Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
- (a)
- Papers from 2005 to present.
- (b)
- Use of collection isolates or isolates that had been previously identified through genotyping; papers without a clear identification of the strains or with strains identified only through phenotyping were excluded.
- (c)
- Assays performed in the optimal broth for each microorganism, not containing restrictive ingredients or conditions (antibiotics, salt, or sub-optimal pH), which could affect the response of the test strains to carvacrol and thymol.
- (d)
- Use of thymol and carvacrol with a degree of purity of at least 96%.
- (e)
- Only research papers with a clear distinction between MIC and MBC (minimum bactericidal concentration) were used. The articles where the threshold values of thymol and carvacrol could be either MIC or MBC were excluded.
- (f)
- Use of both positive (for example, chloramphenicol, cycloheximide, or other antibiotics able to exert inhibition against the tested microorganism) and negative controls (distilled water and the solvent used to prepare thymol and carvacrol solution). Research papers not containing negative controls were excluded.
- (g)
- Experiments conducted using the micro-dilution approach; it was not possible to include papers that used CLSI protocols, as they are generally used for antibiotics, and only in recent years has a proposal for a protocol for EOS been released.
- (h)
- For the analyses, some papers with an agar diffusion approach were retained if the experiments had been conducted with at least 10 different concentrations of thymol and carvacrol and positive and negative controls were obtained, as reported for point “f”.
- (i)
- Only species (bacteria or fungi) for which there were at least three independent evaluations of MICs were retained for this study.
2.2. Standardization and Data Analysis
3. Results and Discussion
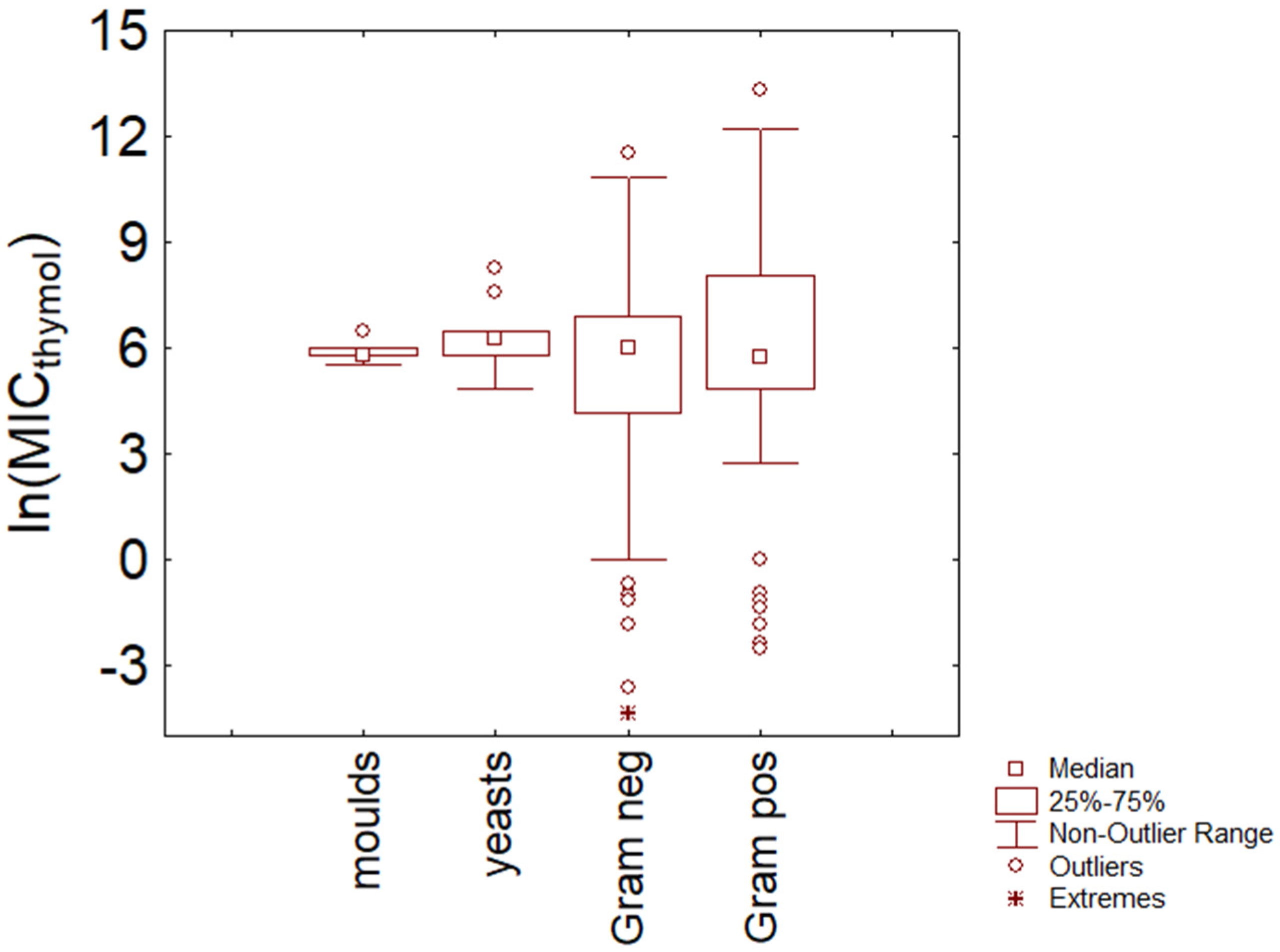
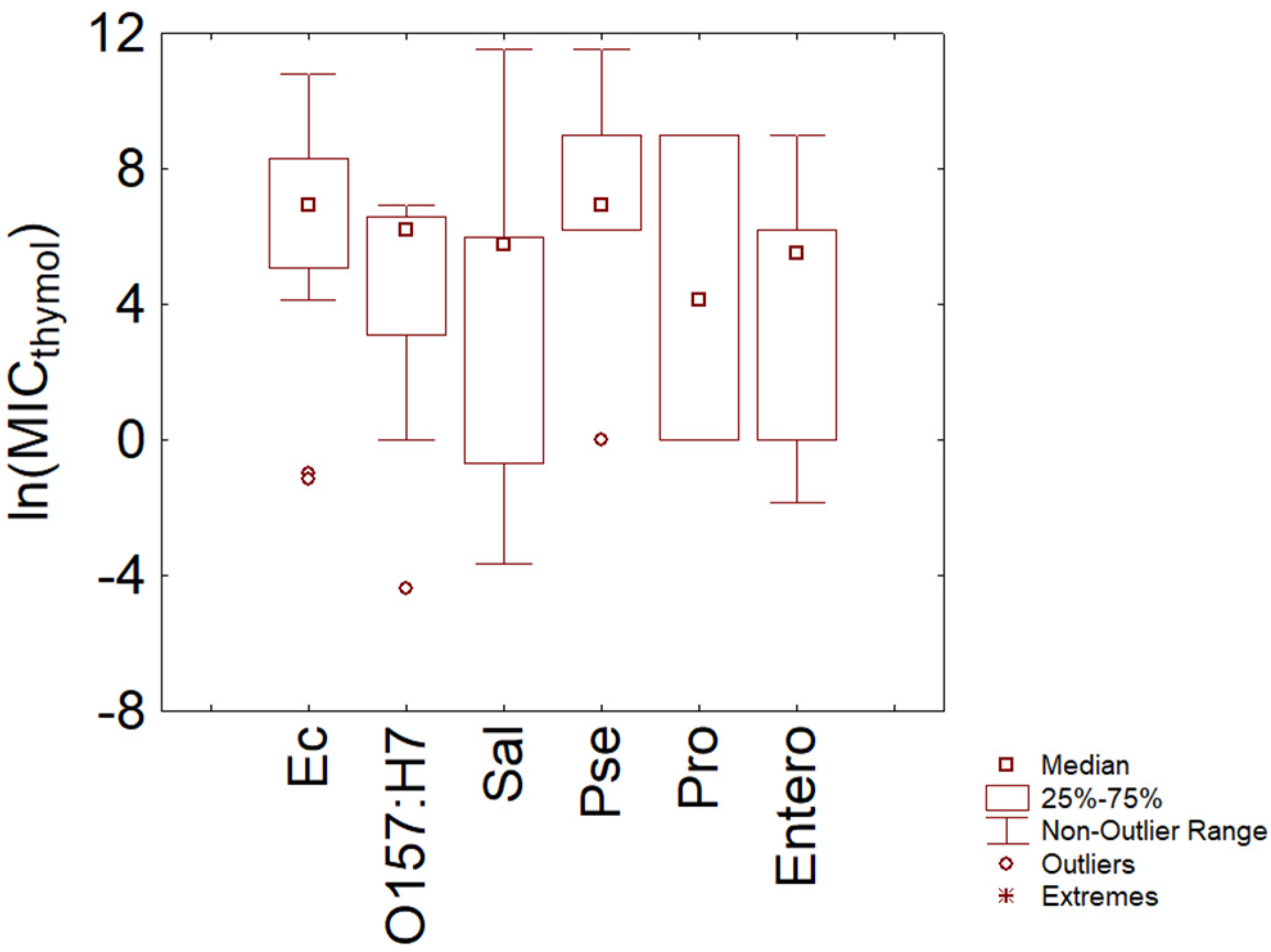
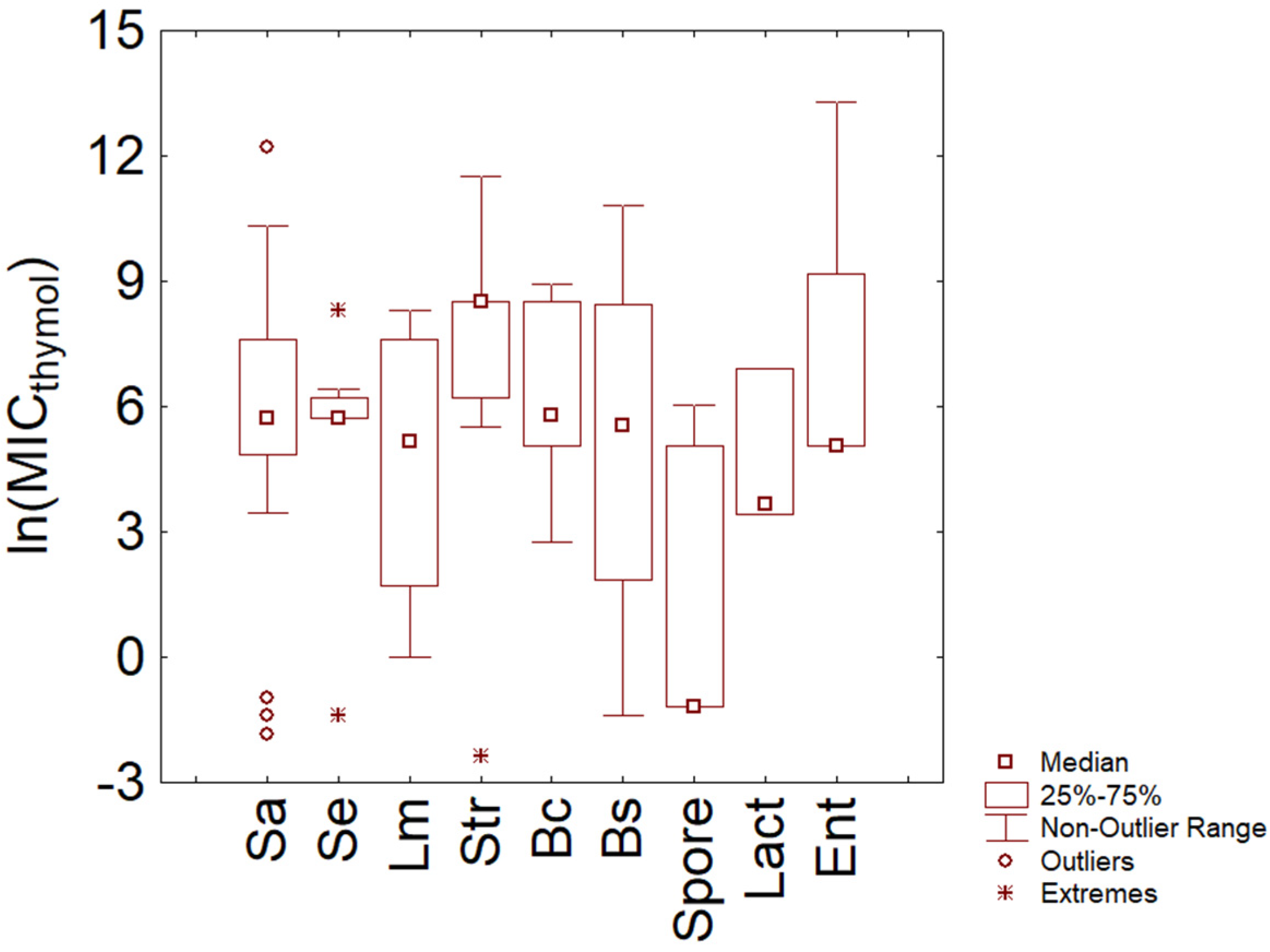
3.1. Thymol
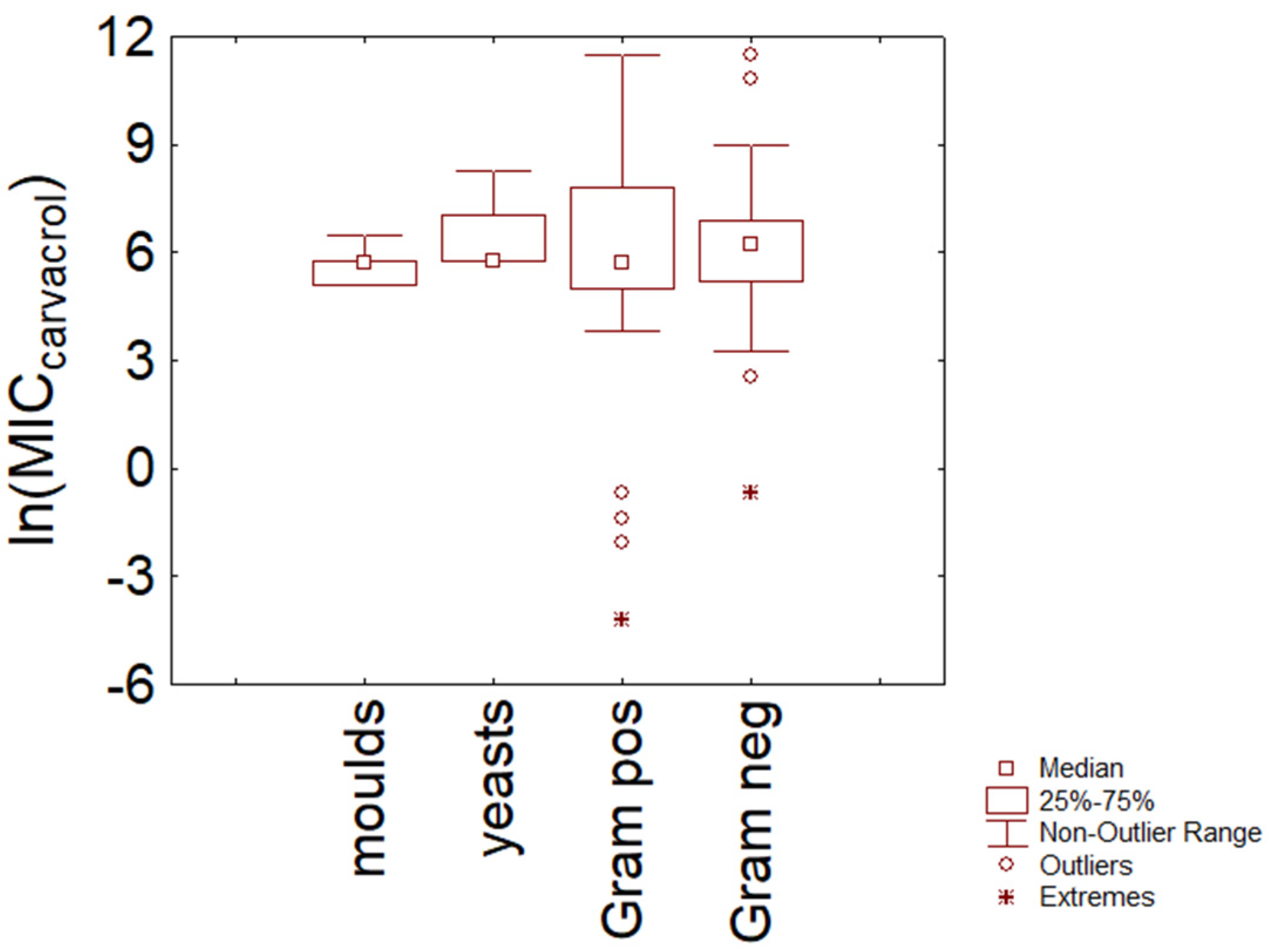
3.2. Carvacrol
3.3. Comparison
4. Conclusions
- (a)
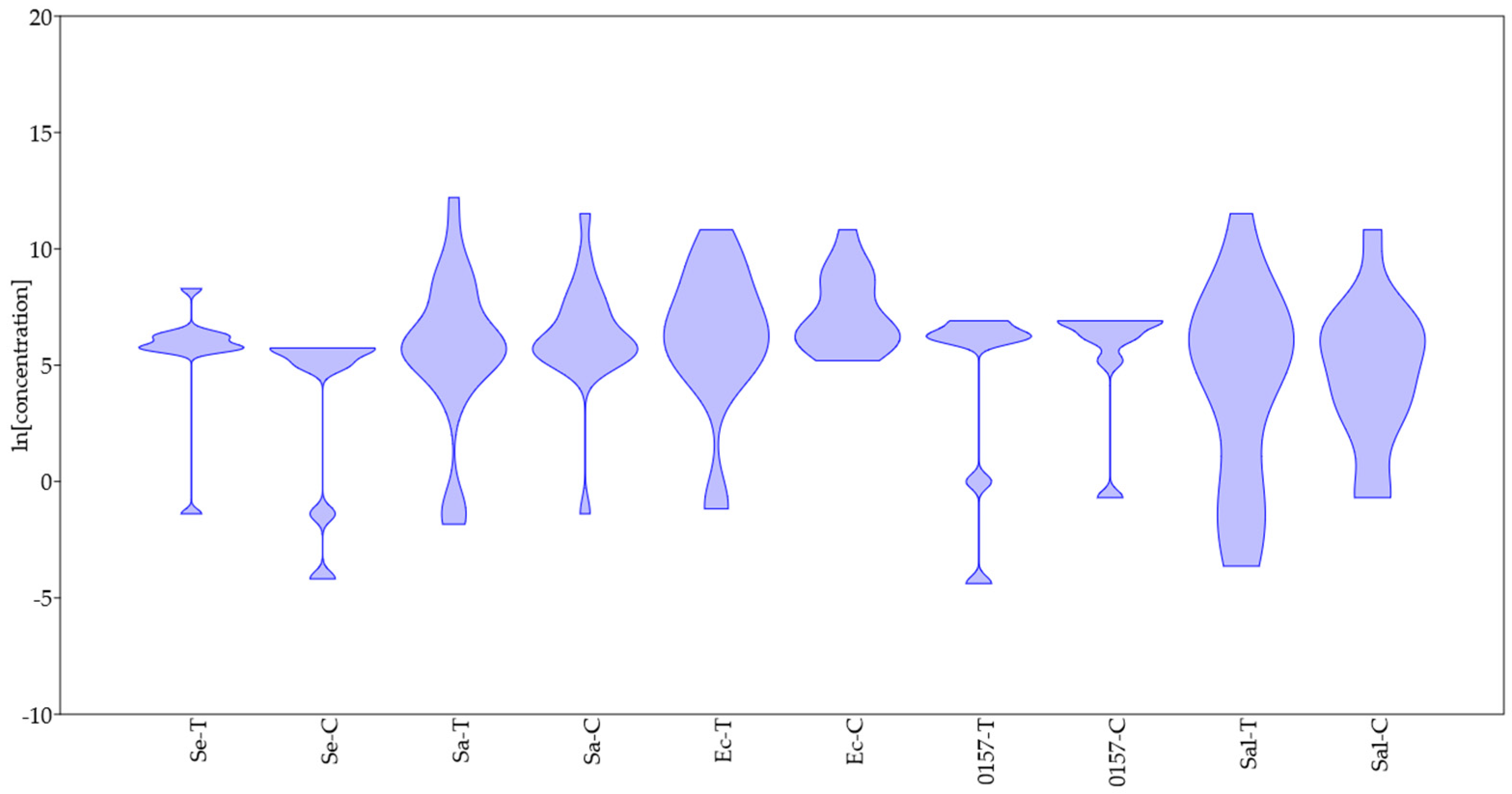
- There is no evidence of a different resistance between Gram-positive and Gram-negative bacteria and fungi, at least for thymol and carvacrol and at least for the data used in this paper. Some differences were found at the species level (for example, between S. epidermidis and E. coli).
- (b)
- The probability distribution found through the violin plot pointed out a possible homogeneous trend toward resistance to thymol and carvacrol for some species (S. epidermidis, E. coli O157:H7), while for other species (for example, Salmonella sp.), the trend was strongly variable, and this could be an issue to think for an effective industrial use of some Eos.
- (c)
- Some species could show different profiles of resistance/sensitivity, as suggested, for example, for E. coli in the case of thymol. An open question is whether these different profiles depend on the existence of sub-species or on different experiment set-ups.
- (d)
- There is a well-defined range for thymol and carvacrol concentrations (5–6 ln (mg/L); 150–400 mg/L), where the probability of finding MIC for many species is high.
- (e)
- Finally, the statistical treatment of data is crucial, as most of the evidence and new perspectives in this paper were found only after a standardization of the data as a natural logarithm and using both non-parametric tests and probability distributions (i.e., a violin graph).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of actions, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akdag, A.; Ozturk, E. Distillation methods of essential oils. Nisan 2019, 45, 22–31. [Google Scholar]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods–A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils. A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, F.; Ge, J.; Li, H.; Xia, F.; Bai, H.; Piao, H. Potential of aromatic plant-derived essential oils for the control of foodborne bacteria and antibiotic resistance in animal production: A review. Antibiotics 2022, 11, 1673. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Zhang, J.R.; Farha, A.K.; Li, H.B.; Zhu, F.; Wang, X.H.; Corke, H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1018–1055. [Google Scholar] [CrossRef] [Green Version]
- Elmi, A.; Prosperi, A.; Zannoni, A.; Bertocchi, M.; Scorpio, D.G.; Forni, M.; Foni, E.; Bacci, M.L.; Ventrella, D. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res. Vet. Sci. 2019, 127, 76–81. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef] [Green Version]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [PubMed]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Definitive Document. Methods for the determination of susceptibility of bacteria to antimicrobial agents. Terminol. Clin. Microbiol. Infect. 1998, 4, 291–296. [CrossRef] [Green Version]
- Vanegas, D.; Abril-Novillo, A.; Khachatryan, A.; Jerves-Andrade, L.; Peñaherrera, E.; Cuzco, N.; Wilches, I.; Calle, J.; León-Tamariz, F. Validation of a method of broth microdilution for the determination of antibacterial activity of essential oils. BMR Res. Notes 2021, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Carta, A.; Rappelli, P.; Diaz, N.; et al. Biological activities of essential oils from leaves of Paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (miq.) Swingle. Antibiotics 2020, 9, 207. [Google Scholar]
- Oliveira-Ribeiro, S.; Fraselle, S.; Baudoux, D.; Zhiri, A.; Stévigny, C. Proposal for antimicrobial testing guidelines applied on Ajowan and Spanish lavender essential oils. Planta Med. 2021, 87, 754–763. [Google Scholar]
- Yammine, J.; Chihib, N.-E.; Gharsalloui, A.; Dumas, E.; Imsail, A. Essential oils and their active components applied as: Free, encapsulated and in hurdle technology to fight microbial contaminations. A review. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising antimicrobial properties of bioactive compounds from different honeybee products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–438. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Pook, C.; Sadooghy-Saraby, S.; Gite, A.; Rush, E. Volatile components and preliminary antibacterial activity of tamarillo (Solanum betaceum Cav.). Foods 2021, 10, 2212. [Google Scholar] [CrossRef]
- El-Hawary, S.; Taha, K.; Abdel-Monem, A.; Kirollos, F.; Mohamed, A. Chemical composition and biological activities of peels and leaves essential oils of four cultivars of Citrus deliciosa var. tangarina. Am. J. Essent. Oils Nat. Prod. 2013, 1, 1–6. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: Un updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Corbo, M.R. Essential oils for preserving perishable foods: Possibilities and limitations. In Application of Alternative Food-Preservation Technologies to Enhance Food Safety & Stability; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Bentham Publisher: Sharjah, United Arab Emirates, 2010; pp. 35–57. [Google Scholar]
- Nostro, A.; Sudano Roccaro, A.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Vidacs, A.; Kerekes, E.; Rajko, R.; Petkovits, T.; Alharbi, N.S.; Khaled, J.M.; Vagvolgyi, C.; Krisch, J. Optimization of essential oil-based natural disinfectants against Listeria monocytogenes and Escherichia coli biofilms formed on polypropylene surfaces. J. Mol. Liq. 2018, 255, 257–262. [Google Scholar] [CrossRef]


| Predictor | Sum of Sqrs | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Species | 103.85 | 4 | 25.96 | 2.32 | 0.03 |
| Antimicrobial | 4.80 | 1 | 4.80 | 0.43 | 0.48 |
| Interaction | −133.69 | 4 | −33.42 | −2.99 | 0.31 |
| Residual | 1452.10 | 130 | 11.17 | ||
| Total | 1427.10 | 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, B.; Bevilacqua, A.; Campaniello, D.; Altieri, C.; Corbo, M.R.; Sinigaglia, M. Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends. Microorganisms 2023, 11, 1774. https://doi.org/10.3390/microorganisms11071774
Speranza B, Bevilacqua A, Campaniello D, Altieri C, Corbo MR, Sinigaglia M. Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends. Microorganisms. 2023; 11(7):1774. https://doi.org/10.3390/microorganisms11071774
Chicago/Turabian StyleSperanza, Barbara, Antonio Bevilacqua, Daniela Campaniello, Clelia Altieri, Maria Rosaria Corbo, and Milena Sinigaglia. 2023. "Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends" Microorganisms 11, no. 7: 1774. https://doi.org/10.3390/microorganisms11071774






