Foodborne Microbial Communities as Potential Reservoirs of Antimicrobial Resistance Genes for Pathogens: A Critical Review of the Recent Literature
Abstract
:1. Antimicrobial Resistance (AMR) within Environmental Niches
2. The Foodborne Reservoir of AMR
3. Horizontal Gene Transfer (HGT)
4. AMR Detection within the Foodborne Bacterial Reservoir
Horizontal Gene Transfer among Foodborne Bacteria
| Donor | Source | AMR Gene(s) | Genomic Context (Chromosome/ Mobile Element/ Plasmid) | Recipient | Donor: Recipient Ratio | Conjugation Method | Frequency | Confirmation of Transconjugants | Other AMR Genes Not Transferred | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Pediococcus pentosaceus OBK05 | Buttermilk | Trimethoprim (gene not identified) | Plasmid | Enterococcus faecalis Staphylococcus aureus Klebsiella pneumoniae Escherichia coli | 1:1 | Filter mating | 2 × 10−4 1 × 10−6 2 × 10−5 3 × 10−4 | Phenotypic (Kirby-Bauer disc diffusion method) | aph(3″)-III, strA, vanA, norfloxacin | [29] |
| Enterococcus faecalis Staphylococcus aureus Klebsiella pneumoniae Escherichia coli | 1:1 | Food mating (cheese) | 2.5 × 10−4 2 × 10−6 3 × 10−5 3.4 × 10−4 | |||||||
| Enterococcus faecalis f1 | Fermented pork | aadE (Streptomycin) | not indicated | Enterococcus faecalis f8 | 1:9 | Solid agar mating | 1.32 × 10−7 | Molecular (RAPD, Rep-PCR; PCR detection of AMR genes) | None | [45] |
| Lactobacillus delbrueckii subsp. bulgaricus R6 | Yogurt | tetM (Tetracycline) | not indicated | Listeria monocytogenes L82 | 1:1 | Filter mating | 7.3 × 10−7 | Phenotypic (Listeria monocytogenes biochemical identification kit); Molecular (PCR detection of AMR genes) | ermB, aac(6′)-aph(2″), ant(6), sulI, sulII, tetM, tetS from 18 strains | [24] |
| Lactiplantibacillus plantarum R41 | tetS (Tetracycline) | not indicated | Listeria monocytogenes L82 | 1:1 | Filter mating | 2.9 × 10−6 | ||||
| Enterococcus faecalis (14 strains) Enterococcus faecium (5 strains) | Ready-to-eat dishes | tetM (Tetracycline) | Tn916/Tn1545 | Enterococcus faecalis JH2-2 (LMG 19456) | not indicated | Filter mating | 1.3 × 10−6 to 8.7 × 10−7 | Molecular (genotyping by PCR melting profile; PCR detection of AMR genes) | aph(2″)-Ib, aph(2″)-Ic, aph(2″)-Id, ant(4′)-Ia, tetK, tetW, ermC | [64] |
| ermB (Erithromycin) | not indicated | 3.2 × 10−6 to 2.4 × 10−8 | ||||||||
| aac(6′)-Ie-aph(2″) (Aminoglycosides) | not indicated | 1.7 × 10−6 to 3.2 × 10−8 | ||||||||
| ant(6′)-Ia, tetL, tetO, ermA, ermB, msrC, mefAB | not indicated | Various ranges/different combinations | ||||||||
| Enterococcus faecium UC7251 (multi-resistant strain) | Fermented dry sausage | tetM (Tetracycline) | Tn916/Tn1545 | Enterococcus faecalis OG1RF Listeria innocua L7 Listeria monocytogenes DSM 15675 Staphylococcus aureus UC7180 Lacticaseibacillus rhamnosus UC8647 | 1:1 | Filter mating | 6 × 10−3 5.7 × 10−6 8.4 × 10−4 3.8 × 10−2 6.8 × 10−5 | Molecular (PCR detection of AMR genes | TetL and unknown gene conferring erythromycin resistance on a mobilizable but non-conjugative plasmid lacking the complete conjugation apparatus. No gene transfer observed toward Gram-negative recipient species | [60] |
| Limosilactobacillus fermentum DVM 95.7 and Limosilactobacillus fermentum NIFTEM 95.8 | Fermented milk | tetM, ermB | Chromosomal (tetM); plasmid (ermB) | Enterococcus faecalis ATCC 14506, Escherichia coli ATCC 11229, Staphylococcus aureus NCDC 109 | 1:10 | Filter mating | 6.0–6.4 × 10−6 with E. coli; no transconjugants with other recipients | none | none | [35] |
| 1:1 | Food mating (fermented milk, idli batter, fermented minced chicken, plant model) | 2.0 × 10−1–3 × 10−2 with E. coli and E. faecalis in minced chicken and in plant model; no transconjugants with S. aureus | ||||||||
| Pediococcus pentosaceus (15 isolates) | Fermented dairy and meat | ermB, msrC | Plasmid | Enterococcus faecalis JH2-2 | 50:1 | Filter mating | 1.0 × 10−6 (only for 1 strain, only ermB transferred) | Molecular (genotyping by RAPD-PCR; detection of AMR genes by PCR and Southern hybridization) | none | [32] |
| Ligilactobacillus salivarius (CHS-1E and CH7-1E strains) and Limosilactobacillus reuteri (CH2-2 strain) | Fermented chicken sausage | ermB, tetW, tetM, tetL | Plasmid | Enterococcus faecalis JH2-2 | 10:1, 25:1 and 50:1 | Filter mating | 2 × 10−3–1 × 10−4 (only 50:1 ratio) | Molecular (genotyping by RAPD-PCR; | none | [33] |
| Enterococcus faecalis JH2-2 | 1:10 | In vivo mating (Wistar rats) | not indicated (although transconjugants were recovered) | detection of AMR genes by Southern hybridization and/or PCR) | ||||||
| Enterococcus faecalis JH2-2, Listeria monocytogenes Scott A, Micrococcus luteus, Yersinia enterocolitica, Bacillus cereus F4453, Staphylococcus aureus, pathogenic E. coli MTCC118 | 1:10 | Food mating (chicken sausage, fermented milk, idli batter) | 1.9 × 10−6–2.9 × 10−7 (Listeria), 0.8 × 10−6–0.8 × 10−9 (Yersinia) | |||||||
| Staphylococcus saprophyticus KM1053 | Jeotgal (High-salt fermented seafood) | tetK | Plasmid | Enterococcus faecalis OG1RF | 1:10 | Broth mating | 5.8 × 10−3 | Molecular (16S rRNA gene sequence analysis; PCR detection of AMR genes) | none | [43] |
| Staphylococcus equorum KM1031 | 9.5 × 102 | |||||||||
| Staphylococcus equorum KS1039 | not transferred | |||||||||
| Staphylococcus aureus USA300 LAC | not transferred |
5. Global/Regional Policies to Tackle AMR Spread
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meier, H.; Spinner, K.; Crump, L.; Kuenzli, E.; Schuepbach, G.; Zinsstag, J. State of Knowledge on the Acquisition, Diversity, Interspecies Attribution and Spread of Antimicrobial Resistance between Humans, Animals and the Environment: A Systematic Review. Antibiotics 2022, 12, 73. [Google Scholar] [CrossRef]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef]
- Huddleston, J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug. Resist. 2014, 7, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Selvarajan, R.; Obize, C.; Sibanda, T.; Abia, A.L.K.; Long, H. Evolution and Emergence of Antibiotic Resistance in Given Ecosystems: Possible Strategies for Addressing the Challenge of Antibiotic Resistance. Antibiotics 2022, 12, 28. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Ganzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton-Pimentel, K.J.; Vergeres, G.; Feskens, E.J.M. A systematic review to identify biomarkers of intake for fermented food products. Genes Nutr. 2021, 16, 5. [Google Scholar] [CrossRef]
- Zinno, P.; Calabrese, F.M.; Schifano, E.; Sorino, P.; Di Cagno, R.; Gobbetti, M.; Parente, E.; De Angelis, M.; Devirgiliis, C. FDF-DB: A Database of Traditional Fermented Dairy Foods and Their Associated Microbiota. Nutrients 2022, 14, 4581. [Google Scholar] [CrossRef]
- Walsh, A.M.; Macori, G.; Kilcawley, K.N.; Cotter, P.D. Meta-analysis of cheese microbiomes highlights contributions to multiple aspects of quality. Nat. Food 2020, 1, 500–510. [Google Scholar] [CrossRef]
- Rajendran, S.; Silcock, P.; Bremer, P. Flavour Volatiles of Fermented Vegetable and Fruit Substrates: A Review. Molecules 2023, 28, 3236. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef]
- Billington, C.; Kingsbury, J.M.; Rivas, L. Metagenomics Approaches for Improving Food Safety: A Review. J. Food Prot. 2022, 85, 448–464. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Baiken, Y.; Fasler-Kan, E.; Alibek, K.; Wang, S.; Maltsev, N.; Ponomarev, E.D.; Sautbayeva, Z.; Kauanova, S.; Moore, A.; et al. Extracellular vesicles in gastrointestinal cancer in conjunction with microbiota: On the border of Kingdoms. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 372–393. [Google Scholar] [CrossRef]
- Lai, F.W.; Lichty, B.D.; Bowdish, D.M. Microvesicles: Ubiquitous contributors to infection and immunity. J. Leukoc. Biol. 2015, 97, 237–245. [Google Scholar] [CrossRef]
- de Brito, F.A.E.; de Freitas, A.P.P.; Nascimento, M.S. Multidrug-Resistant Biofilms (MDR): Main Mechanisms of Tolerance and Resistance in the Food Supply Chain. Pathogens 2022, 11, 1416. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Muniesa, M.; Colomer-Lluch, M.; Jofre, J. Could bacteriophages transfer antibiotic resistance genes from environmental bacteria to human-body associated bacterial populations? Mob. Genet. Elements 2013, 3, e25847. [Google Scholar] [CrossRef] [Green Version]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family—A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [Green Version]
- Roselli, M.; Natella, F.; Zinno, P.; Guantario, B.; Canali, R.; Schifano, E.; De Angelis, M.; Nikoloudaki, O.; Gobbetti, M.; Perozzi, G.; et al. Colonization Ability and Impact on Human Gut Microbiota of Foodborne Microbes from Traditional or Probiotic-Added Fermented Foods: A Systematic Review. Front. Nutr. 2021, 8, 689084. [Google Scholar] [CrossRef]
- Yao, J.; Gao, J.; Guo, J.; Wang, H.; Zhang, E.N.; Lin, Y.; Chen, Z.; Li, S.; Tao, S. Characterization of Bacteria and Antibiotic Resistance in Commercially Produced Cheeses Sold in China. J. Food Prot. 2022, 85, 484–493. [Google Scholar] [CrossRef]
- Jin, Z.; Ding, G.; Yang, G.; Li, G.; Zhang, W.; Yang, L.; Li, W. Rapid detection of antibiotic resistance genes in lactic acid bacteria using PMMA-based microreactor arrays. Appl. Microbiol. Biotechnol. 2020, 104, 6375–6383. [Google Scholar] [CrossRef]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of Diversity and Probiotic Efficiency of the Autochthonous Lactic Acid Bacteria in the Fermentation of Selected Raw Fruit and Vegetable Juices. Front. Microbiol. 2018, 9, 2539. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Yu, T. Characterization and transfer of antimicrobial resistance in lactic acid bacteria from fermented dairy products in China. J. Infect. Dev. Ctries. 2019, 13, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Han, Z.; Wu, Y.; Jiang, S.; Ma, C.; Zhang, Y.; Zhang, J. Metagenomics assembled genome scale analysis revealed the microbial diversity and genetic polymorphism of Lactiplantibacillus plantarum in traditional fermented foods of Hainan, China. Food Res. Int. 2021, 150, 110785. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Zhao, C.; Zhu, Y. Metagenomic insights into the changes in microbial community and antimicrobial resistance genes associated with different salt content of red pepper (Capsicum annuum L.) sauce. Food Microbiol. 2020, 85, 103295. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Wang, J.; Liu, M.; Yang, K.; Zhang, J.; Fan, M.; Wei, X. Antibiotic Resistance of Coagulase-Negative Staphylococci and Lactic Acid Bacteria Isolated from Naturally Fermented Chinese Cured Beef. J. Food Prot. 2018, 81, 2054–2063. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, L.; Li, W.; Wang, Y.; Zheng, H.; Sun, T.; Zhang, H.; Xi, R.; Liu, W.; Sun, Z. Genomics landscape of 185 Streptococcus thermophilus and identification of fermentation biomarkers. Food Res. Int. 2021, 150, 110711. [Google Scholar] [CrossRef]
- Bhukya, K.K.; Bhukya, B. Unraveling the probiotic efficiency of bacterium Pediococcus pentosaceus OBK05 isolated from buttermilk: An in vitro study for cholesterol assimilation potential and antibiotic resistance status. PLoS ONE 2021, 16, e0259702. [Google Scholar] [CrossRef]
- Biswas, K.; Sharma, P.; Joshi, S.R. Co-occurrence of antimicrobial resistance and virulence determinants in enterococci isolated from traditionally fermented fish products. J. Glob. Antimicrob. Resist. 2019, 17, 79–83. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.V.; Sreenivasa, M.Y. Probiotic Properties of Lactic Acid Bacteria Isolated from Neera: A Naturally Fermenting Coconut Palm Nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Thumu, S.C.R.; Halami, P.M. Heterogeneity of macrolide-lincosamide-streptogramin phenotype & conjugal transfer of erm(B) in Pediococcus pentosaceus. Indian J. Med. Res. 2019, 149, 270–275. [Google Scholar] [CrossRef]
- Thumu, S.C.R.; Halami, P.M. Conjugal transfer of erm(B) and multiple tet genes from Lactobacillus spp. to bacterial pathogens in animal gut, in vitro and during food fermentation. Food Res. Int. 2019, 116, 1066–1075. [Google Scholar] [CrossRef]
- Singla, V.; Mandal, S.; Sharma, P.; Anand, S.; Tomar, S.K. Antibiotic susceptibility profile of Pediococcus spp. from diverse sources. 3 Biotech 2018, 8, 489. [Google Scholar] [CrossRef]
- Ojha, A.K.; Shah, N.P.; Mishra, V. Characterization and Transferability of erm and tet Antibiotic Resistance Genes in Lactobacillus spp. Isolated from Traditional Fermented Milk. Curr. Microbiol. 2022, 79, 339. [Google Scholar] [CrossRef]
- Fitriani, V.Y.; Suprapti, B.; Amin, M. The characteristics of lactic acid bacteria isolated from fermented food as potential probiotics. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 743–749. [Google Scholar] [CrossRef]
- Jahansepas, A.; Sharifi, Y.; Aghazadeh, M.; Ahangarzadeh Rezaee, M. Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: Antimicrobial susceptibility and virulence traits. Arch. Microbiol. 2020, 202, 765–772. [Google Scholar] [CrossRef]
- Jahansepas, A.; Aghazadeh, M.; Rezaee, M.A.; Heidarzadeh, S.; Mardaneh, J.; Mohammadzadeh, A.; Pouresmaeil, O. Prevalence, Antibiotic Resistance and Virulence of Enterococcus spp. Isolated from Traditional Cheese Types. Ethiop. J. Health Sci. 2022, 32, 799–808. [Google Scholar] [CrossRef]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Aziz, M.N.M.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and Characterization of Lactobacillus spp. from Kefir Samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.U.; Nayab, H.; Shafique, F.; Williamson, M.P.; Almansouri, T.S.; Asim, N.; Shafi, N.; Attacha, S.; Khalid, M.; Ali, N.; et al. Probiotic Properties of Lactobacillus helveticus and Lactobacillus plantarum Isolated from Traditional Pakistani Yoghurt. Biomed. Res. Int. 2020, 2020, 8889198. [Google Scholar] [CrossRef]
- Arellano, K.; Vazquez, J.; Park, H.; Lim, J.; Ji, Y.; Kang, H.J.; Cho, D.; Jeong, H.W.; Holzapfel, W.H. Safety Evaluation and Whole-Genome Annotation of Lactobacillus plantarum Strains from Different Sources with Special Focus on Isolates from Green Tea. Probiotics Antimicrob. Proteins 2020, 12, 1057–1070. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, S.A.; Yang, S.H.; Kim, D.H.; Cheng, Y.Y.; Kang, J.I.; Lee, S.Y.; Han, N.S. Integrated genome-based assessment of safety and probiotic characteristics of Lactiplantibacillus plantarum PMO 08 isolated from kimchi. PLoS ONE 2022, 17, e0273986. [Google Scholar] [CrossRef]
- Lee, J.H.; Heo, S.; Jeong, M.; Jeong, D.W. Transfer of a mobile Staphylococcus saprophyticus plasmid isolated from fermented seafood that confers tetracycline resistance. PLoS ONE 2019, 14, e0213289. [Google Scholar] [CrossRef]
- Chokesajjawatee, N.; Santiyanont, P.; Chantarasakha, K.; Kocharin, K.; Thammarongtham, C.; Lertampaiporn, S.; Vorapreeda, T.; Srisuk, T.; Wongsurawat, T.; Jenjaroenpun, P.; et al. Safety Assessment of a Nham Starter Culture Lactobacillus plantarum BCC9546 via Whole-genome Analysis. Sci. Rep. 2020, 10, 10241. [Google Scholar] [CrossRef]
- Chotinantakul, K.; Chansiw, N.; Okada, S. Biofilm formation and transfer of a streptomycin resistance gene in enterococci from fermented pork. J. Glob. Antimicrob. Resist. 2020, 22, 434–440. [Google Scholar] [CrossRef]
- Ozkan, E.R.; Demirci, T.; Ozturk, H.I.; Akin, N. Screening Lactobacillus strains from artisanal Turkish goatskin casing Tulum cheeses produced by nomads via molecular and in vitro probiotic characteristics. J. Sci. Food Agric. 2021, 101, 2799–2808. [Google Scholar] [CrossRef]
- Ozdemir, R.; Tuncer, Y. Detection of antibiotic resistance profiles and aminoglycoside-modifying enzyme (AME) genes in high-level aminoglycoside-resistant (HLAR) enterococci isolated from raw milk and traditional cheeses in Turkey. Mol. Biol. Rep. 2020, 47, 1703–1712. [Google Scholar] [CrossRef]
- Houngbedji, M.; Padonou, S.W.; Parkouda, C.; Johansen, P.G.; Hounsou, M.; Agbobatinkpo, B.P.; Sawadogo-Lingani, H.; Jespersen, L.; Hounhouigan, D.J. Multifunctional properties and safety evaluation of lactic acid bacteria and yeasts associated with fermented cereal doughs. World J. Microbiol. Biotechnol. 2021, 37, 34. [Google Scholar] [CrossRef]
- Mariam, S.H. A sampling survey of enterococci within pasteurized, fermented dairy products and their virulence and antibiotic resistance properties. PLoS ONE 2021, 16, e0254390. [Google Scholar] [CrossRef]
- Baccouri, O.; Boukerb, A.M.; Farhat, L.B.; Zebre, A.; Zimmermann, K.; Domann, E.; Cambronel, M.; Barreau, M.; Maillot, O.; Rince, I.; et al. Probiotic Potential and Safety Evaluation of Enterococcus faecalis OB14 and OB15, Isolated from Traditional Tunisian Testouri Cheese and Rigouta, Using Physiological and Genomic Analysis. Front. Microbiol. 2019, 10, 881. [Google Scholar] [CrossRef]
- Pswarayi, F.; Qiao, N.; Gaur, G.; Ganzle, M. Antimicrobial plant secondary metabolites, MDR transporters and antimicrobial resistance in cereal-associated lactobacilli: Is there a connection? Food Microbiol. 2022, 102, 103917. [Google Scholar] [CrossRef] [PubMed]
- Dentice Maidana, S.; Aristimuno Ficoseco, C.; Bassi, D.; Cocconcelli, P.S.; Puglisi, E.; Savoy, G.; Vignolo, G.; Fontana, C. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int. J. Food Microbiol. 2020, 316, 108425. [Google Scholar] [CrossRef] [PubMed]
- Margalho, L.P.; Feliciano, M.D.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Sant’Ana, A.S. Brazilian artisanal cheeses are rich and diverse sources of nonstarter lactic acid bacteria regarding technological, biopreservative, and safety properties-Insights through multivariate analysis. J. Dairy Sci. 2020, 103, 7908–7926. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Camargo, A.C.; Todorov, S.D.; Nero, L.A. Potential Control of Listeria monocytogenes by Bacteriocinogenic Enterococcus hirae ST57ACC and Pediococcus pentosaceus ST65ACC Strains Isolated from Artisanal Cheese. Probiotics Antimicrob. Proteins 2019, 11, 696–704. [Google Scholar] [CrossRef]
- Silva, L.F.; Sunakozawa, T.N.; Amaral, D.M.F.; Casella, T.; Nogueira, M.C.L.; De Dea Lindner, J.; Bottari, B.; Gatti, M.; Penna, A.L.B. Safety and technological application of autochthonous Streptococcus thermophilus cultures in the buffalo Mozzarella cheese. Food Microbiol. 2020, 87, 103383. [Google Scholar] [CrossRef]
- Kothe, C.I.; Mohellibi, N.; Renault, P. Revealing the microbial heritage of traditional Brazilian cheeses through metagenomics. Food Res. Int. 2022, 157, 111265. [Google Scholar] [CrossRef]
- Russo, N.; Caggia, C.; Pino, A.; Coque, T.M.; Arioli, S.; Randazzo, C.L. Enterococcus spp. in Ragusano PDO and Pecorino Siciliano cheese types: A snapshot of their antibiotic resistance distribution. Food Chem. Toxicol. 2018, 120, 277–286. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Joyce, S.A.; Corsetti, A. Genotypic and phenotypic characterization of food-associated Lactobacillus plantarum isolates for potential probiotic activities. FEMS Microbiol. Lett. 2020, 367, fnaa076. [Google Scholar] [CrossRef]
- Tarrah, A.; da Silva Duarte, V.; Pakroo, S.; Corich, V.; Giacomini, A. Genomic and phenotypic assessments of safety and probiotic properties of Streptococcus macedonicus strains of dairy origin. Food Res. Int. 2020, 130, 108931. [Google Scholar] [CrossRef]
- Belloso Daza, M.V.; Milani, G.; Cortimiglia, C.; Pietta, E.; Bassi, D.; Cocconcelli, P.S. Genomic Insights of Enterococcus faecium UC7251, a Multi-Drug Resistant Strain from Ready-to-Eat Food, Highlight the Risk of Antimicrobial Resistance in the Food Chain. Front. Microbiol. 2022, 13, 894241. [Google Scholar] [CrossRef]
- Fontana, C.; Patrone, V.; Lopez, C.M.; Morelli, L.; Rebecchi, A. Incidence of Tetracycline and Erythromycin Resistance in Meat-Associated Bacteria: Impact of Different Livestock Management Strategies. Microorganisms 2021, 9, 2111. [Google Scholar] [CrossRef]
- Kondrotiene, K.; Lauciene, L.; Andruleviciute, V.; Kasetiene, N.; Serniene, L.; Sekmokiene, D.; Malakauskas, M. Safety Assessment and Preliminary In Vitro Evaluation of Probiotic Potential of Lactococcus lactis Strains Naturally Present in Raw and Fermented Milk. Curr. Microbiol. 2020, 77, 3013–3023. [Google Scholar] [CrossRef]
- Chajecka-Wierzchowska, W.; Zadernowska, A.; Garcia-Solache, M. Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: Phenotypic and genotypic characteristics. J. Dairy Sci. 2020, 103, 4068–4077. [Google Scholar] [CrossRef]
- Chajecka-Wierzchowska, W.; Zadernowska, A.; Zarzecka, U.; Zakrzewski, A.; Gajewska, J. Enterococci from ready-to-eat food—Horizontal gene transfer of antibiotic resistance genes and genotypic characterization by PCR melting profile. J. Sci. Food Agric. 2019, 99, 1172–1179. [Google Scholar] [CrossRef]
- Markuskova, B.; Lichvarikova, A.; Szemes, T.; Korenova, J.; Kuchta, T.; Drahovska, H. Genome analysis of lactic acid bacterial strains selected as potential starters for traditional Slovakian bryndza cheese. FEMS Microbiol. Lett. 2018, 365, fny257. [Google Scholar] [CrossRef]
- Rodriguez, J.; Gonzalez-Guerra, A.; Vazquez, L.; Fernandez-Lopez, R.; Florez, A.B.; de la Cruz, F.; Mayo, B. Isolation and phenotypic and genomic characterization of Tetragenococcus spp. from two Spanish traditional blue-veined cheeses made of raw milk. Int. J. Food Microbiol. 2022, 371, 109670. [Google Scholar] [CrossRef]
- Labba, I.M.; Andlid, T.; Lindgren, A.; Sandberg, A.S.; Sjoberg, F. Isolation, identification, and selection of strains as candidate probiotics and starters for fermentation of Swedish legumes. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Shani, N.; Oberhaensli, S.; Arias-Roth, E. Antibiotic Susceptibility Profiles of Pediococcus pentosaceus from Various Origins and Their Implications for the Safety Assessment of Strains with Food-Technology Applications. J. Food Prot. 2021, 84, 1160–1168. [Google Scholar] [CrossRef]
- Ludin, P.; Roetschi, A.; Wuthrich, D.; Bruggmann, R.; Berthoud, H.; Shani, N. Update on Tetracycline Susceptibility of Pediococcus acidilactici Based on Strains Isolated from Swiss Cheese and Whey. J. Food Prot. 2018, 81, 1582–1589. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Zinno, P.; Perozzi, G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Front. Microbiol. 2013, 4, 301. [Google Scholar] [CrossRef] [Green Version]
- Colautti, A.; Arnoldi, M.; Comi, G.; Iacumin, L. Antibiotic resistance and virulence factors in lactobacilli: Something to carefully consider. Food Microbiol. 2022, 103, 103934. [Google Scholar] [CrossRef] [PubMed]
- Nunziata, L.; Brasca, M.; Morandi, S.; Silvetti, T. Antibiotic resistance in wild and commercial non-enterococcal Lactic Acid Bacteria and Bifidobacteria strains of dairy origin: An update. Food Microbiol. 2022, 104, 103999. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Solymosi, N. Review and Comparison of Antimicrobial Resistance Gene Databases. Antibiotics 2022, 11, 339. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Bortolaia, V.; Tate, H.; Tyson, G.H.; Aarestrup, F.M.; McDermott, P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public. Health 2019, 7, 242. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Roberts, A.P.; Mullany, P. Tn916-like genetic elements: A diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 856–871. [Google Scholar] [CrossRef] [Green Version]
- Niegowska, M.; Wogerbauer, M. Improving the risk assessment of antimicrobial resistance (AMR) along the food/feed chain and from environmental reservoirs using qMRA and probabilistic modelling. EFSA J. 2022, 20, e200407. [Google Scholar] [CrossRef]
- EMA Committee for Medicinal Products for Veterinary Use (CVMP) and EFSA Panel on Biological Hazards (BIOHAZ); Murphy, D.; Ricci, A.; Auce, Z.; Beechinor, J.G.; Bergendahl, H.; Breathnach, R.; Bures, J.; Duarte Da Silva, J.P.; Hederová, J.; et al. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. In WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals: Policy Brief; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Xiao, Y.; Li, L. China’s national plan to combat antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 1216–1218. [Google Scholar] [CrossRef]
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rappuoli, R.; Young, P.; Ron, E.; Pecetta, S.; Pizza, M. Save the microbes to save the planet. A call to action of the International Union of the Microbiological Societies (IUMS). One Health Outlook 2023, 5, 5. [Google Scholar] [CrossRef]
- Additives, E.P.o.; Products or Substances used in Animal, F.; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- European Food Safety, A. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2021, 19, e06506. [Google Scholar] [CrossRef] [PubMed]
- Agamennone, V.; Abuja, P.M.; Basic, M.; De Angelis, M.; Gessner, A.; Keijser, B.; Larsen, M.; Pinart, M.; Nimptsch, K.; Pujos-Guillot, E.; et al. HDHL-INTIMIC: A European Knowledge Platform on Food, Diet, Intestinal Microbiomics, and Human Health. Nutrients 2022, 14, 1881. [Google Scholar] [CrossRef] [PubMed]
- Cernava, T.; Rybakova, D.; Buscot, F.; Clavel, T.; McHardy, A.C.; Meyer, F.; Meyer, F.; Overmann, J.; Stecher, B.; Sessitsch, A.; et al. Metadata harmonization-Standards are the key for a better usage of omics data for integrative microbiome analysis. Environ. Microbiome 2022, 17, 33. [Google Scholar] [CrossRef] [PubMed]
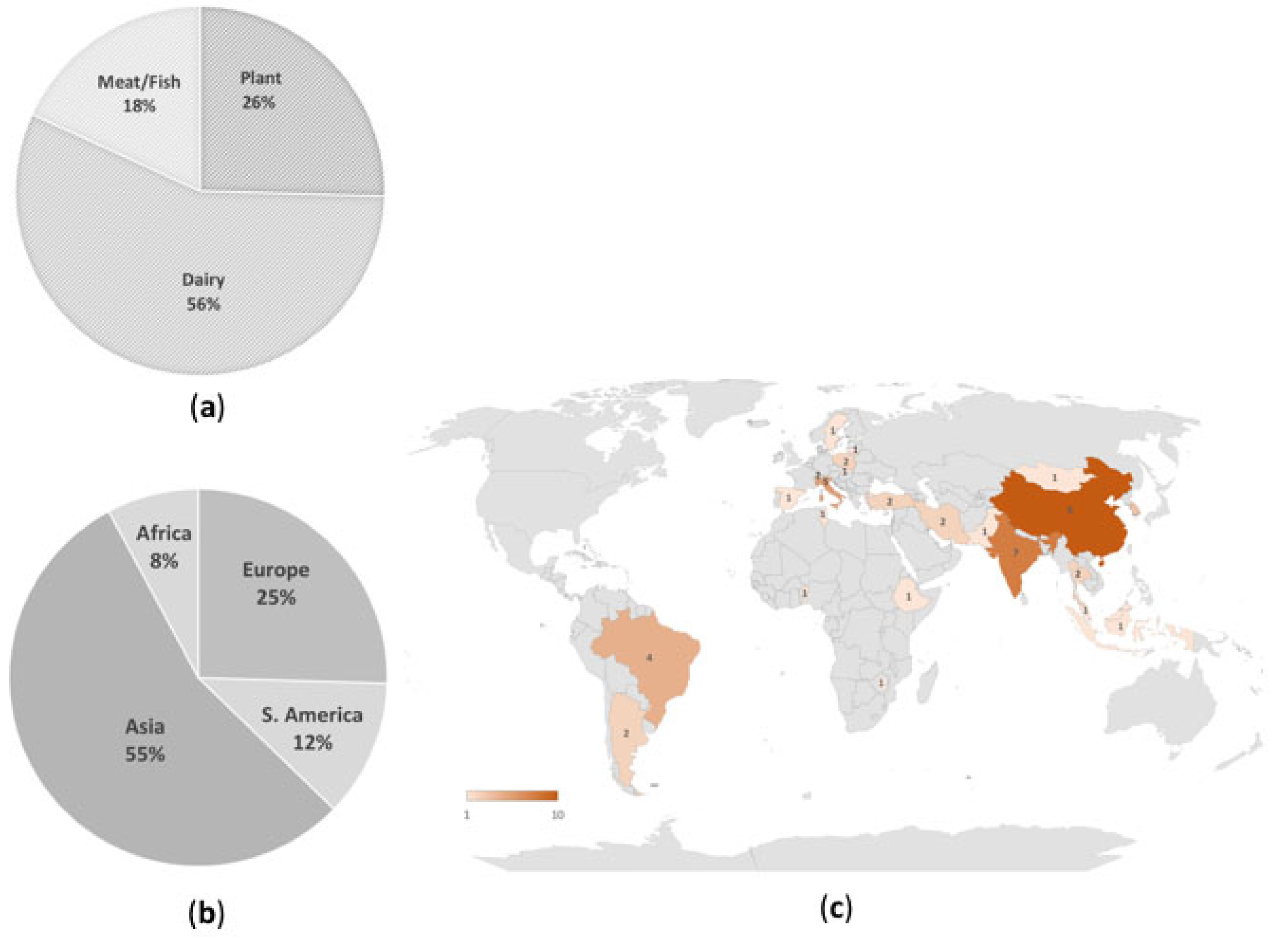
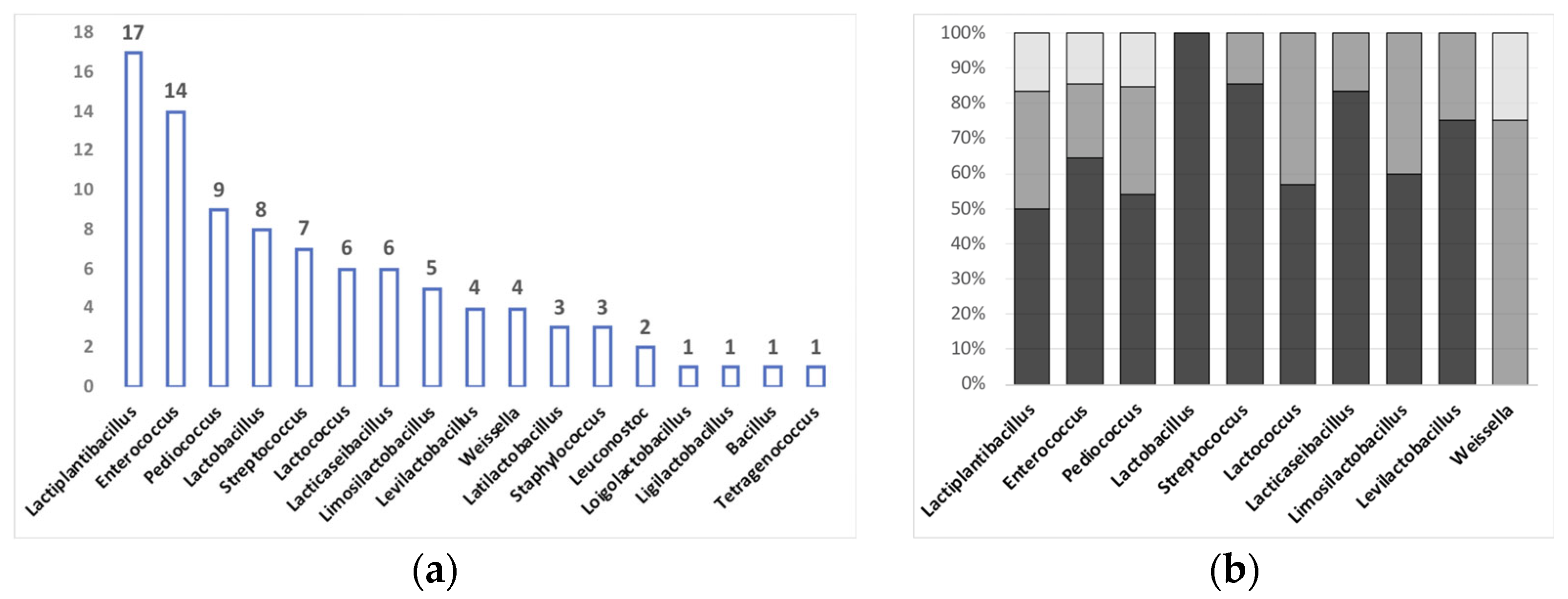
| AMR Species Identified | Food Source | Food Origin | Characterization of Bacterial Isolates | Phenotypic Resistance | Detected AMR Genes | Detection Method of AMR Gene(s) | Reference |
|---|---|---|---|---|---|---|---|
| Asia | |||||||
| Lactococcus lactis, Lactobacillus gallinarum, Lacticaseibacillus casei, Lacticaseibacillus paracasei, Lactiplantibacillus plantarum, Streptococcus thermophilus | Fermented dairy products (Mozzarella, Traditional milk tofu, Cheddar, Cream, Mimolette Wedge, Emmentaler, Brie, Feta) | China | 16S-DNA-based metagenomics (cheese metagenome); 16S rRNA gene sequencing (isolates) | Streptomycin, sulfamethoxazole, clindamycin, tetracycline, penicillin, norfloxacin, ciprofloxacin | aadE, strA, strB, sul1, sul2 | qPCR | [21] |
| Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Lactiplantibacillus plantarum | Fermented dairy products (Yogurt) | China | 16S rRNA gene sequencing | Streptomycin, tetracycline, vancomycin | not determined | not determined | [22] |
| Lactococcus, Leuconostoc mesenteroides, Weissella cibaria/soli/confusa, Enterococcus gallinarum/durans/hirae, Pediococcus pentosaceus, Bacillus coagulans, Lactococcus garvieae/lactis | Fermented vegetable products (Broccoli, Cherry, Ginger, White radish, White-fleshed pitaya juices) | China | 16S rRNA gene sequencing | Ampicillin, penicillin, amoxycillin, orfloxacine, levoflacin, gentamycin, streptomycin, amikacin, erythromycin | not determined | not determined | [23] |
| Lactobacillus delbrueckii subsp. bulgaricus, Lactiplantibacillus plantarum, Streptococcus thermophilus | Fermented dairy products (Yogurt) | China | 16S rRNA gene sequencing; PFGE | Erythromycin, gentamycin, streptomycin, sulfamethoxazole, tetracycline | ermB, aac(6′)-aph(2″), ant(6), sulI and sulII, tetM, tetS | PCR | * [24] |
| Lactiplantibacillus plantarum, Lactiplantibacillus fermentum | Fermented vegetable products (Yucha) | China | Shotgun metagenomics; 16S rRNA gene sequencing of isolates | not determined | AMR genes (not specified) | Shotgun metagenomics, Comprehensive Antibiotic Resistance Database (CARD) | [25] |
| Food metagenome | Fermented vegetable products | China | Shotgun metagenomics | not determined | Multidrug resistance genes | Shotgun metagenomics, SARG database | [26] |
| Staphylococcus carnosus, Lactiplantibacillus plantarum, Latilactobacillus sakei, Weissella cibaria, Weissella confusa | Fermented meat products | China | RAPD; 16S rRNA gene sequencing | Streptomycin, vancomycin, erythromycin, roxithromycin, lincomycin, kanamycin | tetM, ereA, strA, strB | PCR | [27] |
| Streptococcus thermophilus | Fermented dairy products (Koumiss, Kurut, Fermented cow milk, Fermented goat milk, Qula) | China, Mongolia | WGS | Chloramphenicol | vanH, vanU, catB8 | WGS, Comprehensive Antibiotic Resistance Database (CARD); droplet digital PCR | [28] |
| Pediococcus pentosaceus | Fermented dairy products | India | 16S rRNA gene sequencing | Kanamycin, streptomycin, vancomycin, ciprofloxacin, norfloxacin (chromosomal); trimethoprim (plasmid) | aph (3″)-III, strA, vanA, gyrA | PCR | * [29] |
| Enterococcus faecalis | Fermented fish products (Tungtap) | India | 16S rRNA gene sequencing | Kanamycin, gentamycin, streptomycin, ciprofloxacin, vancomycin, penicilin G, ampicillin, tetracyclin, rifampycin, chloraphenicol, polymyxin b | vanA, vanB | PCR | [30] |
| Levilactobacillus brevis, Enterococcus durans, Enterococcus lactis, Enterococcus faecium, Leuconostoc lactis | Fermented vegetable products (Coconut palm nectar) | India | 16S rRNA gene sequencing | Ampicillin, vancomycin, gentamicin, kanamycin, chloramphenicol, erythromycin, clindamycin | not determined | not determined | [31] |
| Pediococcus pentosaceus | Fermented foods (Idli, Dosa batter, Dahi, Fermented dry sausage) | India | not indicated | Azitromycin, clarithromycin, clyndamicin, lyncomincin, piramicin, pristinamycin, streptograminB | ermB, msrC | PCR, Southern blot | * [32] |
| Ligilactobacillus salivarius | Fermented poultry products | India | 16S rRNA gene sequencing | Erythromycin, tetraciclyne | ermB, tetW, tetM, tetL | PCR, Southern blot | * [33] |
| Pediococcus pentosaceus, Pediococcus acidilactici | Fermented food products | India | species-specific PCR | Vancomycin, nalidixic acid | not determined | not determined | [34] |
| Limosilactobacillus fermentum | Fermented dairy products | India, China | Phenotipic tests | Ampicillin, ciprofloxacin, vancomycin, streptomycin, trimethoprim | tetM, ermB | PCR | * [35] |
| Lactobacillus acidophilus, Limosilactobacillus reuteri | Fermented vegetable and dairy products | Indonesia | not indicated | Streptomycin, kanamycin, amikacin, meropenem | not determined | not determined | [36] |
| Enterococcus faecalis, Enterococcus faecium | Fermented dairy products | Iran | species-specific PCR (ddl gene) | Quinupristin/dalfopristin, penicillin G, ampicillin, rifampicin, doxycycline, ciprofloxacin | not determined | not determined | [37] |
| Enterococcus faecalis, Enterococcus faecium | Fermented dairy products | Iran | 16S rRNA gene sequencing | Rifampicin, quinupristin, dalfopristin | ace, gelE, asa1 | PCR | [38] |
| Lactobacillus harbinensis, Lacticaseibacillus paracasei, Lactiplantibacillus plantarum | Fermented dairy products (Kefir) | Malaysia | 16S rRNA gene sequencing | Ampicillin, penicillin, tetracycline | not determined | not determined | [39] |
| Lactiplantibacillus plantarum, Lactobacillus helveticus | Fermented dairy products (Yogurt) | Pakistan | 16S rRNA gene sequencing | Ampicillin, trimethoprim, vancomycin, nitrofurantoin | not determined | not determined | [40] |
| Lactiplantibacillus plantarum | Fermented vegetable products | Republic of Korea | 16S rRNA sequencing | Chloramphenicol, tetracycline | not determined | not determined | [41] |
| Lactiplantibacillus plantarum | Fermented vegetable products | Republic of Korea | WGS | Ampicillin | ermB | WGS, Comprehensive Antibiotic Resistance Database (CARD) | [42] |
| Staphylococcus saprophyticus | Fermented seafood products (Jeotgal) | Republic of Korea | 16S rRNA gene sequencing | Penicillin G, tetracycline | tetK | PCR | * [43] |
| Lactiplantibacillus plantarum | Fermented meat products (Nham) | Thailand | WGS | Chloramphenicol, kanamycin | cat | PCR | [44] |
| Enterococcus faecium, Enterococcus faecalis | Fermented meat products | Thailand | not indicated | Streptomycin | aadE | PCR | * [45] |
| Levilactobacillus brevis, Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, Loigolactobacillus coryniformis, Lacticaseibacillus rhamnosus, Lactobacillus helveticus | Fermented dairy products (Tulum) | Turkey | 16S rRNA gene sequencing | Ampicillin, chloraphenicol, erytromycin, gentamycin, kanamycin, penicillin, streptomycin, tetracycline, vancomycin | not determined | not determined | [46] |
| Enterococcus faecalis, Enterococcus faecium, Enterococcus durans, Enterococcus gallinarum | Fermented dairy products | Turkey | species-specific PCR | Streptomycin, gentamicin | aph(3′)-IIIa, ant(4′)-Ia, ant(6′)-Ia, aph(2″)-Ic | PCR | [47] |
| Africa | |||||||
| Limosilactobacillus fermentum, Weissella confusa, Lactiplantibacillus plantarum, Pediococcus pentosaceus, Pediococcus acidilactici | Fermented cereal products (Mawé) | Benin | 16S rRNA gene sequencing | Chloramphenicol, erythromycin, tetracycline, ampicillin, streptomycin, kanamicin | aph(3)I, aph(3)III, tetS, tetM, tetL, strA, strB, aadA, aadE, strA, strB, aadA, aadE | PCR | [48] |
| Enterococcus faecium, Enterococcus faecalis | Fermented dairy products (Yogurt, Cheese, Milk) | Ethiopia | species-specific PCR | Erythromycin, tetracycline, ampicillin, oxacillin, ciprofloxacin, azithromycin, vancomycin | vanC1, vanC2/C3, ermB, ermC | PCR | [49] |
| Enterococcus fecalis | Fermented dairy products (Testouri cheese, Rigouta, Yogurt, Leben, Rayeb) | Tunisia | MALDI TOF, species-specific PCR, WGS | Gentamicin, erythromycin | Tetracycline resistance determinants (not specified) | WGS | [50] |
| Lactiplantibacillus plantarum, Limosilactobacillus fermentum | Fermented cereal products (Mahewu) | Zimbawe | WGS | Streptomycin, norfloxacin, erythromycin, chloramphenico, tetracycline | tetB(48), tetA(48), efrA, efrB, dfrE, dfrF, dfrI, lmrB, lmrD, mdtG, vanRM, vanRF, vanHF, patA, patB, qacH, emeA, mprF, bacA, taeA, fusF | WGS, Comprehensive Antibiotic Resistance Database (CARD) | [51] |
| South America | |||||||
| Enterococcus faecium, Lactococcus lactis, Lacticaseibacillus rhamnosus, Weissella cibaria | Fermented cereal products (Chia sourdough) | Argentina | 16S rRNA gene sequencing | Clindamycin, erythromycin, gentamycin, vancomycin | not determined | not determined | [52] |
| Levilactobacillus brevis, Lacticaseibacillus paracasei, Lactococcus lactis, Lacticaseibacillus rhamnosus, Lactobacillus pentosaceus, Lactilactobacillus curvatus, Lactiplantibacillus paraplantarum, Pediococcus acidilactici | Fermented dairy products | Brasil | MALDI-TOF | Gentamycin, ciprofloxacin, vancomycin, streptomycin | not determined | not determined | [53] |
| Enterococcus hirae, Pediococcus pentosaceus | Fermented dairy products | Brazil | RAPD; 16S rRNA gene sequencing | Ampicillin, penicillin G, oxacillin, clindamycin, erythromycin, imipenem, rifampicin, chloramphenicol, tetracycline, trimethoprim/sulfamethoxazole vancomycin | ermA, ermB, ermC, bcrB, tetO, vatE | PCR | [54] |
| Streptococcus thermophilus | Fermented dairy products (Buffalo mozzarella cheese) | Brazil | 16S rRNA gene sequencing | Oxacillin | not determined | not determined | [55] |
| Food metagenome of different cheese samples: Lactococcus lactis, Streptococcus thermophilus-salivarius, Streptococcus equinus-lutetiensis-infantarius complex (dominant species); Lacticaseibacillus casei, Lactobacillus delbrueckii, Lactiplantibacillus plantarum, Lactococcus piscium, Leuconostoc mesenteroides, Streptococcus spp., Lactococcus spp. (less abundant species) | Fermented dairy products | Brazil | 16S-DNA-based metagenomics; Shotgun metagenomics | not determined | 30 AMR genes identified, belonging to nine different classes of antibiotics (tetK, tetS most prevalent) | Shotgun metagenomics, Comprehensive Antibiotic Resistance Database (CARD) | [56] |
| Europe | |||||||
| Enterococcus faecalis, Enterococcus durans, Enterococcus faecium | Fermented dairy products (Ragusano, Pecorino) | Italy | MALDI-TOF, species-specific multiplex PCR | Tetracycline, erythromycin, streptomycin, gentamycin, ampicillin, rifampicin, penicillin G, sulphametoxazol, chloramphenicol, vancomycin, kanamycin | not determined | not determined | [57] |
| Lactiplantibacillus plantarum | Fermented foods (Table olives, Pickled cabbage, Sourdough, Raw milk cheese) | Italy | multilocus sequence typing (MLST) | Vancomycin, ciprofloxacin; streptomycin, kanamycin; gentamicin, penicillin | not determined | not determined | [58] |
| Streptococcus macedonicus | Fermented dairy products | Italy | WGS | Kanamycin | not determined | not determined | [59] |
| Enterococcus faecium | Fermented dry sausage | Italy | species-specific PCR | Ampicillin, streptomycin, kanamycin, erythromycin, clindamycin, tylosin, tetracycline | ermB, tetM, tetL, aph3-IIIa, satA, ant(6)-Ia, aadE | PCR | * [60] |
| Latilactobacillus sakei, Lactiplantibacillus plantarum, Staphylococcus xylosus, Staphylococcus equorum, Staphylococcus saprophyticus | Fermented meat products (Italian Piacentino Salami DOP; Fermented pork and llama meat products) | Italy, ** Argentina | RAPD; 16S RNA gene sequencing | Tetracycline, erythromycin | tetM, tetK, tetW, tetS, tetL, ermA, ermB, ermC | PCR | [61] |
| Lactococcus lactis | Raw milk | Lithuania | species-specific PCR | Clindamycin, streptomycin, gentamycin, tetracycline erytromycin, ampicillin, vancomycin, kanamycin | not determined | not determined | [62] |
| Enterococcus faecium, Enterococcus faecalis | Fermented dairy products | Poland | species-specific PCR | Streptomycin, erythromycin, tetracycline, rifampicin, tigecycline, vancomycin, linezolid | ant(6′)-Ia, aac(6′)-Ie-aph(2″)-Ia, aph(3″)-IIIa; tetM, tetL; ermA, ermB | PCR | * [63] |
| Enterococcus faecium, Enterococcus faecalis | Ready-to-eat foods | Poland | RAPD | Erythromycin, gentamicin, streptomycin, tetracycline, tigecycline, fosfomycin, ciprofloxacin, levofloxacin, norfloxacin, rifampicin, linezolid, quinupristin/dalfopristin | aac(6′)-Ie-aph(2″)-Ia, aph(2″)-Ib, aph(2″)-Ic, aph(2″)-Id, ant(4′)-Ia, ant(6′)-Ia; tetM, tetL, tetK, tetO, tetW, ermA, ermB, ermC, msrC, mefAB | PCR | [64] |
| Enterococcus faecium, Levilactobacillus brevis, Limosilactobacillus fermentum, Lacticaseibacillus paracasei, Lactiplactibacillus paraplantarum, Lactococcus lactis, Leuconostoc mesenteroides, Leuconostoc pseudomesenteroides | Fermented dairy products | Slovakia | WGS | not determined | aac(6′)-li, msrC, efmA, lmrCD | WGS, Comprehensive Antibiotic Resistance Database (CARD) | [65] |
| Tetragenococcus koreensis, Tetragenococcus halophilus | Fermented dairy products | Spain | 16S rRNA gene sequencing | Erythromycin, clindamycin | not determined | not determined | [66] |
| Enterococcus faecium, Enterococcus mundtii, Enterococcus hirae | Fermented legumes | Sweden | MALDI-TOF | Ampicillin, trimethoprim, vancomycin, nitrofurantoin | not determined | not determined | [67] |
| Pediococcus pentosaceus | Fermented dairy and meat products | Switzerland | WGS, MALDI-TOF | Ampicillin, gentamycin, erytromycin, clindamycin, tetracycline, chloramphenico, streptomycin, vancomicin, kanamycin | not determined | WGS AMRFin-derPlus, CARD, ARG-ANNOT, and Resfinder databases | [68] |
| Pediococcus acidilactici | Fermented dairy products (Gruyere, Emmental, Tete de Moine, Sbrinz, Tilsit) | Switzerland | MALDI-TOF | Clindamycin, tetracycline, streptomycin, penicillin, chloramphenicol, kanamycin, vancomycin, gentamycin, erythromycin | not determined | not determined | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinno, P.; Perozzi, G.; Devirgiliis, C. Foodborne Microbial Communities as Potential Reservoirs of Antimicrobial Resistance Genes for Pathogens: A Critical Review of the Recent Literature. Microorganisms 2023, 11, 1696. https://doi.org/10.3390/microorganisms11071696
Zinno P, Perozzi G, Devirgiliis C. Foodborne Microbial Communities as Potential Reservoirs of Antimicrobial Resistance Genes for Pathogens: A Critical Review of the Recent Literature. Microorganisms. 2023; 11(7):1696. https://doi.org/10.3390/microorganisms11071696
Chicago/Turabian StyleZinno, Paola, Giuditta Perozzi, and Chiara Devirgiliis. 2023. "Foodborne Microbial Communities as Potential Reservoirs of Antimicrobial Resistance Genes for Pathogens: A Critical Review of the Recent Literature" Microorganisms 11, no. 7: 1696. https://doi.org/10.3390/microorganisms11071696





