Magnesium Binding by Cyberlindnera jadinii Yeast in Media from Potato Wastewater and Glycerol
Abstract
:1. Introduction
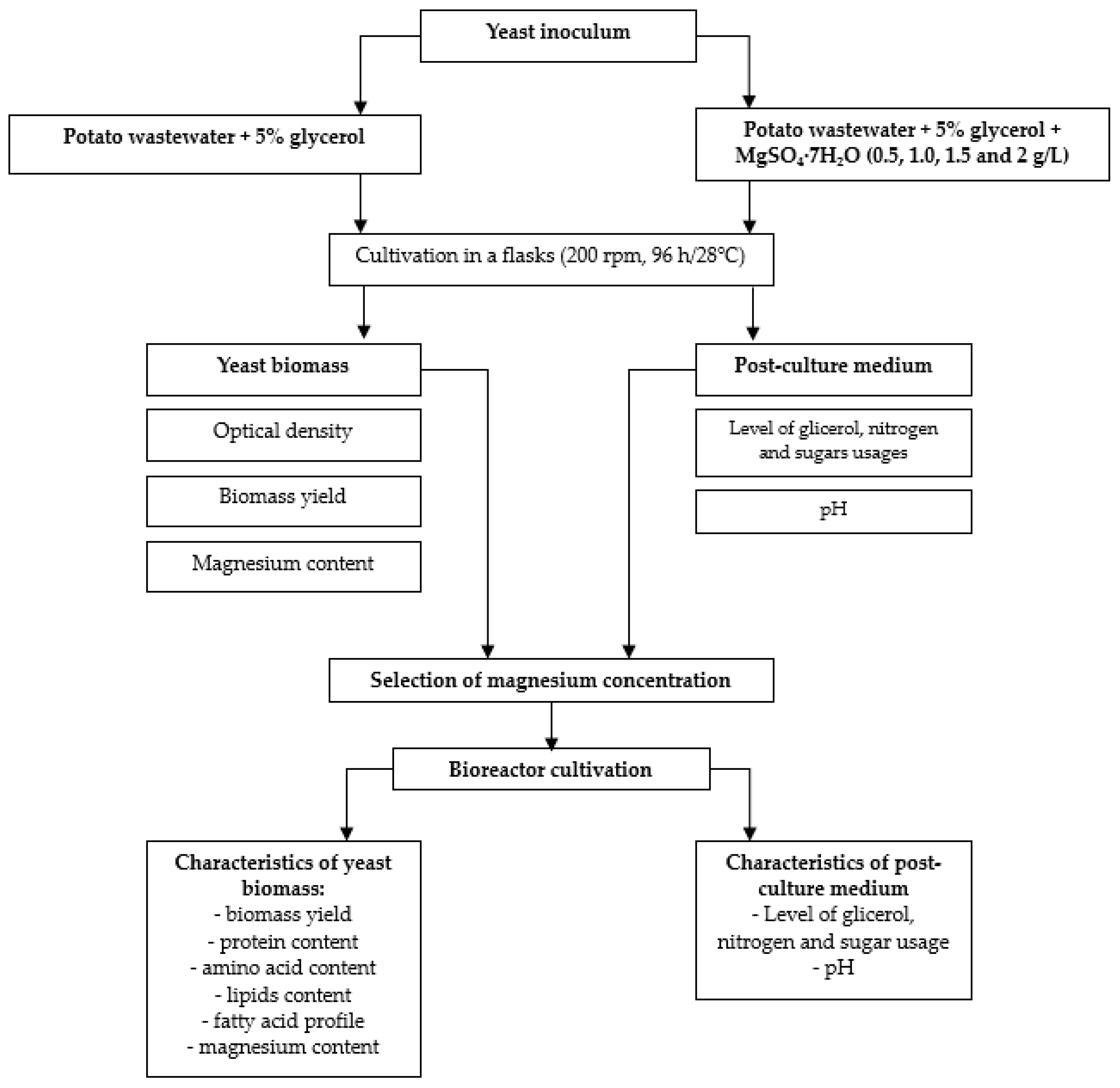
2. Materials and Methods
2.1. Biological Material
2.2. Glycerol from Biodiesel Production
2.3. Deproteinized Potato Wastewater
2.4. Culture Media
2.5. Inoculum Preparation
2.6. Culture Conditions
2.7. Biomass Yield and Optical Density
2.8. Analysis of the Composition of the Culture Media
2.9. Determination of the Magnesium Content
2.10. The Protein Content in the Yeast Biomass
2.11. The Amino Acid Content in the Yeast Protein
2.12. The Lipids Content and Their Profile
2.13. Statistical Analysis of the Results
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Kettler, M. Magnesium basics. Clin. Kidney J. 2012, 5, 3–14. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.; O’Keefe, J.; Wilson, W. Subclinical magnesium deficiency: A principle driver of cardiovascular disease and a publice health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Kurek, E. Binding and conversion of selenium in Candida utilis ATCC 9950 yeasts in bioreactor culture. Molecules 2017, 22, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gniewosz, M.; Błażejak, S.; Roman, J.; Duszkiewicz-Reinhard, W. A study on Saccharomyces cerevisiae and Candida utilis cell wall capacity for binding magnesium. Eur. Food Res. Technol. 2006, 224, 49–54. [Google Scholar]
- Błażejak, S.; Duszkiewicz-Reinhard, W.; Gniewosz, M.; Wiatrzyk, P. Impact of magnesium and mannose in the cultivation media on the magnesium biosorption, the biomass yield and on the cell wall structure of Candida utilis yeast. Eur. Food Res. Technol. 2008, 227, 695–700. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Tkacz, K. Cell wall structure of selected yeast species as a factor of magnesium binding ability. Eur. Food Res. Technol. 2012, 235, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Kot, A.M.; Piwowarek, K.; Błażejak, S. Accumulation of selenium in Candida utilis growing in media of increasing concentration of this element. Appl. Sci. 2020, 10, 1439. [Google Scholar] [CrossRef] [Green Version]
- Watanasrisin, W.; Iwatani, S.; Oura, T.; Tomita, Y.; Ikushima, S.; Chindamporn, A.; Niimi, M.; Niimi, K.; Lamping, E.; Cannon, R.D.; et al. Identification and characterization of Candida utilis multidrug efflux transporter CuCde1p. FEMS Yeast Res. 2016, 16, fow042. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Fell, J.W. The Yeasts a Taxonomic Study, 4th ed.; Elsevier: New York, NY, USA, 1998. [Google Scholar]
- Tomita, Y.; Ikeo, K.; Tamakawa, H.; Gojobori, T.; Ikushima, S. Genome and transcriptome analysis of the food-yeast Candida utilis. PLoS ONE 2012, 7, e37226. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.J.; Singleton, I.; Tobin, J.M. Metal cation uptake by yeast: A review. Appl. Microbiol. Biotechnol. 1995, 43, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [Green Version]
- Gniewosz, M.; Duszkiewicz-Reinhard, W.; Błażejak, S.; Sobiecka, J.; Zarzecka, M. Investigations into magnesium biosorption by waste brewery yeast Saccharomyces uvarum. Acta Sci. Pol. Technol. Aliment. 2007, 6, 57–67. [Google Scholar]
- Gardner, R.C. Genes for magnesium transport. Curr. Opin. Plant Biol. 2003, 6, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Knoop, V.; Groth-Malonek, M.; Gebert, M.; Eifler, K.; Weyand, K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 2005, 274, 205–216. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Pobiega, K.; Błażejak, S.; Kieliszek, M. The scale-up cultivation of Candida utilis in waste potato juice water with glycerol affects biomass and β(1,3)/(1,6)-glucan characteristic and yield. Appl. Microbiol. Biotechnol. 2018, 102, 9131–9145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass Valorization 2018, 9, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Bzducha-Wróbel, A.; Koczoń, P.; Błażejak, S.; Kozera, J.; Kieliszek, M. Valorization of deproteinated potato juice water into β-Glucan preparation of C. utilis origin: Comparative study of preparations obtained by two isolation methods. Waste Biomass Valorization 2019, 11, 3257–3271. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.J.; Luo, X.L.; Wan, C.X.; Li, Y.B. Characterization of crude glycerol from biodiesel plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Dos Santos, R.G. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological methods of management and utilization of potato industry waste—A review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Molenda, M.; Reczek, L. Biosynthesis of β(1,3)/(1,6)-glucans of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. Eur. Food Res. Technol. 2015, 240, 1023–1034. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 10 June 2023).
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Darbre, A. Analytical methods. In Practical Protein Chemistry—A Handbook; Darbre, A., Ed.; John Wiley: Chchester, UK, 1986. [Google Scholar]
- Cieślik, E.; Gębusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote Zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. [Google Scholar]
- Davidson, I. Hydrolysis of samples for amino acid analysis. In Protein Sequencing Protocols; Smith, B.J., Ed.; Humana: Totowa, NJ, USA, 2003. [Google Scholar]
- Carne, A.F. Chemical modifications of proteins as an aid to sequence analysis. In Protein Sequencing Protocols; Smith, B.J., Ed.; Humana: Totowa, NJ, USA, 2003. [Google Scholar]
- Kot, A.M.; Gientka, I.; Bzducha-Wróbel, A.; Błażejak, S.; Kurcz, A. Comparison of simple and rapid cell wall disruption methods for improving lipid extraction from yeast cells. J. Microbiol. Methods 2020, 176, 105999. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Jędrzejczak, R. Wiązanie selenu przez drożdże paszowe Candida utilis ATCC 9950. Bromatol. Chem. Toksykol. 2012, 3, 628–633. [Google Scholar]
- Błażejak, S.; Duszkiewicz-Reinhard, W.; Gniewosz, M.; Kamiński, T. Badanie zdolności wiązania magnezu przez drożdże paszowe Candida utilis ATCC 9950 w warunkach hodowli wgłębnej. Acta Sci. Pol. Technol. Aliment. 2003, 2, 109–123. [Google Scholar]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzyme Microb Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Błażejak, S.; Duszkiewicz-Reinhard, W.; Gniewosz, M.; Mazurkiewicz, B. Lokalizacja magnezu w komórkach drożdży paszowych Candida utilis ATCC 9950 wzbogaconych o ten pierwiastek. Acta Sci. Pol. Technol. Aliment. 2004, 3, 95–110. [Google Scholar]
- Goksungur, Y.; Uren, S.; Guvenc, U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gientka, I.; Kieliszek, M.; Jermacz, K.; Błażejak, S. Identification and characterization of oleaginous yeast isolated from kefir and its aility to accumulate intercellular fats in deproteinated potato wastewater with diffrent carbon sources. BioMed Res. Int. 2017, 2017, 6061042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous production of lipids and carotenoids by the red yeast Rhodotorula from waste glycerol fraction and potato wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef]
- Błażejak, S.; Duszkiewicz-Reinhard, W.; Gniewosz, M.; Chojnacka, M. Wpływ pH na zdolność wiązania magnezu przez drożdże paszowe Candida utilis ATCC 9950 podczas hodowli wgłębnej. Acta Sci. Pol. Technol. Aliment. 2005, 4, 47–57. [Google Scholar]
- Nowak, D.; Kasiak, T.; Lewicki, P.P.; Duszkiewicz-Reinhard, W. Pilot-plant cultivation of brewery’s yeast Saccharomyces cerevisiae enriched with magnesium. Pol. J. Food Nutr. Sci. 2005, 55, 177–182. [Google Scholar]
- Ratledge, C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 2002, 30, 1047–1050. [Google Scholar] [CrossRef] [Green Version]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast protein as an easily accessible food source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO); United Nations University (UNU). Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation; World Health Organization (WHO): Geneva, Switzerland, 2007. [Google Scholar]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A. Production of single cell protein (SCP) and essentials amino acids from Candida utilis FMJ12 by solid state fermentation using mango waste supplemented with nitrogen sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar]
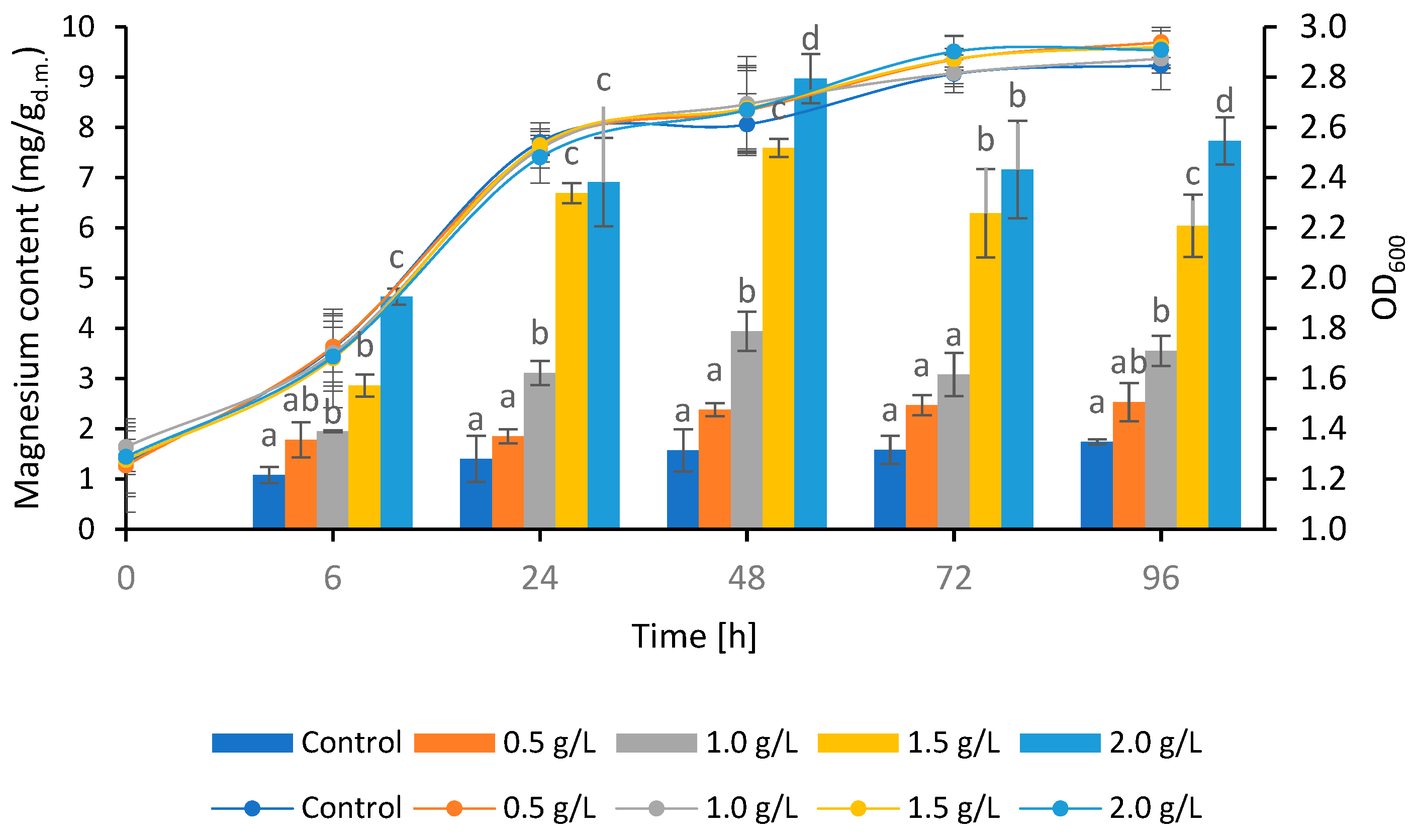
| Time [h] | Control | 0.5 g Mg2+/L | 1.0 g Mg2+/L | 1.5 g Mg2+/L | 2.0 g Mg2+/L |
|---|---|---|---|---|---|
| 0 | 0.99 ± 0.08 | 0.90 ± 0.04 | 0.88 ± 0.05 | 1.00 ± 0.03 | 0.88 ± 0.01 |
| 6 | 5.57 ± 0.45 | 5.04 ± 0.32 | 5.54 ± 0.59 | 5.86 ± 0.53 | 6.05 ± 0.06 |
| 24 | 13.51 ± 1.56 | 16.03 ± 1.52 | 16.25 ± 1.86 | 14.15 ± 3.59 | 15.02 ± 2.05 |
| 48 | 25.70 ± 3.49 | 24.91 ± 2.03 | 26.63 ± 0.87 | 25.65 ± 2.26 | 25.44 ± 1.87 |
| 72 | 33.83 ± 3.61 | 34.59 ± 2.03 | 35.71 ± 1.16 | 34.54 ± 3.40 | 33.70 ± 3.13 |
| 96 | 36.10 ± 4.78 | 35.83 ± 3.83 | 35.86 ± 2.07 | 36.04 ± 2.75 | 35.29 ± 2.75 |
| Type of Medium | Level of Glycerol Usage (%) * | Level of Nitrogen Usage (%) * | Level of Reducing Sugar Usage (%) * | pH of Medium | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h * | 6 h * | 24 h * | 48 h * | 72 h | 96 h | ||||
| Control | 98.4 ± 1.4 | 58.3 ± 8.3 | 72.6 ± 2.3 | 5.0 ± 0.1 | 5.68 ± 0.04 | 8.24 ± 0.35 | 7.96 ± 0.20 | 8.01 ± 0.12 a | 7.93 ± 0.10 a |
| 0.5 g Mg2+/L | 97.5 ± 0.1 | 61.0 ± 4.8 | 71.8 ± 2.2 | 5.0 ± 0.1 | 5.54 ± 0.03 | 8.22 ± 0.37 | 7.81 ± 0.24 | 7.89 ± 0.03 ab | 7.80 ± 0.15 a |
| 1.0 g Mg2+/L | 99.2 ± 0.4 | 64.8 ± 2.5 | 73.8 ± 1.8 | 5.0 ± 0.1 | 5.67 ± 0.03 | 8.16 ± 0.36 | 7.74 ± 0.10 | 7.59 ± 0.31 b | 7.62 ± 0.08 ab |
| 1.5 g Mg2+/L | 99.1 ± 0.5 | 64.1 ± 2.1 | 72.6 ± 1.6 | 5.0 ± 0.1 | 5.51 ± 0.05 | 7.86 ± 0.10 | 7.72 ± 0.20 | 7.72 ± 0.13 ab | 7,52 ± 0.08 ab |
| 2.0 g Mg2+/L | 99.2 ± 0.4 | 65.3 ± 6.8 | 72.9 ± 1.1 | 5.0 ± 0.1 | 5.95 ± 0.06 | 7.86 ± 0.14 | 7.58 ± 0.24 | 7.62 ± 0.21 b | 7.45 ± 0.09 b |
| Time [h] | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Biomass yield (gd.m./L) | 0.61 ± 0.09 a | 19.31 ± 0.73 b | 30.23 ± 0.85 c | 50.58 ± 1.27 d |
| Level of glycerol usage (%) | - | 15.0 ± 1.5 a | 60.2 ± 3.6 b | 93.2 ± 1.8 c |
| Level of nitrogen usage (%) | - | 11.53 ± 2.4 a | 39.3 ± 1.5 b | 55.8 ± 2.1 c |
| Level of reducing sugar usage (%) | - | 56.1 ± 2.7 a | 70.7 ± 1.6 b | 76.1 ± 0.8 c |
| Medium pH | 5.0 ± 0.1 a | 7.12 ± 0.21 b | 7.68 ± 0.23 c | 8.21 ± 0.09 d |
| Magnesium content in yeast biomass (mg/gd.m.) | - | 7.89 ± 0.24 c | 4.65 ± 0.38 b | 2.83 ± 0.64 a |
| Time [h] | 24 h | 48 h | 72 h |
|---|---|---|---|
| Lipid content (g/100 gd.m.) | 6.35 ± 0.68 a | 6.26 ± 0.42 a | 6.90 ± 0.95 a |
| Proportions of fatty acids (%): | |||
| C16:0 | 6.61 ± 0.85 a | 5.80 ± 0.53 a | 6.24 ± 0.71 a |
| C17:0 | 8.45 ± 0.96 ab | 7.16 ± 1.12 b | 8.94 ± 0.93 a |
| C18:0 | 8.03 ± 0.87 b | 10.92 ± 1.63 a | 7.89 ± 0.72 b |
| C18:1 | 59.38 ± 3.13 a | 55.77 ± 2.06 b | 56.46 ± 2.17 b |
| C18:2 | 8.56 ± 1.03 a | 9.05 ± 0.95 a | 9.13 ± 0.47 a |
| C20:0 | 0.86 ± 0.10 a | 0.40 ± 0.07 b | 0.90 ± 0.24 a |
| C24:0 | 1.25 ± 0.25 b | 2.56 ± 0.64 a | 1.53 ± 0.30 b |
| Protein content (g/100 gd.m.) | 43.73 ± 2.24 a | 33.75 ± 1.82 b | 26.94 ± 1.61 c |
| Proportions of amino acids (%): | |||
| Aspartic acid | 10.98 ± 0.42 a | 10.84 ± 0.14 a | 10.67 ± 0.16 a |
| Threonine | 5.21 ± 0.09 a | 5.37 ± 0.04 a | 5.44 ± 0.06 a |
| Serine | 5.53 ± 0.13 a | 5.47 ± 0.10 a | 5.59 ± 0.12 a |
| Glutamic acid | 13.72 ± 0.41 a | 14.46 ± 0.41 a | 14.70 ± 0.09 a |
| Proline | 4.07 ± 0.11 a | 3.57 ± 0.04 b | 3.63 ± 0.27 b |
| Glycine | 4.83 ± 0.12 a | 4.70 ± 0.05 a | 4.85 ± 0.02 a |
| Alanine | 6.43 ± 0.23 a | 6.28 ± 0.07 a | 6.64 ± 0.01 a |
| Valine | 5.82 ± 0.12 a | 4.98 ± 0.14 b | 4.81 ± 0.02 b |
| Isoleucine | 5.22 ± 0.08 a | 5.16 ± 0.10 a | 5.02 ± 0.08 a |
| Leucine | 7.35 ± 0.21 a | 7.05 ± 0.18 ab | 6.76 ± 0.07 b |
| Tyrosine | 2.83 ± 0.08 a | 2.94 ± 0.32 a | 2.67 ± 0.22 a |
| Phenylalanine | 3.91 ± 0.05 a | 3.68 ± 0.20 a | 3.36 ± 0.11 b |
| Histidine | 2.69 ± 0.14 a | 2.59 ± 0.13 a | 2.53 ± 0.02 a |
| Lysine | 6.23 ± 0.20 a | 5.76 ± 0.10 b | 6.16 ± 0.03 a |
| Arginine | 5.17 ± 0.31 a | 4.29 ± 0.07 b | 3.30 ± 0.01 c |
| Cysteine | 1.64 ± 0.03 b | 1.47 ± 0.38 b | 1.81 ± 0.04 a |
| Amino Acid (g/100 g Protein) | Yeast Protein after 24 h of Cultivation in the Bioreactor | FAO/WHO Standard (2007) |
|---|---|---|
| Histidine | 2.7 | 1.5 |
| Valine | 5.8 | 3.9 |
| Isoleucine | 5.2 | 3.0 |
| Leucine | 7.3 | 5.9 |
| Lysine | 6.2 | 4.5 |
| Methionine + cysteine | 1.6 | 2.2 |
| Phenylalanine + tyrosine | 6.7 | 3.8 |
| Threonine | 5.2 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, A.M.; Błażejak, S.; Nosek, K.; Synowiec, A.; Chlebowska-Śmigiel, A.; Pobiega, K. Magnesium Binding by Cyberlindnera jadinii Yeast in Media from Potato Wastewater and Glycerol. Microorganisms 2023, 11, 1923. https://doi.org/10.3390/microorganisms11081923
Kot AM, Błażejak S, Nosek K, Synowiec A, Chlebowska-Śmigiel A, Pobiega K. Magnesium Binding by Cyberlindnera jadinii Yeast in Media from Potato Wastewater and Glycerol. Microorganisms. 2023; 11(8):1923. https://doi.org/10.3390/microorganisms11081923
Chicago/Turabian StyleKot, Anna M., Stanisław Błażejak, Klaudia Nosek, Alicja Synowiec, Anna Chlebowska-Śmigiel, and Katarzyna Pobiega. 2023. "Magnesium Binding by Cyberlindnera jadinii Yeast in Media from Potato Wastewater and Glycerol" Microorganisms 11, no. 8: 1923. https://doi.org/10.3390/microorganisms11081923






