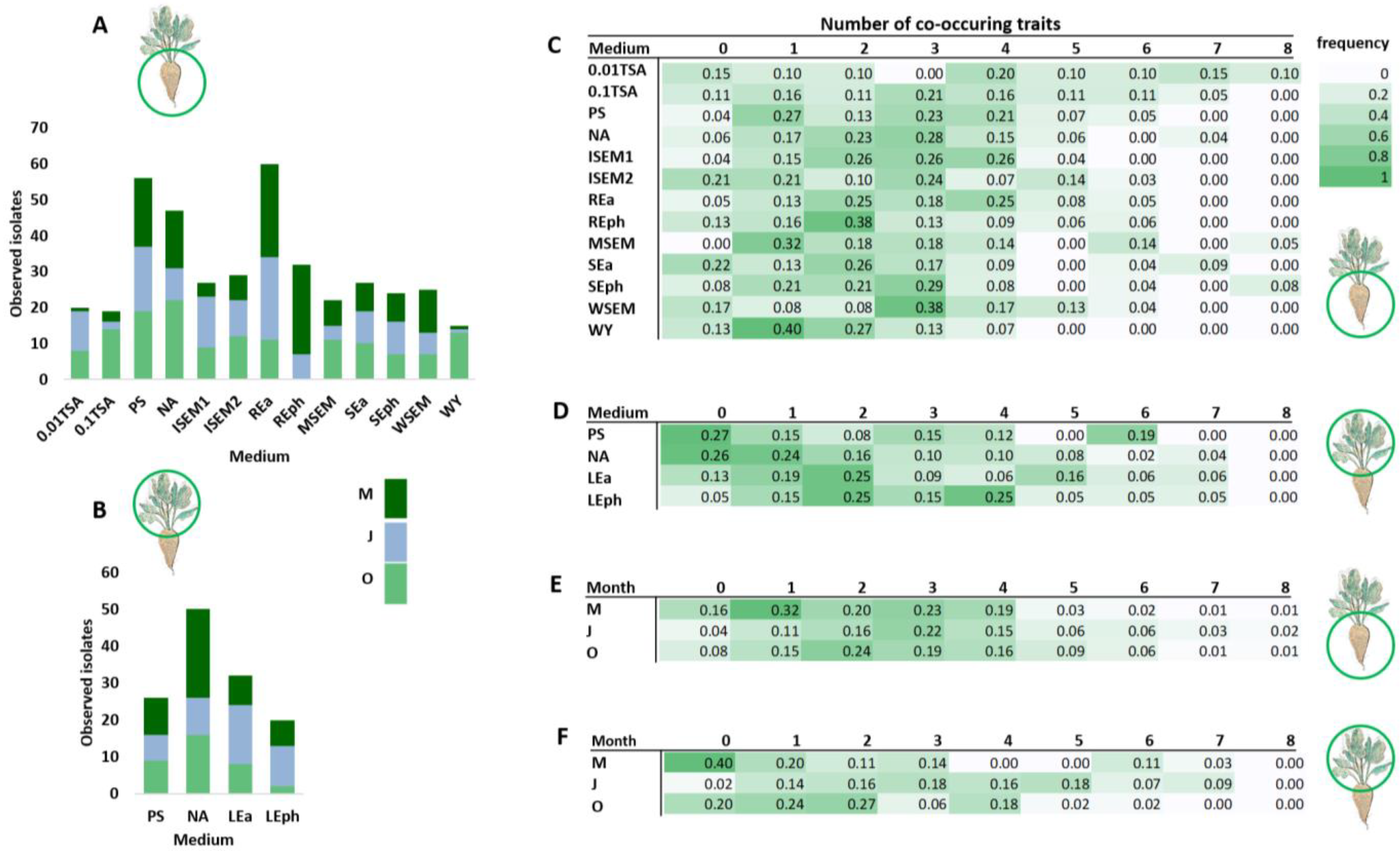
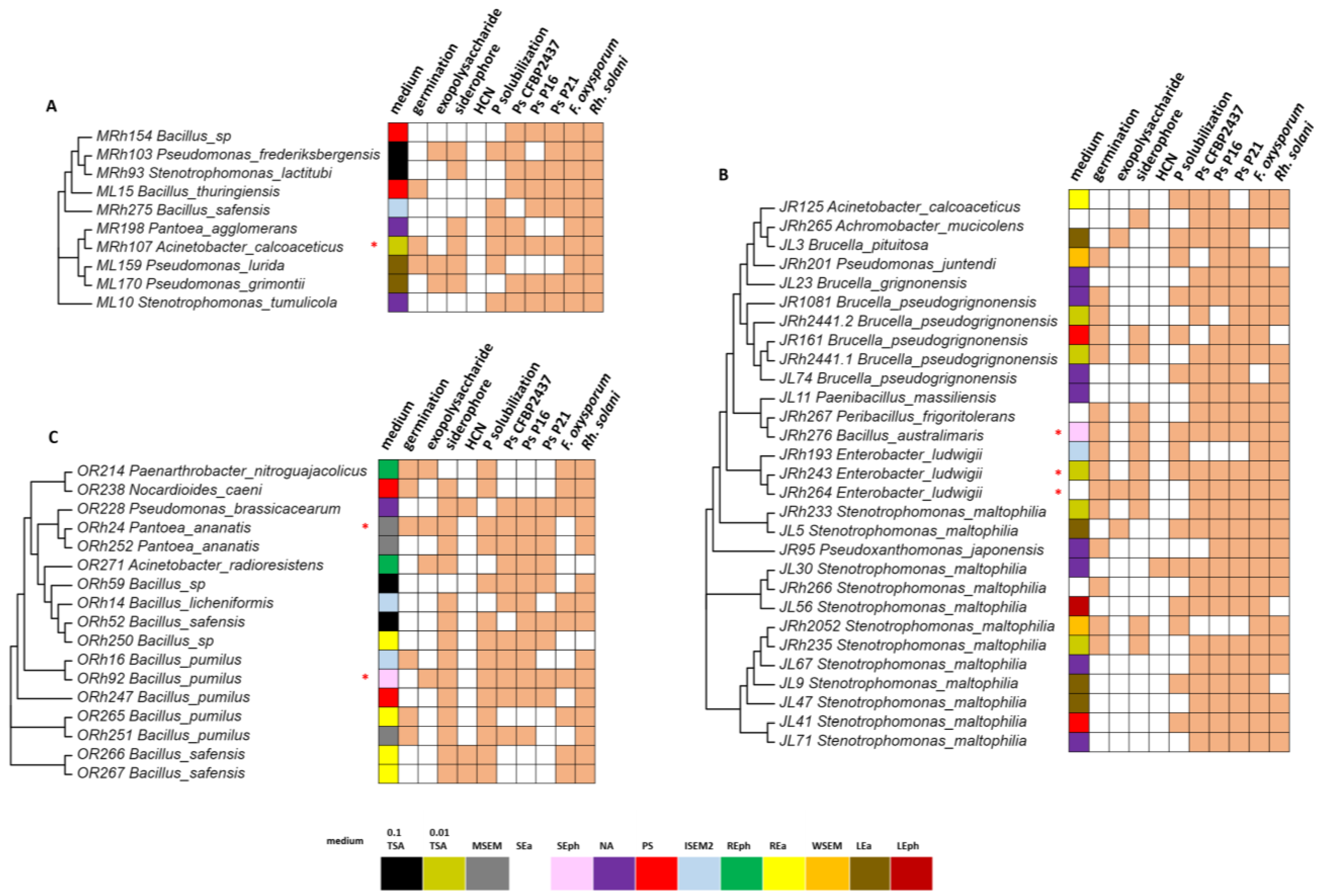
3.2. Isolation and Characterization of Plant-Beneficial Bacteria from the Sugar Beet Microbiome
3.3. Comparison of Culture-Dependent and -Independent Approaches in the Detection of Plant-Beneficial Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Lentendu, G.; Lewin, S.; Kolb, S. DNA Metabarcoding for the Characterization of Terrestrial Microbiota—Pitfalls and Solutions. Microorganisms 2021, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.H.; Hamza, M.A.; Fayez, M.; Mourad, E.F.; Saleh, M.Y.; Sarhan, M.S.; Suker, R.M.; Eltahlawy, A.A.; Nemr, R.A.; El-Tahan, M.; et al. Plant-based culture media: Efficiently support culturing rhizobacteria and correctly mirror their in-situ diversity. J. Adv. Res. 2016, 7, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.M.; Seo, C.; Ji, M.; Paik, M.J.; Myung, S.W.; Kim, J. Effective soil extraction method for cultivating previously uncultured soil bacteria. Appl. Environ. Microbiol. 2018, 84, e01145-18. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Okazaki, K.; Takahashi, H.; Tsurumaru, H.; Minamisawa, K. Seasonal Shifts in Bacterial Community Structures in the Lateral Root of Sugar Beet Grown in an Andosol Field in Japan. Microbes Environ. 2023, 38, ME22071. [Google Scholar] [CrossRef]
- FAOSTAT. Crops—Production/Yield Quantities of Sugar Beet. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 23 February 2023).
- Okazaki, K.; Tsurumaru, H.; Hashimoto, M.; Takahashi, H.; Okubo, T.; Ohwada, T.; Minamisawa, K.; Ikeda, S. Community Analysis-based Screening of Plant Growth-promoting Bacteria for Sugar Beet. Microbes Environ. 2021, 36, ME20137. [Google Scholar] [CrossRef]
- Çakmakçi, R.; Dönmez, F.; Aydın, A.; Şahin, F. Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]
- Shi, Y.; Lou, K.; Li, C. Promotion of plant growth by phytohormone-producing endophytic microbes of sugar beet. Biol. Fertil. Soils 2009, 45, 645–653. [Google Scholar] [CrossRef]
- Kato, S.; Yamagishi, A.; Daimon, S.; Kawasaki, K.; Tamaki, H.; Kitagawa, W.; Abe, A.; Tanaka, M.; Sone, T.; Asano, K.; et al. Isolation of Previously Uncultured Slow growing Bacteria by Using a Simple Modification in the Preparation of Agar Media. Appl. Environ. Microbiol. 2018, 84, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Mourad, E.F.; Sarhan, M.S.; Daanaa, H.S.A.; Abdou, M.; Morsi, A.T.; Abdelfadeel, M.R.; Elsawey, H.; Nemr, R.; El-Tahan, M.; Hamza, M.A.; et al. Plant materials are sustainable substrates supporting new technologies of plant-only-based culture media for in vitro culturing of the plant microbiota. Microbes Environ. 2018, 33, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.; Stanković, S.; Dimkić, I.; Berić, T.; Stojšin, V.; Janse, J.; Popović, T. Genetic diversity and pathogenicity of Pseudomonas syringae pv. aptata isolated from sugar beet. Plant Pathol. 2018, 67, 1194–1207. [Google Scholar]
- Hanson, L.; De Lucchi, C.; Stevanato, P.; McGrath, M.; Panella, L.; Sella, L.; De Biaggi, M.; Concheria, G. Root rot symptoms in sugar beet lines caused by Fusarium oxysporum f. sp. betae. Eur. J. Plant Pathol. 2018, 150, 589–593. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia solani in Sugar Beet and Effect of Fungicide Application and Plant Cultivar on Inoculum Potential in the Soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 19, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Costa, L.E.; de Queiroz, M.V.; Borges, A.C.; de Moraes, C.A.; de Araújo, E.F. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz. J. Microbiol. 2012, 43, 1562–1575. [Google Scholar] [CrossRef]
- Berić, T.; Urdaci, M.C.; Stanković, S.; Knežević-Vukčević, J. Rapd Analysis of Genetic Diversity and Qualitative Assessment of Hydrolytic Activities In a Collection of Bacillus sp. Isolate. Arch. Biol. Sci. 2009, 61, 645–652. [Google Scholar] [CrossRef]
- Kurm, V.; Van Der Putten, W.H.; Hol, W.H.G. Cultivation-success of rare soil bacteria is not influenced by incubation time and growth medium. PLoS ONE 2019, 14, 0210073. [Google Scholar] [CrossRef]
- Draganić, V.; Lozo, J.; Biočanin, M.; Dimkić, I.; Garalejić, E.; Fira, D.J.; Stanković, S.; Berić, T. Genotyping of Bacillus Spp. Isolate Collection from Natural Samples. Genetika 2017, 2, 445–456. [Google Scholar] [CrossRef]
- Sarkar, S.F.; Guttman, D.S. Evolution of the core genome of Pseudomonas syringae, a highly clonal, endemic plant pathogen. Appl. Environ. Microbiol. 2004, 70, 1999–2012, Erratum in Appl. Environ. Microbiol. 2008, 74, 1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1997, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Lopes, A.C.; Mendes, L.W.; Brito, K.A.S.B.; da Silva, J.L.; Rocha, S.M.B.; Antunes, J.E.L.; de Souza Oliveira, L.M.; Melo, V.M.M.; Oliveira, F.A.S.; de Araujo Pereira, A.P.; et al. Rhizospheric microbial community in plant species from the Phaseolus genus. Appl. Soil Ecol. 2023, 182, 104731. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, X.; Fang, Y.; Ogunyemi, S.O.; Ahmed, T.; Li, X.; Yang, Y.; Yan, C.; Chen, J.; Li, B. Metabarcoding reveals response of rice rhizosphere bacterial community to rice bacterial leaf blight. Microbiol. Res. 2023, 270, 127344. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, H.; Zhang, T.; Sun, J.; Lou, K. Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of Tianshan mountain. Appl. Microbiol. Biotechnol. 2014, 98, 6375–6385. [Google Scholar] [CrossRef] [PubMed]
- Hudz, S.O.; Skivka, L.M. Formation of the eubacterial complex in the rhizosphere of sugar beet (Beta vulgaris) under different fertilization systems. Biotechnol. Acta 2021, 14, 81–86. [Google Scholar] [CrossRef]
- Majumdar, R.; Strausbaugh, C.A.; Vincill, E.D.; Eujayl, I.; Galewski, P.J. Leaf bacteriome in Sugar beet shows differential response against beet curly top virus during resistant and susceptible interactions. Int. J. Mol. Sci. 2022, 23, 8073. [Google Scholar] [CrossRef]
- Broccanello, C.; Ravi, S.; Deb, S.; Bolton, M.; Secor, G.; Richards, C.; Maretto, L.; Lucia, M.C.D.; Bertoldo, G.; Orsini, E.; et al. Bacterial endophytes as indicators of susceptibility to Cercospora Leaf Spot (CLS) disease in Beta vulgaris L. Sci Rep. 2022, 23, 10719. [Google Scholar] [CrossRef]
- Thompson, I.P.; Bailey, M.J.; Fenlon, J.S.; Fermor, T.R.; Lilley, A.K.; Lynch, J.M.; McCormack, P.J.; McQuilken, M.P.; Purdy, K.J.; Rainey, P.B.; et al. Quantitative and qualitative seasonal changes in the microbial community from the phyllosphere of sugar beet (Beta vulgaris). Plant Soil 1993, 150, 177–191. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Narayanan, M.; Pugazhendhi, A.; Ma, Y. Assessment of PGP traits of Bacillus cereus NDRMN001 and its influence on Cajanus cajan (L.) Millsp. phytoremediation potential on metal-polluted soil under controlled conditions. Front. Plant Sci. 2022, 13, 1017043. [Google Scholar] [CrossRef]
- De Oliveira, S.P.A.; do Nascimento, H.M.A.; Sampaio, K.B.; de Souza, E.L. A review on bioactive compounds of beet (Beta vulgaris L. subsp. vulgaris) with special emphasis on their beneficial effects on gut microbiota and gastrointestinal health. Crit. Rev. Food Sci. Nutr. 2021, 61, 2022–2033. [Google Scholar] [CrossRef]
- Senger, M.; Moresco, E.; Dalbosco, M.; Santin, R.; Inderbitzin, P.; Barrocas, E. Methods to quantify Bacillus simplex-based inoculant and its effect as a seed treatment on field-grown corn and soybean in Brazil. J. Seed Sci. 2022, 44, e202244040. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Martínez-Goñi, X.S.; Liñero, O.; Muñoz-Colmenero, M.; Aguirre, M.; Abad, D.; Baroja-Careaga, I.; Diego, A.d.; Gilbert, J.A.; Estonba, A. Response of horticultural soil microbiota to different fertilization practices. Plants 2020, 9, 1501. [Google Scholar] [CrossRef]
- Patz, S.; Witzel, K.; Scherwinski, A.-C.; Ruppel, S. Culture dependent and independent analysis of potential probiotic bacterial genera and species present in the phyllosphere of raw eaten produce. Int. J. Mol. Sci. 2019, 20, 3661. [Google Scholar] [CrossRef] [Green Version]
- Barbu, V.; Cotârleț, M.; Bolea, C.A.; Cantaragiu, A.; Andronoiu, D.G.; Bahrim, G.E.; Enachi, E. Three types of beetroot products enriched with lactic acid bacteria. Foods 2020, 9, 786. [Google Scholar] [CrossRef]
- Alotaibi, F.; St-Arnaud, M.; Hijri, M. In-depth characterization of plant growth promotion potentials of selected alkanes-degrading plant growth-promoting bacterial isolates. Front. Microbiol. 2022, 29, 863702. [Google Scholar] [CrossRef]
- Omar, A.F.; Abdelmageed, A.H.A.; Al-Turki, A.; Abdelhameid, N.M.; Sayyed, R.Z.; Rehan, M. Exploring the plant growth-promotion of four Streptomyces strains from rhizosphere soil to enhance Cucumber growth and yield. Plants 2022, 11, 3316. [Google Scholar] [CrossRef] [PubMed]
- Benaissa, A.; Djebbar, R.; Abderrahmani, A. Diversity of plant growth promoting rhizobacteria of Rhus tripartitus in arid soil of Algeria (Ahaggar) and their physiological properties under abiotic atresses. Adv. Hort. Sci. 2018, 32, 525–534. [Google Scholar]
- Youseif, S.H.; Abd El-Megeed, F.H.; Humm, E.A.; Maymon, M.; Mohamed, A.H.; Saleh, S.A.; Hirsch, A.M. Comparative analysis of the cultured and total bacterial community in the wheat rhizosphere microbiome using culture-dependent and culture-independent approaches. Microbiol. Spectr. 2021, 9, e0067821. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, R.C. Plant growth promoting endophytic bacteria Bacillus australimaris BLR41 and Enterobacter kobei BLR45 enhance the growth of medicinal plant Barleria lupulina Lindl. J. Pure Appl. Microbiol. 2022, 16, 2647–2658. [Google Scholar] [CrossRef]
- Shoebitz, M.; Ribaudo, C.M.; Pardo, M.A.; Cantore, M.L.; Ciampi, L.; Curá, J.A. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biol. Biochem. 2009, 41, 1768–1774. [Google Scholar] [CrossRef]
- Bendaha, M.E.A.; Belaouni, H.A. Effect of the endophytic plant growth promoting Enterobacter ludwigii EB4B on tomato growth. Hellenic Plant Prot. J. 2020, 13, 54–65. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A.; Jha, B. Plant growth promoting rhizobacterium Stenotrophomonas maltophilia BJ01 augments endurance against N2 starvation by modulating physiology and biochemical activities of Arachis hypogea. PLoS ONE 2019, 14, e0222405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 9, 1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, W.; Sugawara, M.; Miwa, K.; Morikawa, M. Plant growth-promoting bacterium Acinetobacter calcoaceticus P23 increases the chlorophyll content of the monocot Lemna minor (duckweed) and the dicot Lactuca sativa (lettuce). J. Biosci. Bioeng. 2014, 118, 41–44. [Google Scholar] [CrossRef]
- Weller-Stuart, T.; De Maayer, P.; Coutinho, T. Pantoea ananatis: Genomic insights into a versatile pathogen. Mol. Plant Pathol. 2017, 18, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Liu, J.; Guo, W.H.; Li, X.B.; Xia, J.B.; Xie, W.J.; Yao, Z.G.; Zhang, Y.M.; Wang, R.Q. Characterization and initial application of endophytic Bacillus safensis strain ZY16 for improving phytoremediation of oil-contaminated saline soils. Front. Microbiol. 2019, 10, 991. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić Tomić, T.; Atanasković, I.; Nikolić, I.; Joković, N.; Stević, T.; Stanković, S.; Berić, T.; Lozo, J. Culture-Dependent and Metabarcoding Characterization of the Sugar Beet (Beta vulgaris L.) Microbiome for High-Yield Isolation of Bacteria with Plant Growth-Promoting Traits. Microorganisms 2023, 11, 1538. https://doi.org/10.3390/microorganisms11061538
Krstić Tomić T, Atanasković I, Nikolić I, Joković N, Stević T, Stanković S, Berić T, Lozo J. Culture-Dependent and Metabarcoding Characterization of the Sugar Beet (Beta vulgaris L.) Microbiome for High-Yield Isolation of Bacteria with Plant Growth-Promoting Traits. Microorganisms. 2023; 11(6):1538. https://doi.org/10.3390/microorganisms11061538
Chicago/Turabian StyleKrstić Tomić, Tamara, Iva Atanasković, Ivan Nikolić, Nataša Joković, Tatjana Stević, Slaviša Stanković, Tanja Berić, and Jelena Lozo. 2023. "Culture-Dependent and Metabarcoding Characterization of the Sugar Beet (Beta vulgaris L.) Microbiome for High-Yield Isolation of Bacteria with Plant Growth-Promoting Traits" Microorganisms 11, no. 6: 1538. https://doi.org/10.3390/microorganisms11061538







