Isolation of Bacillus subtilis and Bacillus pumilus with Anti-Vibrio parahaemolyticus Activity and Identification of the Anti-Vibrio parahaemolyticus Substance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Bacteria Isolation
2.2. Screening of Anti-V. parahaemolyticus Bacteria
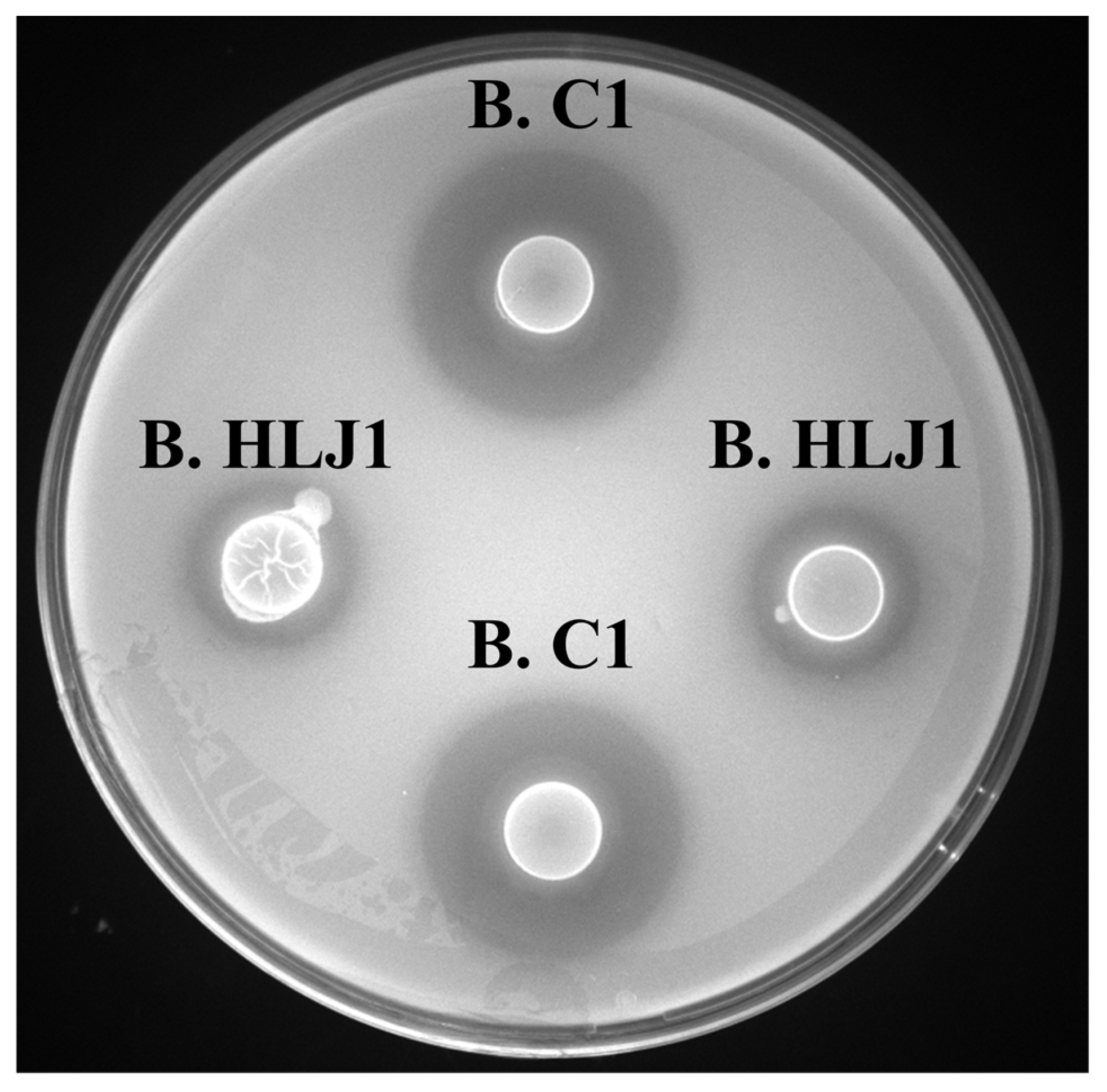
2.2.1. Primary Screening for Strains by Double-Layer Plate Method
2.2.2. Second Screening for Strains by Co-Culture Method
2.3. Identification of Strains
2.4. Safety of the Candidate Strains
2.4.1. Antibiotic Susceptibility
2.4.2. Virulence Factor Genes
2.5. Acid Tolerance and Spore Production of the Candidate Strains
2.5.1. Acid Tolerance Assay
2.5.2. Spore Production Assay
2.6. Extracellular Enzyme Activity and Aggregation Capacity
2.6.1. Extracellular Enzyme Activity
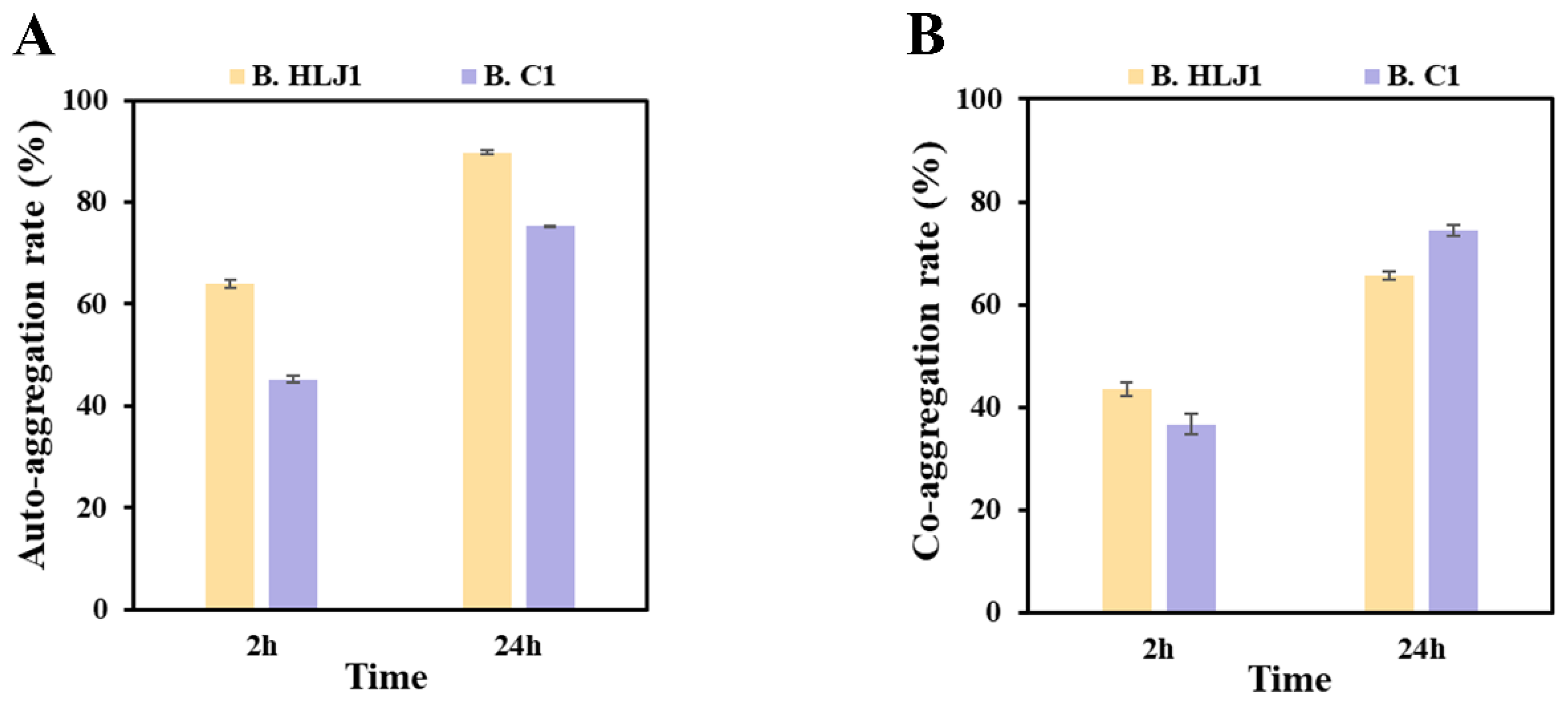
2.6.2. Auto-Aggregation and Co-Aggregation Assay
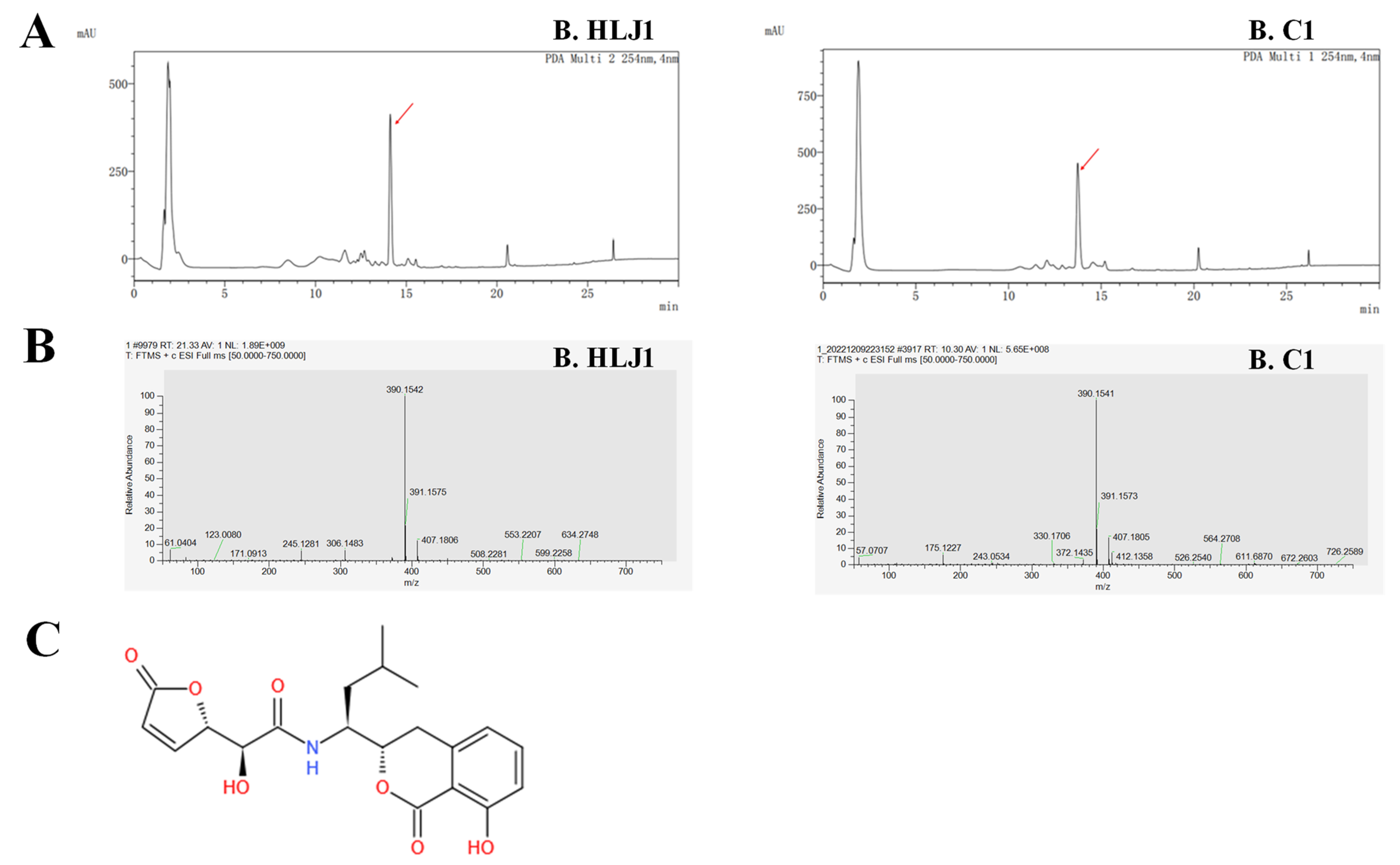
2.7. Identification of Anti-V. parahaemolyticus Substance
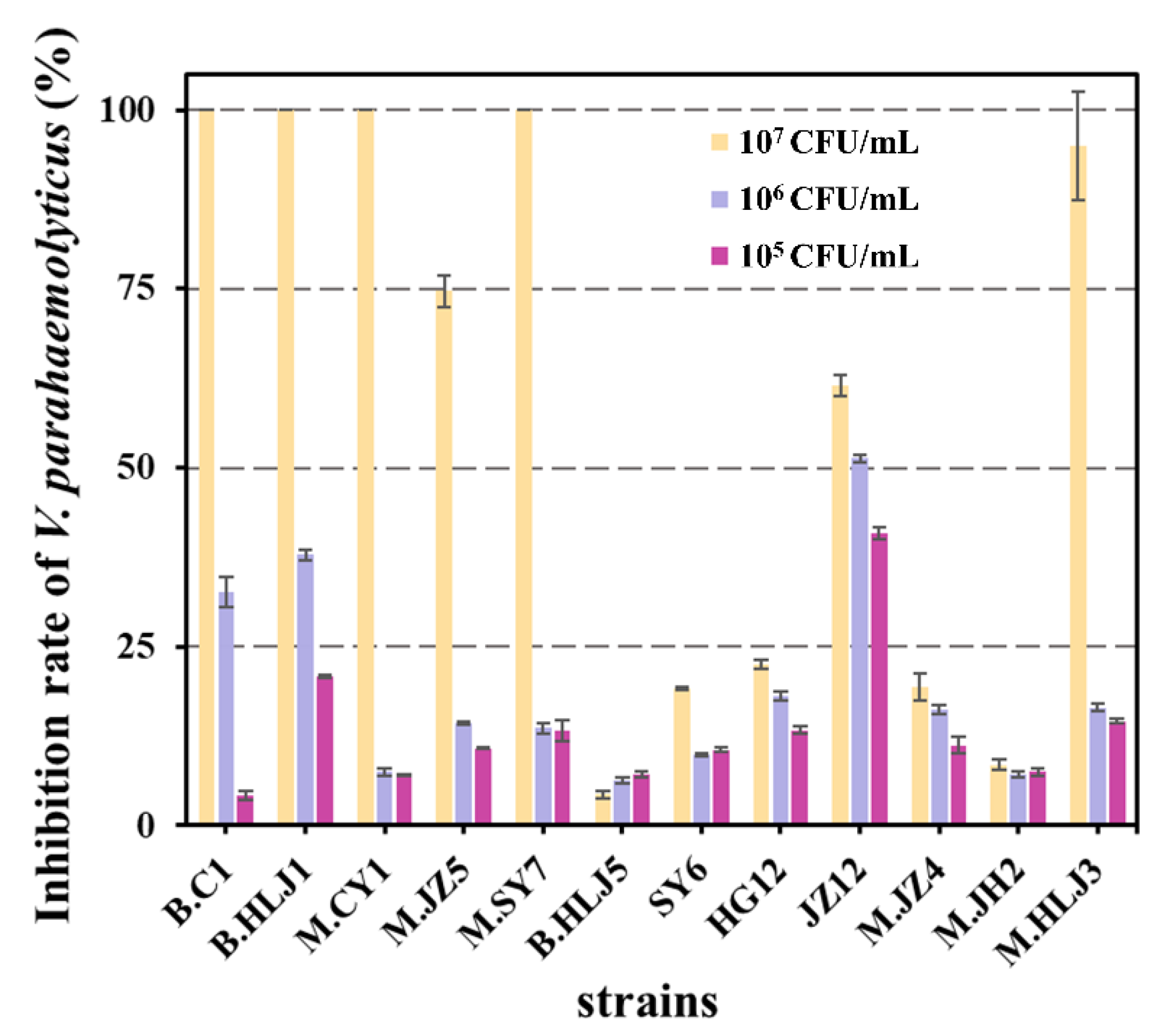
2.8. Inhibitory Activity against Different V. parahaemolyticus
2.9. Anti-V. parahaemolyticus Effect in Simulated Aquaculture Wastewater
3. Results
3.1. Screening of Anti-V. parahaemolyticus Bacteria
3.1.1. Primary Screening by Double-Layer Plate Method
3.1.2. Second Screening for Strains by Co-Culture Method
3.2. Identification of Strains
3.3. Safety of the Candidate Strains
3.4. Acid Tolerance and Spore Production of the Candidate Strains
3.5. Extracellular Enzyme Activity and Aggregation Capacity
3.6. Identification of Anti-V. parahaemolyticus Substance
3.7. Inhibitory Activity against Different V. parahaemolyticus
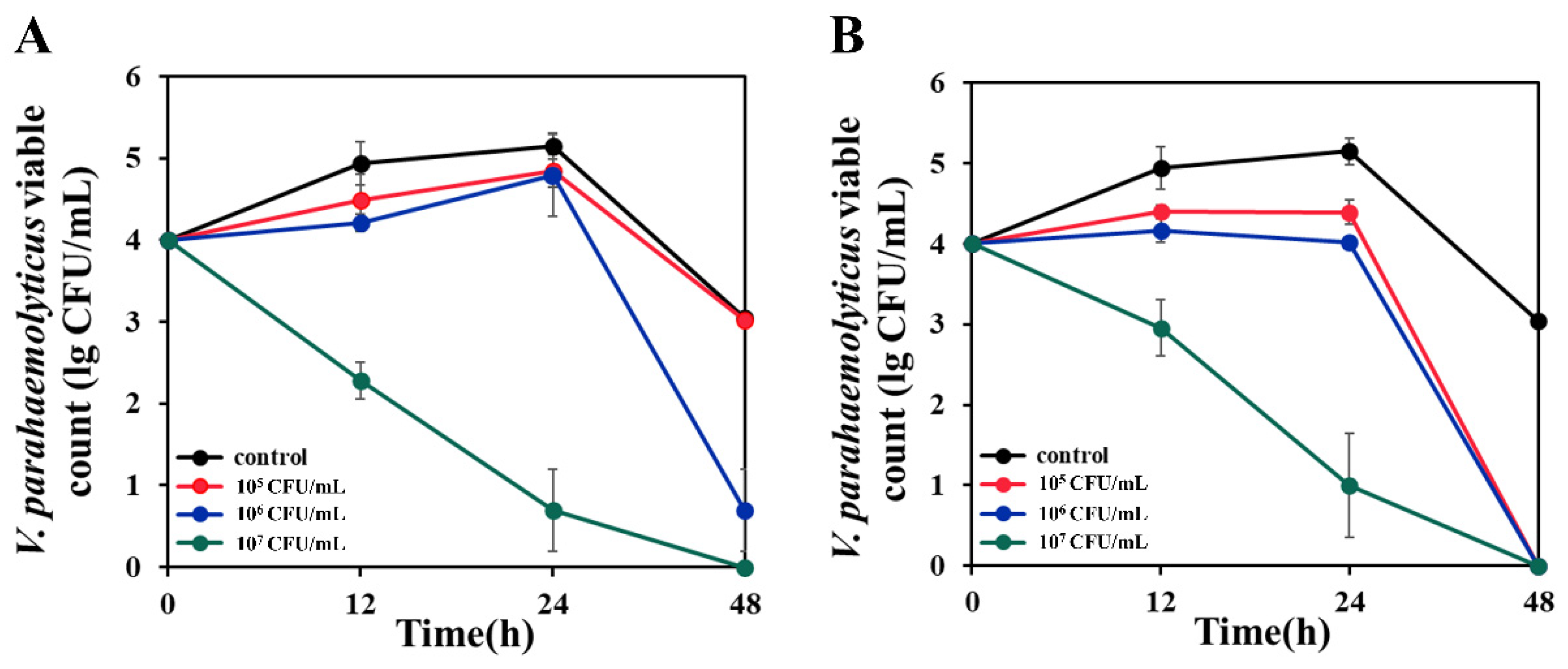
3.8. Anti-V. parahaemolyticus Effect in Simulated Aquaculture Wastewater
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus Subtilis. Rev. Aquac. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.R.; Zhou, Z.G. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Chau, K.M.; Van, T.T.H.; Quyen, D.V.; Le, H.D.; Phan, T.H.T.; Ngo, N.D.T.; Vo, T.D.T.; Dinh, T.T.; Le, H.T.; Khanh, H.H.N. Molecular Identification and Characterization of Probiotic Bacillus Species with the Ability to Control Vibrio spp. in Wild Fish Intestines and Sponges from the Vietnam Sea. Microorganisms 2021, 9, 16. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Alfaro, A.; Arroyo, B.B.; Leon, J.A.R.; Sonnenholzner, S. Metabolic responses of penaeid shrimp to acute hepatopancreatic necrosis disease caused by Vibrio parahaemolyticus. Aquaculture 2021, 533, 8. [Google Scholar] [CrossRef]
- Shinn, A.; Pratoomyot, J.; Griffiths, D.; Trọng, T.; Nguyen, V.; Jiravanichpaisal, P.; Briggs, M. Asian Shrimp Production and the Economic Costs of Disease. Asian Fish. Sci. 2018, 31S, 29–58. [Google Scholar] [CrossRef]
- Chen, J.M.; Sun, R.X.; Pan, C.G.; Sun, Y.; Mai, B.X.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Amin Jahari, M.; Shuhaimi, M.; Akhmal, M.; Roslan, H.; Lamasudin, D.U.; Manap, Y.; Amin, M. Encapsulation of Lactobacillus plantarum with Mannan and Sodium Alginate Improves its Cell Production. J. Biochem. Microbiol. Biotechnol. 2019, 7, 17–22. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef]
- Chomwong, S.; Charoensapsri, W.; Amparyup, P.; Tassanakajon, A. Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannarnei. Dev. Comp. Immunol. 2018, 89, 54–65. [Google Scholar] [CrossRef]
- Xi, D.Y.; Liu, C.C.; Su, Y.C. Effects of green tea extract on reducing Vibrio parahaemolyticus and increasing shelf life of oyster meats. Food Control 2012, 25, 368–373. [Google Scholar] [CrossRef]
- Kewcharoen, W.; Srisapoome, P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 2019, 94, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Huang, Q.C.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Dong, X.H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z.; Zhang, H.T. Administration of probiotic Bacillus licheniformis induces growth, immune and antioxidant enzyme activities, gut microbiota assembly and resistance to Vibrio parahaemolyticus in Litopenaeus vannamei. Aquac. Nutr. 2020, 26, 1604–1622. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Bae, J.H.; Jang, W.J.; Lee, S.; Bai, S.C. Evaluation of Potential Probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moyanoa, S.; dos Santos, M.; Galvan, A.I.; Merchan, A.V.; Gonzalez, E.; Cordoba, M.D.; Benito, M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: Probiotic characteristics and prebiotic metabolism. Lwt Food Sci. Technol. 2019, 114, 7. [Google Scholar] [CrossRef]
- Priyodip, P.; Balaji, S. A Preliminary Study on Probiotic Characteristics of Sporosarcina spp. for Poultry Applications. Curr. Microbiol. 2019, 76, 448–461. [Google Scholar] [CrossRef]
- Kavitha, M.; Raja, M.; Perumal, P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac. Rep. 2018, 11, 59–69. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Hasan, N.A.; Huq, A.; Colwell, R.R. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front. Cell. Infect. Microbiol. 2013, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.R.; Wang, Y.H.; Hong, B.; Li, Y.M.; Ma, Y.; Wang, J.F. Isolation and Characterization of a Lytic Vibrio parahaemolyticus Phage vB_VpaP_GHSM17 from Sewage Samples. Viruses 2022, 14, 15. [Google Scholar] [CrossRef]
- Schillinger, U.; Lucke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulden, E.F.; Hall, M.R.; Pereg, L.L.; Høj, L. Identification of an Antagonistic Probiotic Combination Protecting Ornate Spiny Lobster (Panulirus ornatus) Larvae against Vibrio owensii Infection. PLoS ONE 2012, 7, e39667. [Google Scholar] [CrossRef]
- Wattiau, P.; Renard, M.E.; Ledent, P.; Debois, V.; Blackman, G.; Agathos, S.N. A PCR test to identify Bacillus subtilis and closely related species and its application to the monitoring of wastewater biotreatment. Appl. Microbiol. Biotechnol. 2001, 56, 816–819. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, Q.; Goodwin, P.H.; Deng, X.; Xu, W.; Xia, M.; Zhang, J.; Sun, R.; Wu, C.; Wang, Q.; et al. Isolation and Genome-Based Characterization of Biocontrol Potential of Bacillus siamensis YB-1631 against Wheat Crown Rot Caused by Fusarium pseudograminearum. J. Fungi 2023, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Valencia-Marin, M.F.; Chavez-Avila, S.; Ramirez-Diaz, M.I.; de Los Santos-Villalobos, S.; Meza-Carmen, V.; Orozco-Mosqueda, M.D.C.; Santoyo, G. Genome mining, phylogenetic, and functional analysis of arsenic (As) resistance operons in Bacillus strains, isolated from As-rich hot spring microbial mats. Microbiol. Res. 2022, 264, 127158. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Kong, W.L.; Liao, Y.C.Z.; Zhu, L.H. Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae. J. Fungi 2022, 8, 18. [Google Scholar] [CrossRef]
- Lee, G.; Heo, S.; Kim, T.; Na, H.E.; Park, J.; Lee, E.; Lee, J.H.; Jeong, D.W. Discrimination of Bacillus subtilis from Other Bacillus Species Using Specific Oligonucleotide Primers for the Pyruvate Carboxylase and Shikimate Dehydrogenase Genes. J. Microbiol. Biotechnol. 2022, 32, 1011–1016. [Google Scholar] [CrossRef]
- Mazlan, S.; Jaafar, M.N.; Wahab, A.; Sulaiman, Z.; Rajandas, H.; Zulperi, D. Molecular characterization and phylogenetic analysis of Bacillus pumilus causing trunk bulges of RRIM 3001 superclone rubber tree in Malaysia. Eur. J. Plant Pathol. 2021, 159, 825–838. [Google Scholar] [CrossRef]
- Kabore, D.; Gagnon, M.; Roy, D.; Sawadogo-Lingani, H.; Diawara, B.; LaPointe, G. Rapid screening of starter cultures for maari based on antifungal properties. Microbiol. Res. 2018, 207, 66–74. [Google Scholar] [CrossRef]
- Anokyewaa, M.A.; Amoah, K.; Li, Y.; Lu, Y.S.; Kuebutornye, F.K.A.; Asiedu, B.; Seidu, I. Prevalence of virulence genes and antibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China. Aquac. Rep. 2021, 21, 8. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. Lwt-Food Sci. Technol. 2017, 86, 438–446. [Google Scholar] [CrossRef]
- Zhou, S.X.; Xia, Y.; Zhu, C.M.; Chu, W.H. Isolation of Marine Bacillus sp with Antagonistic and Organic-Substances-Degrading Activities and Its Potential Application as a Fish Probiotic. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, S.; Abbasiliasi, S.; Zulkifly, S.; Sam-on, M.F.S.; Yusof, M.T.; Hashim, A.M.; Jahari, M.A.; Roslan, M.A.H. Evaluation of three Bacillus spp. isolated from the gut of giant freshwater prawn as potential probiotics against pathogens causing Vibriosis and Aeromonosis. Microb. Pathog. 2022, 164, 12. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Pan, L.Q.; Su, C.; Liu, L.P.; Dou, L. Simultaneous aerobic removal of phosphorus and nitrogen by a novel salt-tolerant phosphate-accumulating organism and the application potential in treatment of domestic sewage and aquaculture sewage. Sci. Total Environ. 2021, 758, 12. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, K.; Schrempf, H.; Goebel, W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J. Bacteriol. 1978, 133, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Chen, D.D.; Peng, K.S.; Cui, Z.W.; Zhang, X.J.; Li, S.; Zhang, Y.A. Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish. Immunol. 2016, 52, 74–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Yu, M.; Zhang, D.; Wang, Q.; Lin, L.; Wang, G.; Elsadek, M.M.; Yao, Q.; Chen, Y.; et al. Evaluating the probiotic potential and adhesion characteristics of Bacillus spp. isolated from the intestine of Rhynchocypris lagowskii Dybowski. Aquac. Int. 2022, 30, 747–772. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, Z.C.; Wang, S.; Wang, X.; Zhang, D.M.; Wang, Q.J.; Lin, L.L.; Wang, G.Q.; Guo, Z.X.; Chen, Y.K. Inhibitory effect of probiotic Bacillus spp. isolated from the digestive tract of Rhynchocypris Lagowskii on the adhesion of common pathogenic bacteria in the intestinal model. Microb. Pathog. 2022, 169, 11. [Google Scholar] [CrossRef]
- Bairagi, A.; Ghosh, K.S.; Sen, S.K.; Ray, A.K. Evaluation of the nutritive value of Leucaena leucocephala leaf meal, inoculated with fish intestinal bacteria Bacillus subtilis and Bacillus circulans in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings. Aquac. Res. 2004, 35, 436–446. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J.; Allison, E.; Jescovitch, L.N.; Wilson, A.E.; Terhune, J.S.; et al. Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar] [CrossRef]
- Fernandes, S.; Kerkar, S.; Leitao, J.; Mishra, A. Probiotic Role of Salt Pan Bacteria in Enhancing the Growth of Whiteleg Shrimp, Litopenaeus vannamei. Probiotics Antimicrob. Proteins 2019, 11, 1309–1323. [Google Scholar] [CrossRef]
- Canzi, E.; Guglielmetti, S.; Mora, D.; Tamagnini, I.; Parini, C. Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2005, 88, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Suskovic, J.; Vukovic, S.; Simpraga, M.; Frece, J.; Matosic, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Amoah, K.; Dong, X.H.; Tan, B.P.; Zhang, S.; Kuebutornye, F.K.A.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Zhang, H.T.; Yang, Y.Z. In vitro Assessment of the Safety and Potential Probiotic Characteristics of Three Bacillus Strains Isolated from the Intestine of Hybrid Grouper (Epinephelus fuscoguttatus female x Epinephelus lanceolatus male). Front. Vet. Sci. 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Knipe, H.; Temperton, B.; Lange, A.; Bass, D.; Tyler, C.R. Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev. Aquac. 2021, 13, 324–352. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, Y.; Miao, L.L.; Li, E.W.; Hou, T.T.; Liu, Z.P. Mechanism of anti-Vibrio activity of marine probiotic strain Bacillus pumilus H2, and characterization of the active substance. AMB Express 2017, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Itoh, J.; Omoto, S.; Shomura, T.; Nishizawa, N.; Miyado, S.; Yuda, Y.; Shibata, U.; Inouye, S. Amicoumacin-A, a new antibiotic with strong anti-inflammatory and antiulcer activity. J. Antibiot. 1981, 34, 611–613. [Google Scholar] [CrossRef] [Green Version]
- Shimojima, Y.; Hayashi, H.; Ooka, T.; Shibukawa, M.; Iitaka, Y. (Studies on AI-77s, microbial products with pharmacological activity) structures and the chemical nature of AI-77s. Tetrahedron Lett. 1982, 23, 5435–5438. [Google Scholar] [CrossRef]
- Huang, Y.F.; Li, L.H.; Tian, L.; Qiao, L.; Hua, H.M.; Pei, Y.H. Sg17-1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17-1. J. Antibiot. 2006, 59, 355–357. [Google Scholar] [CrossRef]
- Boya, C.A.; Herrera, L.; Guzman, H.M.; Gutierrez, M. Antiplasmodial activity of bacilosarcin A isolated from the octocoral-associated bacterium Bacillus sp. collected in Panama. J. Pharm. Bioallied Sci. 2012, 4, 66–69. [Google Scholar] [CrossRef]
- Shi, W.P.; Zeng, H.; Wan, C.X.; Zhou, Z.B. Amicoumacins from a desert bacterium: Quorum sensing inhibitor against Chromobacterium violaceum. Nat. Prod. Res. 2021, 35, 5508–5512. [Google Scholar] [CrossRef]
- Shimojima, Y.; Hayashi, H.; Ooka, T.; Shibukawa, M.; Iitaka, Y. Studies on AI-77s, microbial products with gastroprotective activity. Structures and the chemical nature of AI-77s. Tetrahedron 1984, 40, 2519–2527. [Google Scholar] [CrossRef]
- Shi, L.Y.; Liang, S.; Luo, X.; Ke, C.H.; Zhao, J. Microbial community of Pacific abalone (Haliotis discus hannai) juveniles during a disease outbreak in South China. Aquac. Res. 2017, 48, 1080–1088. [Google Scholar] [CrossRef]
- Jayaprakash, N.S.; Pai, S.S.; Anas, A.; Preetha, R.; Philip, R.; Singh, I.S.B. A marine bacterium, Micrococcus MCCB 104, antagonistic to vibrios in prawn larval rearing systems. Dis. Aquat. Org. 2005, 68, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.O.; Ringo, E.; Ahmadifar, E.; Abdel-Latif, H.M.R. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 15. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, D.; Vinothkanna, A.; Rai, A.K.; Vignesh, V.S. Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 45, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Gao, X.J.; Zhang, S.M.; Jiang, Z.Y.; Yang, H.; Liu, X.D.; Jiang, Q.; Zhang, X.J. Characterization of a Bacillus velezensis with antibacterial activity and inhibitory effect on common aquatic pathogens. Aquaculture 2020, 523, 9. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Ghosh, K. Antagonism against fish pathogens by cellular components and verification of probiotic properties in autochthonous bacteria isolated from the gut of an Indian major carp, Catla catla (Hamilton). Aquac. Res. 2016, 47, 2243–2255. [Google Scholar] [CrossRef]
- Samson, J.S.; Choresca, C.H.; Quiazon, K.M.A. Selection and screening of bacteria from African nightcrawler, Eudrilus eugeniae (Kinberg, 1867) as potential probiotics in aquaculture. World J. Microbiol. Biotechnol. 2020, 36, 16. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.M.; Deng, L.; Hu, N.; Zhao, B.; Liang, Y.X. Cost-effective production of Bacillus licheniformis using simple netting bag solid bioreactor. World J. Microbiol. Biotechnol. 2008, 24, 2859–2863. [Google Scholar] [CrossRef]


| Strains | Inhibitory Activity | Strain | Inhibitory Activity |
|---|---|---|---|
| B. C1 | ++++ | M. CY1 | +++ |
| B. HLJ1 | +++ | M. JZ4 | +++ |
| B. HLJ5 | ++ | M. JZ5 | ++ |
| SY6 | ++ | M. JH2 | ++ |
| HG12 | ++ | M. SY7 | ++ |
| JZ12 | ++ | M. HLJ3 | ++ |
| Antibiotic | Judgement Standard (μg/mL) | B. C1 | B. HLJ1 | ||||
|---|---|---|---|---|---|---|---|
| S | I | R | MIC (μg/mL) | Sensitivity | MIC (μg/mL) | Sensitivity | |
| Teicoplanin | 8 | 16 | 32 | 0.25 | S | 0.25 | S |
| Vancomycin | 4 | 8–16 | 32 | 0.25 | S | 0.25 | S |
| Chloramphenicol | 8 | 16 | 32 | 8 | S | 16 | I |
| Tetracycline | 4 | 8 | 16 | 0.25 | S | 0.25 | S |
| Erythromycin | 0.5 | 1–4 | 8 | 1 | I | 0.25 | S |
| Gentamycin | 4 | 8 | 16 | 4 | S | 2 | S |
| Kanamycin | 16 | 32 | 64 | 16 | S | 2 | S |
| Strain | Virulence Genes | ||||||
|---|---|---|---|---|---|---|---|
| hblA | hblC | hblD | nheA | nheB | nheC | entFM | |
| B. C1 | − | − | − | − | − | − | − |
| B. HLJ1 | − | − | − | − | − | − | − |
| Strain | Ratio of Enzymatic Circle Diameter to Colony Diameter | ||
|---|---|---|---|
| Amylase | Protease | Cellulase | |
| B. HLJ1 | 1.33 ± 0.13 | 1.27 ± 0.11 | 1.23 ± 0.06 |
| B. C1 | − | 2.08 ± 0.32 | 2.14 ± 0.26 |
| Strains | Origin | Diameter of Inhibition Zone (mm) | |
|---|---|---|---|
| B. C1 | B. HLJ1 | ||
| V. parahaemolyticus ATCC17802 | American Type Culture Collection | 23.3 ± 0.3 | 15.3 ± 0.3 |
| V. parahaemolyticus OY14 | American oyster | 16.5 ± 0.5 | 11.5 ± 0.5 |
| V. parahaemolyticus FFTF11 | Frozen fork tail fillets | 15.5 ± 0.5 | 12.0 ± 0.1 |
| V. parahaemolyticus BC21 | Bengali frozen cuttlefish | 22.0 ± 1.0 | 19.5 ± 0.5 |
| V. parahaemolyticus IFH23 | Indonesian frozen hairtail | 17.5 ± 0.5 | 11.5 ± 0.5 |
| V. parahaemolyticus TSG36 | Thai grass shrimp | 18.5 ± 1.0 | 11.5 ± 0.5 |
| V. parahaemolyticus SAL38 | South African lobster | 11.4 ± 0.1 | 10.3 ± 0.3 |
| V. parahaemolyticus HA44 | Haliotis | 19.3 ± 0.2 | 11.1 ± 0.1 |
| V. parahaemolyticus TMS61 | Thai mantis shrimp | 20.1 ± 0.2 | 15.2 ± 0.1 |
| V. parahaemolyticus SC123 | Scallop | 21.0 ± 0.5 | 14.5 ± 0.5 |
| V. parahaemolyticus CR127 | Crab | 19.5 ± 0.5 | 14.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Hong, B.; Luo, K.; Li, Y.; Fu, H.; Wang, J. Isolation of Bacillus subtilis and Bacillus pumilus with Anti-Vibrio parahaemolyticus Activity and Identification of the Anti-Vibrio parahaemolyticus Substance. Microorganisms 2023, 11, 1667. https://doi.org/10.3390/microorganisms11071667
Jiang N, Hong B, Luo K, Li Y, Fu H, Wang J. Isolation of Bacillus subtilis and Bacillus pumilus with Anti-Vibrio parahaemolyticus Activity and Identification of the Anti-Vibrio parahaemolyticus Substance. Microorganisms. 2023; 11(7):1667. https://doi.org/10.3390/microorganisms11071667
Chicago/Turabian StyleJiang, Ning, Bin Hong, Kui Luo, Yanmei Li, Hongxin Fu, and Jufang Wang. 2023. "Isolation of Bacillus subtilis and Bacillus pumilus with Anti-Vibrio parahaemolyticus Activity and Identification of the Anti-Vibrio parahaemolyticus Substance" Microorganisms 11, no. 7: 1667. https://doi.org/10.3390/microorganisms11071667





