Effect of Agave Fructans on Changes in Chemistry, Morphology and Composition in the Biomass Growth of Milk Kefir Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Culture Conditions of Kefir Grain
2.3. Determination of Biomass of Kefir Grains
Optimization through Response Surface Methodology
2.4. Effect of Different Carbon Sources
2.5. Chemical Analysis
2.6. Microstructure Analysis
2.6.1. Environmental Scanning Electron Microscopy
2.6.2. Image Digital Analysis of ESEM
2.7. Gram Staining
2.8. Statistical Analysis
3. Results and Discussion
3.1. Kefir Grain Conditioning
3.2. Analysis of Biomass Increase in Conditioning
3.3. Kefir Grains Conditioning Optimization
3.4. Effect of Carbon Source and Culture Medium
3.5. Chemical Analysis
3.6. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in carbohydrate profile in kefir fermentation. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Pothuraju, R.; Yenuganti, V.R.; Hussain, S.A.; Sharma, M. 2018. Fermented Milk in Protection against Inflammatory Mechanisms in Obesity. In Immunity and Inflammation in Health and Disease; Academic Press: Cambridge, MA, USA, 2018; Chapter 29; pp. 389–401. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef]
- Júnior, L.M.; Vieira, R.P.; Anjos, C.A.R. Kefiran-based films: Fundamental concepts, formulation strategies and properties. Carbohydr. Polym. 2020, 246, 116609. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Ye, T.; Yang, X.; Xue, Y.; Shen, Y.; Zhang, Q.; Zheng, X. Effect of lactic acid bacteria and yeasts on the structure and fermentation properties of Tibetan kefir grains. Int. Dairy J. 2021, 114, 104943. [Google Scholar] [CrossRef]
- Zajšek, K.; Goršek, A.; Kolar, M. Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem. 2013, 139, 970–977. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimada, P.S.; Abraham, A.G. Kefiran improves rheological properties of glucono-δ-lactone induced skim milk gels. Int. Dairy J. 2006, 16, 33–39. [Google Scholar] [CrossRef]
- Piermaria, J.A.; Pinotti, A.; Garcia, M.A.; Abraham, A.G. Films based on kefiran, an exopolysaccharide obtained from kefir grain: Development and characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Suksawang, S.; Yeesang, J.; Boonsawang, P. Co-production of functional exopolysaccharides and lactic acid by Lactobacillus kefiranofaciens originated from fermented milk, kefir. J. Food Sci. Technol. 2018, 55, 331–340. [Google Scholar] [CrossRef]
- Apar, D.K.; Demirhan, E.; Özel, B.; Özbek, B. Kefir Grain Biomass Production: Influence of Different Culturing Conditions and Examination of Growth Kinetic Models. J. Food Process Eng. 2017, 40, e12332. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ghaly, M.F.; El-Naggar, A.K. Some physicochemical analyses of kefir produced under different fermentation conditions. J. Sci. Ind. Res. 2011, 70, 365–372. Available online: http://nopr.niscpr.res.in/handle/123456789/11645 (accessed on 22 March 2023).
- Li, Z.; Han, L.; Ji, Y.; Wang, X.; Tan, T. Fermentative production of l-lactic acid from hydrolysate of wheat bran by Lactobacillus rhamnosus. Biochem. Eng. J. 2010, 49, 138–142. [Google Scholar] [CrossRef]
- Harta, O.; Iconomopoulou, M.; Bekatorou, A.; Nigam, P.; Kontominas, M.; Koutinas, A.A. Effect of various carbohydrate substrates on the production of kefir grains for use as a novel baking starter. Food Chem. 2004, 88, 237–242. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Gradilla-Hernández, M.S.; Ortiz-Basurto, R.I.; García-Reyes, R.A.; González-Avila, M. Assessment of intermediate-and long-chains agave fructan fermentation on the growth of intestinal bacteria cultured in a gastrointestinal tract. Rev. Mex. Ing. Quim. 2020, 19, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Ceja-Medina, L.I.; Medina-Torres, L.; González-Ávila, M.; Martínez-Rodríguez, J.C.; Andrade-González, I.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A.; Ortiz-Basurto, R.I. In vitro synbiotic activity of Lactobacillus plantarum encapsulated with mixtures of Aloe vera mucilage, agave fructans and food additives as wall materials. Rev. Mex. Ing. Quím. 2021, 20, 711–723. [Google Scholar] [CrossRef]
- Velázquez-Martínez, J.R.; González-Cervantes, R.M.; Hernández-Gallegos, M.A.; Mendiola, R.C.; Aparicio, A.R.J.; Ocampo, M.L.A. Prebiotic Potential of Agave angustifolia Haw Fructans with Different Degrees of Polymerization. Molecules 2014, 19, 12660–12675. [Google Scholar] [CrossRef] [Green Version]
- Buitrago-Arias, C.; Londoño-Moreno, A.; Avila-Reyes, S.V.; Arenas-Ocampo, M.L.; Alamilla-Beltran, L.; Jimenez-Aparicio, A.R.; Camacho-Díaz, B.H. Evaluation of the fermentation of acetylated agave fructans (agavins), with Saccharomyces boulardii as a probiotic. Rev. Mex. De Ing. Quim. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Ruiz-Martínez, I.G.; Rodrigue, D.; Arenas-Ocampo, M.L.; Camacho-Díaz, B.H.; Avila-Reyes, S.V.; Solorza-Feria, J. Production and Characterization of Gelatin Biomaterials Based on Agave Microfibers and Bentonite as Reinforcements. Foods 2022, 11, 1573. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; González- Cortázar, M.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Identification and Quantification of β-Sitosterol β-d-Glucoside of an Ethanolic Extract Obtained by Microwave-Assisted Extraction from Agave angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.; Kok-Tas, T.; Ertekin-Filiz, B.; Seydim, A.C. Effect of different growth conditions on biomass increase in kefir grains. J. Dairy Sci. 2011, 94, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, R.; Mendoza, F.; Aguilera, J.M.; Chanona, J.; Gutiérrez-López, G. Determination of senescent spotting in banana (Musa cavendish) using fractal texture Fourier image. J. Food Eng. 2008, 84, 509–515. [Google Scholar] [CrossRef]
- Matos, R.S.; Gonçalves, E.; Pinto, E.P.; Lopes, G.A.; Ferreira, N.S.; Resende, C.X. Nanoscale morphology, structure and fractal study of kefir microbial films grown in natura. Polímeros 2020, 30, e2020033. [Google Scholar] [CrossRef]
- Du, C.J.; Sun, D.W. Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci. Technol. 2004, 15, 230–249. [Google Scholar] [CrossRef]
- Piermaria, J.A.; de la Canal, M.L.; Abraham, A.G. Gelling properties of kefiran, a food-grade polysaccharide obtained from kefir grain. Food Hydrocoll. 2008, 22, 1520–1527. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Ferreira, S.L.C.; Novaes, C.G.; Dos Santos, A.M.P.; Valásques, G.S.; da Mata Cerqueira, U.M.F.; dos Santos Alves, J.P. Simultaneous optimization of multiple responses and its application in analytical chemistry—A review. Talanta 2019, 194, 941–959. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Gharibzahedi, S.M.T. Enhanced Production of Iranian Kefir Grain Biomass by Optimization and Empirical Modeling of Fermentation Conditions Using Response Surface Methodology. Food Bioprocess Technol. 2012, 5, 3230–3235. [Google Scholar] [CrossRef]
- Pop, C.R.; Apostu, S.; Salanţă, L.; Rotar, A.M.; Sindic, M.; Mabon, N.; Socaciu, C. Influence of Different Growth Conditions on the Kefir Grains Production, used in the Kefiran Synthesis. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca J. Food Sci. Technol. 2014, 71, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Bermal, J.M.; Serna-Jimenez, J.A.; Uribe-Bohorquez, M.; Klotz, B.; Quintanilla-Carvajal, M.X. Application of response surface methodology to evaluate the effect of the concentration of sugar and commercials starters on the fermentation kinetics of yogurt. Rev. Mex. Ing. Quim. 2014, 13, 363–371. [Google Scholar]
- International, A. AOAC: Official Methods of Analysis; AOAC: Rockville, MD, USA, 1980; Volume 552. [Google Scholar]
- Nielsen, S.S. Food Analysis Laboratory Manual, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–146. Available online: http://link.springer.com/openurl?genre=book&isbn=978-3-319-44127-6 (accessed on 8 March 2023).
- Furukawa, S.; Nojima, N.; Yoshida, K.; Hirayama, S.; Ogihara, H.; Morinaga, Y. The importance of inter-species cell-cell co-aggregation between lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae BY4741 in mixed-species biofilm formation. Biosci. Biotechnol. Biochem. 2011, 75, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef]
- Golfinopoulos, A.; Papaioannou, L.; Soupioni, M.; Koutinas, A.A. Lactose uptake rate by kefir yeast using 14C-labelled lactose to explain kinetic aspects in its fermentation. Bioresour. Technol. 2009, 100, 5210–5213. [Google Scholar] [CrossRef]
- Gao, J.; Gu, F.; Ruan, H.; Chen, Q.; He, J.; He, G. Culture conditions optimization of tibetan kefir grains by response surface methodology. Procedia Eng. 2012, 37, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Blandón, L.M.; Noseda, M.D.; Islan, G.A.; Castro, G.R.; de Melo-Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Optimization of culture conditions for kefiran production in whey: The structural and biocidal properties of the resulting polysaccharide. Bioact. Carbohydr. Diet. Fibre 2018, 16, 14–21. [Google Scholar] [CrossRef]
- Alves, V.; Scapini, T.; Camargo, A.F.; Bonatto, C.; Stefanski, F.S.; de Jesus, E.; Techi-Diniz, L.G.; Canhadas-Bertan, L.; Resende-Maldonado, R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. LWT Food Sci. Technol. 2021, 145, 111364. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Márquez-Morales, C.E.; Moreno-León, G.R.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Solorza-Feria, J.; García-Armenta, E.; Villalobos-Espinosa, J.C. Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Appl. Sci. 2022, 12, 2459. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Camacho-Díaz, B.H.; Acosta-García, M.C.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. Effect of salt and sugar osmotic stress on the viability and morphology of Saccharomyces boulardii. Int. J. Environ. Agric. Biotechnol. 2016, 1, 593–602. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Leandro, E.D.S.; de Alencar, E.R.; Botelho, R.B.A. Fermentation of chickpea (Cicer arietinum L.) and coconut (Coccus nucifera L.) beverages by Lactobacillus paracasei subsp paracasei LBC 81: The influence of sugar content on growth and stability during storage. LWT Food Sci. Technol. 2020, 132, 109834. [Google Scholar] [CrossRef]
- Schoevers, A.; Britz, T. Influence of different culturing conditions on kefir grain increase Influence of different culturing conditions on kefir grain increase. Int. J. Dairy Technol. 2003, 56, 183–187. [Google Scholar] [CrossRef]
- Arzate-Vázquez, I.; Chanona-Pérez, J.J.; Calderón-Domínguez, G.; Terres-Rojas, E.; Garibay-Febles, V.; Martínez-Rivas, A.; Gutiérrez-López, G.F. Microstructural characterization of chitosan and alginate films by microscopy techniques and texture image analysis. Carbohydr. Polym. 2012, 87, 289–299. [Google Scholar] [CrossRef]
- Malegori, C.; Franzetti, L.; Guidetti, R.; Casiraghi, E.; Rossi, R. GLCM, an image analysis technique for early detection of biofilm. J. Food Eng. 2016, 185, 48–55. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Jia, Y.; Pan, Y.; Wang, Y. Lactobacillus kefiranofaciens, the sole dominant and stable bacterial species, exhibits distinct morphotypes upon colonization in tibetan kefir grains. Heliyon 2018, 4, e00649. [Google Scholar] [CrossRef] [PubMed]
- Păcularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.E. Selection of Wild Lactic Acid Bacteria Strains as Promoters of Postbiotics in Gluten-Free Sourdoughs. Microorganisms 2020, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Satir, G.; Guzel-Seydim, Z.B. How kefir fermentation can affect product composition? Small Rumin. Res. 2016, 134, 1–7. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo-Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; Vandenberghe, L.P.d.S.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Kristensen, K.; Ward, L.M.; Mogensen, M.L.; Cichosz, S.L. Using Image Processing and Automated Classification Models to Classify Microscopic Gram Stain Images. Comput. Methods Programs Biomed. Update 2023, 3, 100091. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S. Exopolysaccharide-producing isolates from Thai milk kefir and their antioxidant activities. LWT Food Sci. Technol. 2016, 73, 592–601. [Google Scholar] [CrossRef]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.M.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Hensing, M.; Vrouwenvelder, H.; Hellinga, C.; Baartmans, R.; Vandijken, H. Production of Extracellular Inulinase in High-Cell-Density Fed-Batch Cultures of Kluyveromyces-Marxianus. Appl. Microbiol. Biotechnol. 1994, 42, 516–521. [Google Scholar] [CrossRef]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. Single-Cell Protein. In Biotechnology & Genetic Engineering Reviews; Academic Press: Cambridge, MA, USA, 2003; pp. 5269–5276. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. Single Cell Protein | Yeasts and Bacteria. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 431–438. ISBN 978-0-12-384733-1. [Google Scholar]
- Cruz-Guerrero, A.; Garcia-Peña, I.; Barzana, E.; García-Garibay, M.; Gómez-Ruíz, L. Kluyveromyces marxianus CDBB-L-278: A wild inulinase hyperproducing strain. J. Ferment. Bioeng. 1995, 80, 159–163. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Oliveira, M.G.; Maugeri, F. Modelling thermal stability and activity of free and immobilized enzymes as a novel tool for enzyme reactor design. Bioresour. Technol. 2007, 98, 3142–3148. [Google Scholar] [CrossRef]
 - -); SM 2% (-□―); LFM 10% (―●―); LFM 5% (-o―); LFM 2% (-
- -); SM 2% (-□―); LFM 10% (―●―); LFM 5% (-o―); LFM 2% (- ̶).
̶).
 - -); SM 2% (-□―); LFM 10% (―●―); LFM 5% (-o―); LFM 2% (-
- -); SM 2% (-□―); LFM 10% (―●―); LFM 5% (-o―); LFM 2% (- ̶).
̶).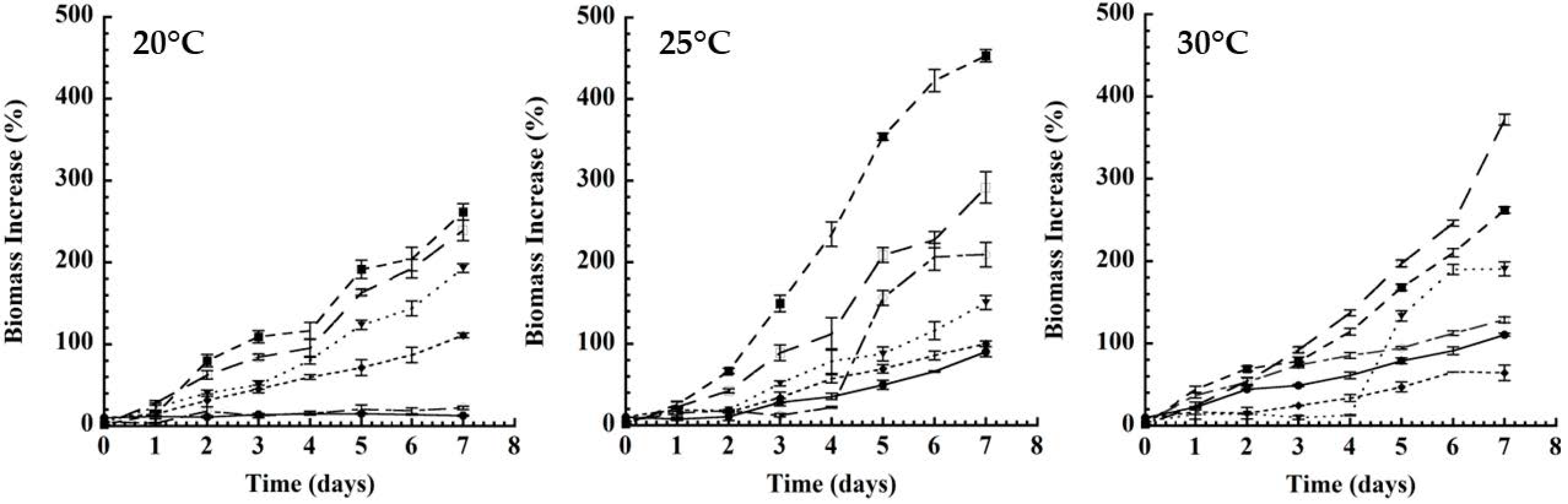

 ), D: dextrose; F: fructose; FT: fructans; G: galactose; I: inulin; L: lactose, in a proportion of 2 (
), D: dextrose; F: fructose; FT: fructans; G: galactose; I: inulin; L: lactose, in a proportion of 2 ( ), 4 (
), 4 ( ) and 6 (
) and 6 ( ) % (w/w), respectively. Results are expressed as mean ± SD; (p ˂ 0.05), Different letters indicates statistically significant differences using an ANOVA followed by Tukey test. (B) Kefir grains; SM: low-fat whole milk; LFM: lactose-free milk; LFM-F: lactose-free milk with fructans.
) % (w/w), respectively. Results are expressed as mean ± SD; (p ˂ 0.05), Different letters indicates statistically significant differences using an ANOVA followed by Tukey test. (B) Kefir grains; SM: low-fat whole milk; LFM: lactose-free milk; LFM-F: lactose-free milk with fructans.
 ), D: dextrose; F: fructose; FT: fructans; G: galactose; I: inulin; L: lactose, in a proportion of 2 (
), D: dextrose; F: fructose; FT: fructans; G: galactose; I: inulin; L: lactose, in a proportion of 2 ( ), 4 (
), 4 ( ) and 6 (
) and 6 ( ) % (w/w), respectively. Results are expressed as mean ± SD; (p ˂ 0.05), Different letters indicates statistically significant differences using an ANOVA followed by Tukey test. (B) Kefir grains; SM: low-fat whole milk; LFM: lactose-free milk; LFM-F: lactose-free milk with fructans.
) % (w/w), respectively. Results are expressed as mean ± SD; (p ˂ 0.05), Different letters indicates statistically significant differences using an ANOVA followed by Tukey test. (B) Kefir grains; SM: low-fat whole milk; LFM: lactose-free milk; LFM-F: lactose-free milk with fructans.

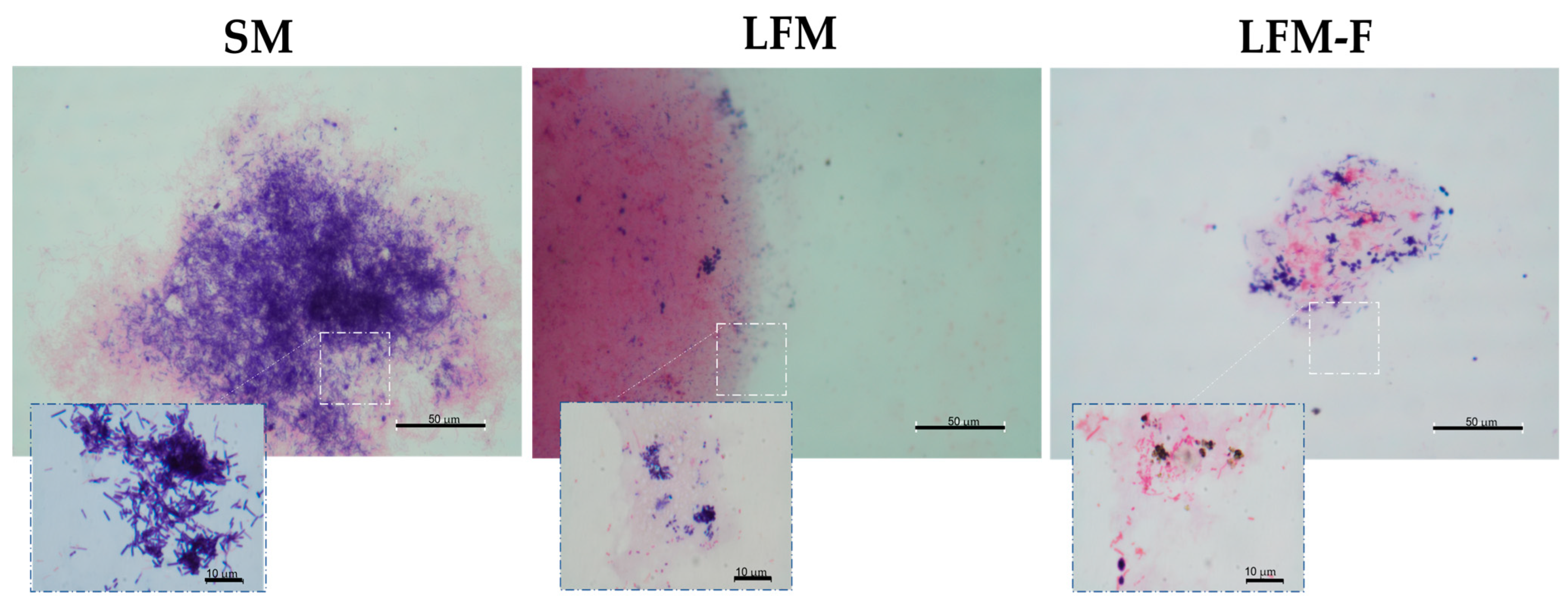
| Culture Medium | Moisture (%) | Fat (%) | Ashes (%) | Carbohydrates (%) | Protein (%) |
|---|---|---|---|---|---|
| SM | 82.5 ± 0.90 a | 1.48 ± 0.39 c | 3.42 ± 0.31 b | 8.08 ± 0.70 b | 4.27 ± 0.71 b |
| LFM | 79.34 ± 1.16 b | 2.33 ± 0.33 b | 2.43 ± 0.12 c | 11.92 ± 0.91 a | 3.93 ± 0.16 b |
| LFM-F | 76.50 ± 2.10 b | 3.76 ± 0.52 a | 5.57 ± 0.21 a | 7.05 ± 0.81 b | 7.12 ± 0.36 a |
| Culture Medium | Contrast | Correlation | IDM | Entropy |
|---|---|---|---|---|
| SM | 233.0488 6.1819 a | 0.0004 0.0001 a | 0.2953 0.0167 a | 7.7130 0.1534 a |
| LFM | 257.4760 13.7788 a,b | 0.0004 0.0000 a | 0.2490 0.0147 b | 7.9323 0.1106 a,b |
| LFM-F | 291.2643 40.0676 b | 0.0003 0.0000 a | 0.2230 0.0216 b | 8.1703 0.2742 b |
| Culture Medium | Contrast | Correlation | IDM | Entropy |
|---|---|---|---|---|
| SM | 203.7258 15.2888 a | 0.0008 0.0002 a | 0.3504 0.0244 a | 6.8348 0.2234 a |
| LFM | 223.7620 2.4241 b | 0.0005 0.0000 b | 0.3070 0.0140 b | 7.5570 0.1208 a |
| LFM-F | 244.5800 6.8698 b | 0.0005 0.0001 b | 0.2423 0.0149 b | 7.7743 0.1148 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-León, G.R.; Avila-Reyes, S.V.; Villalobos-Espinosa, J.C.; Camacho-Díaz, B.H.; Tapia-Maruri, D.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Solorza-Feria, J. Effect of Agave Fructans on Changes in Chemistry, Morphology and Composition in the Biomass Growth of Milk Kefir Grains. Microorganisms 2023, 11, 1570. https://doi.org/10.3390/microorganisms11061570
Moreno-León GR, Avila-Reyes SV, Villalobos-Espinosa JC, Camacho-Díaz BH, Tapia-Maruri D, Jiménez-Aparicio AR, Arenas-Ocampo ML, Solorza-Feria J. Effect of Agave Fructans on Changes in Chemistry, Morphology and Composition in the Biomass Growth of Milk Kefir Grains. Microorganisms. 2023; 11(6):1570. https://doi.org/10.3390/microorganisms11061570
Chicago/Turabian StyleMoreno-León, Germán R., Sandra V. Avila-Reyes, Julieta C. Villalobos-Espinosa, Brenda H. Camacho-Díaz, Daniel Tapia-Maruri, Antonio R. Jiménez-Aparicio, Martha L. Arenas-Ocampo, and Javier Solorza-Feria. 2023. "Effect of Agave Fructans on Changes in Chemistry, Morphology and Composition in the Biomass Growth of Milk Kefir Grains" Microorganisms 11, no. 6: 1570. https://doi.org/10.3390/microorganisms11061570







