Comprehensive Response of Rhodosporidium kratochvilovae to Glucose Starvation: A Transcriptomics-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Determination of the Consumption of Glucose and Total Nitrogen
2.3. RNA Extraction and Sequencing
2.4. Sequence Read Mapping and Differential Expression Analysis
2.5. Functional Annotation of Gene Transcripts
2.6. Cloning the Full-Length Sequence of the Fatty acyl-CoA oxidase Gene
2.7. Plasmid Construction and Yeast Transformation
2.8. Lipid Extraction and Separation
2.9. Fatty Acid Analysis
2.10. Acetyl-CoA Analysis
2.11. Total Carotenoids and Components Analysis
2.12. Determination of Total Intracellular ROS
2.13. Quantitative Real-Time PCR (qPCR) Validation
3. Results
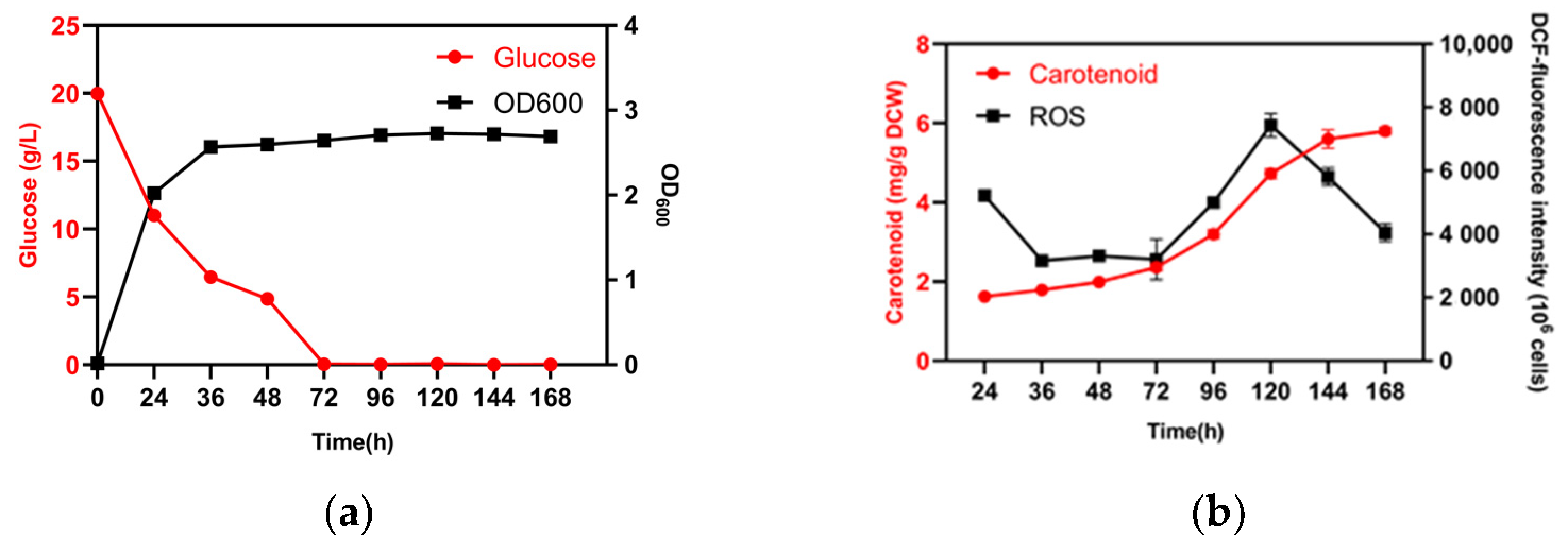
3.1. Effects of Glucose Consumption on Cell Growth, Total ROS Levels, and Carotenoid Biosynthesis
3.2. Transcriptome Sequencing and Assembly
3.3. DEGs under Glucose Starvation Conditions
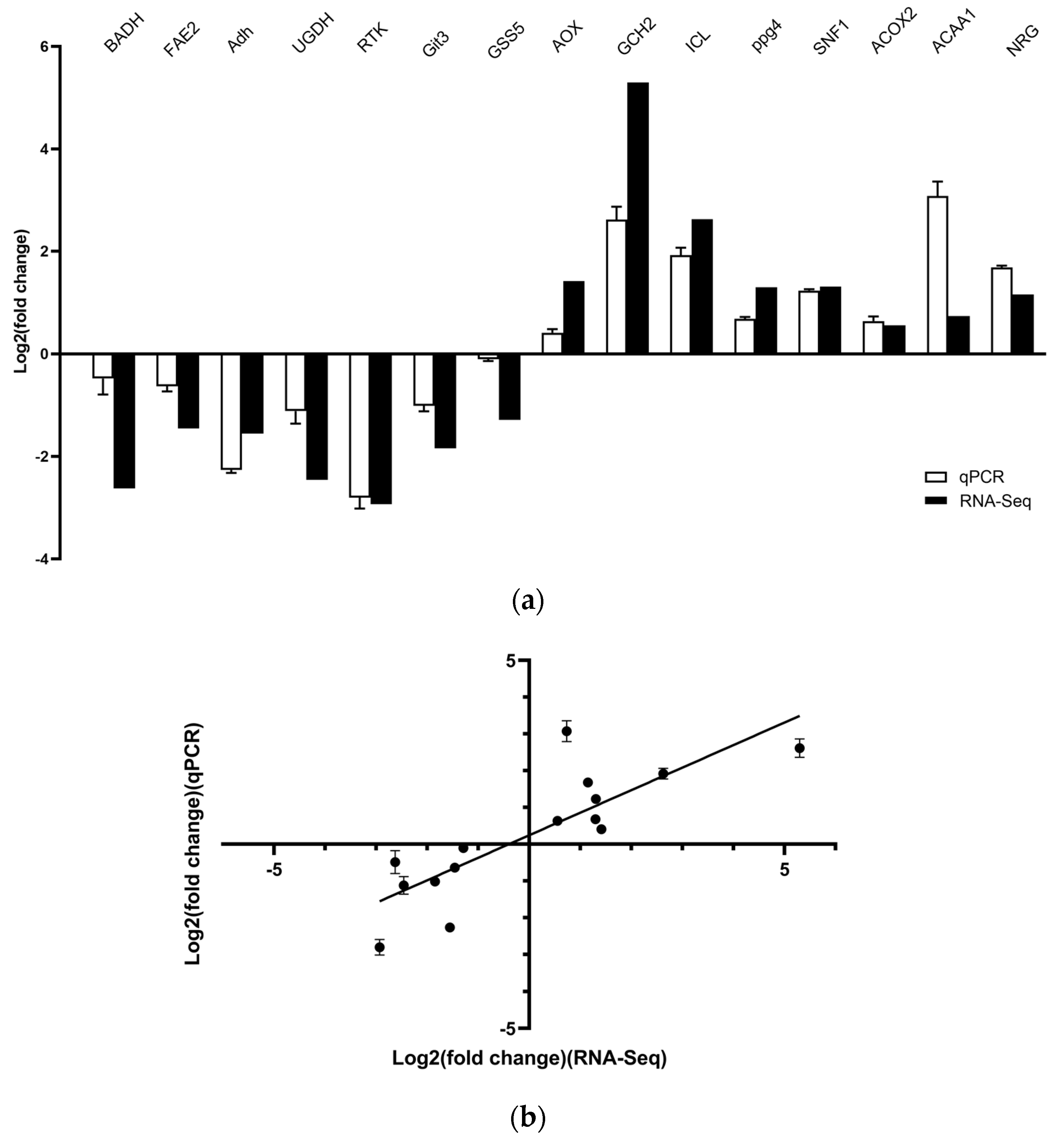
3.4. Verification of RNA-Sequencing Data
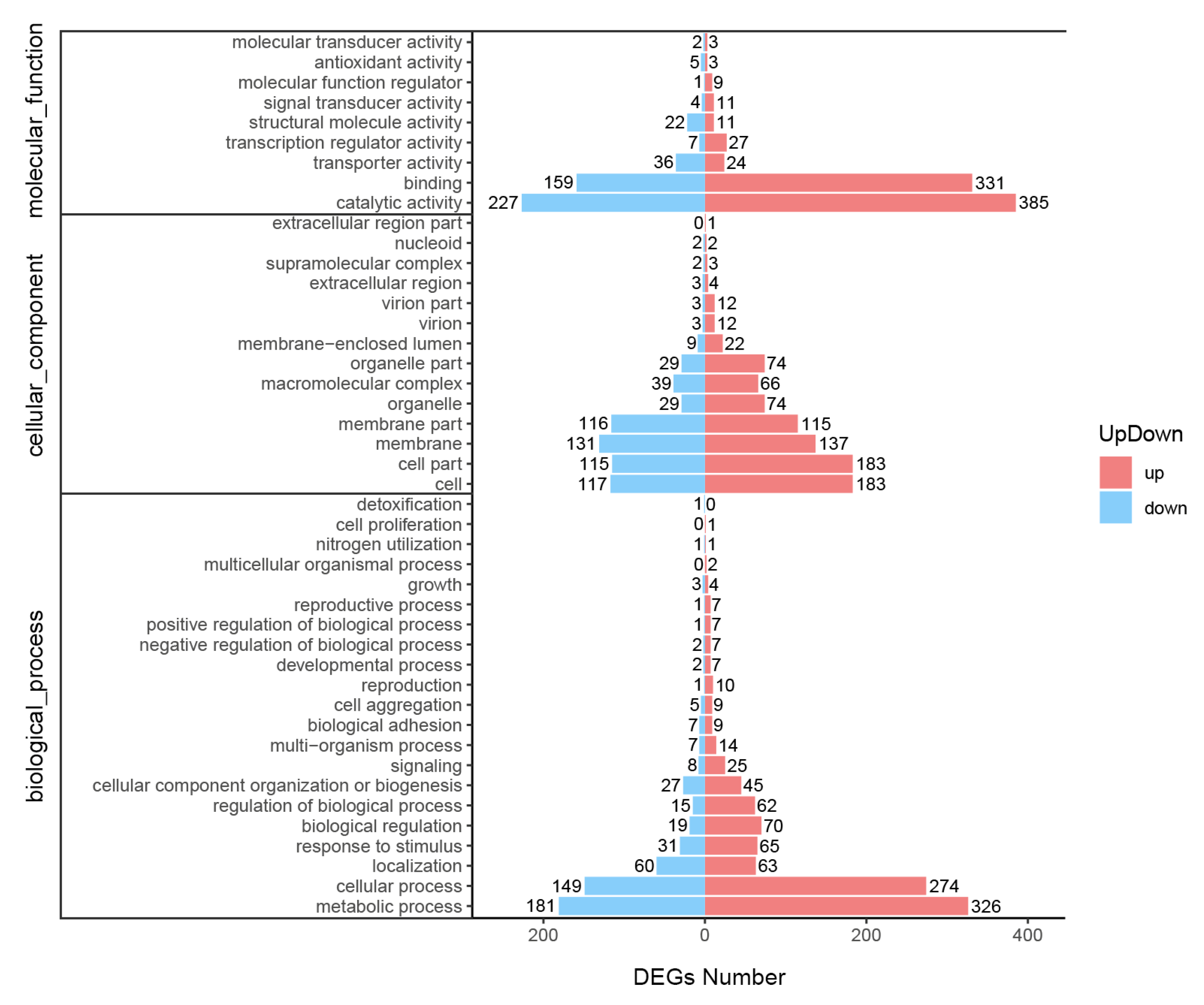
3.5. Functional Enrichment of the DEGs
3.6. Effect of RkACOX2 Overexpression on FFA Levels under Glucose Starvation
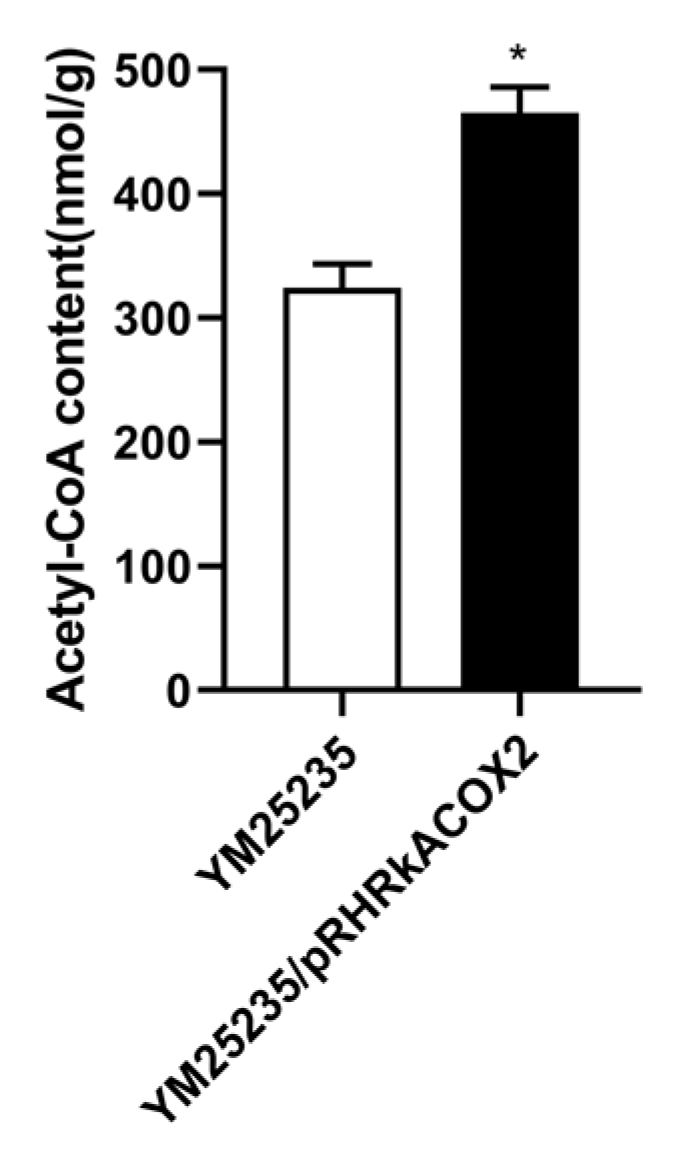
3.7. Effect of RkACOX2 Overexpression on Intracellular acetyl-CoA Levels under Glucose Starvation
3.8. Effect of RkACOX2 Overexpression on Carotenoid Biosynthesis under Glucose Starvation
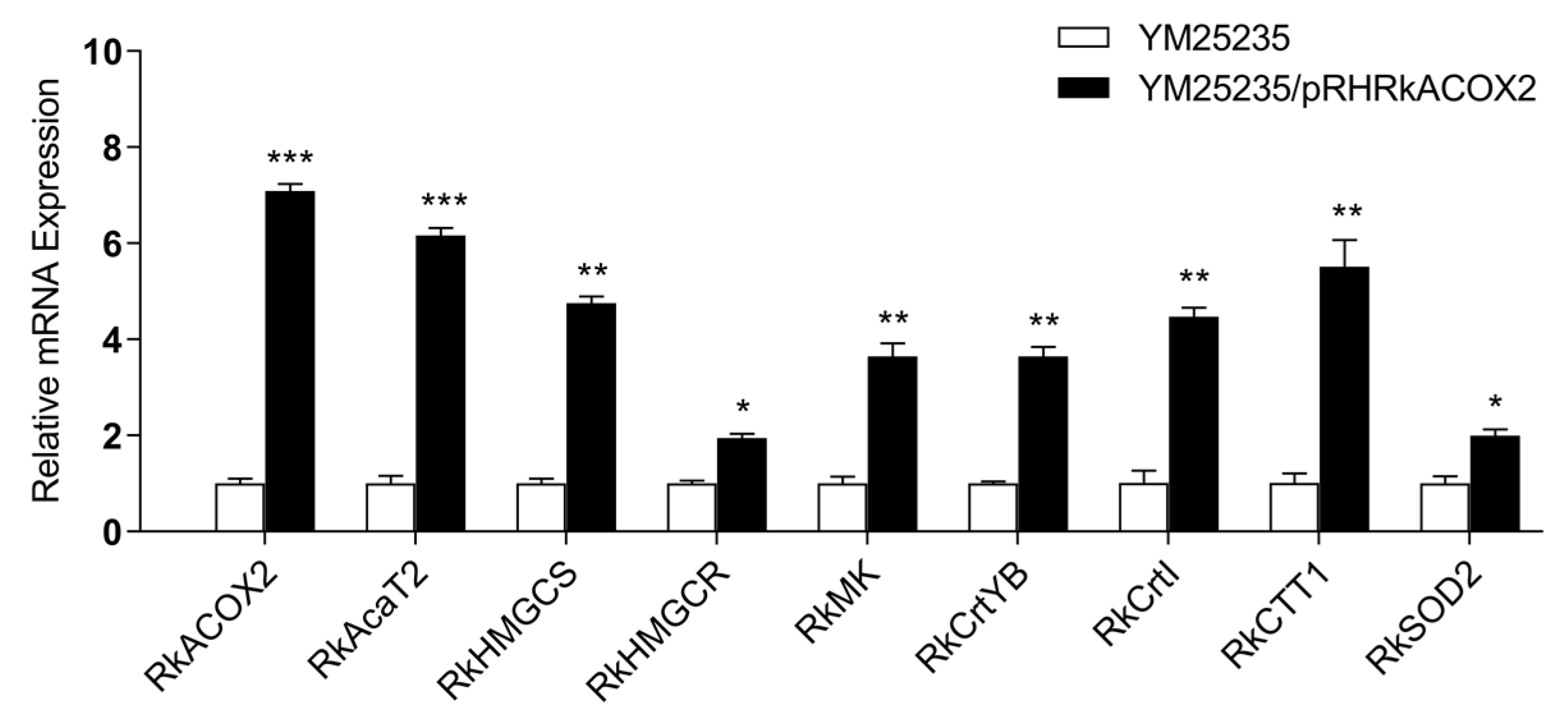
3.9. Effect of RkACOX2 Overexpression on the Transcription of Genes Related to Fatty Acid Oxidation, Carotenoid Biosynthesis, and Antioxidants under Glucose Starvation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simpson-Lavy, K.; Kupiec, M. Carbon catabolite repression in yeast is not limited to glucose. Sci. Rep. 2019, 9, 6491. [Google Scholar] [CrossRef]
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299. [Google Scholar] [CrossRef]
- Stasyk, O.G.; Stasyk, O.V. Glucose sensing and regulation in yeasts. In Non-Conventional Yeasts: From Basic Research to Application; Springer: Cham, Switzerland, 2019; pp. 477–519. [Google Scholar]
- Silva, C.M.; Borba, T.M.; Burkert, C.A.V.; Burkert, J.F.M. Carotenoid production by Phaffia rhodozyma using raw glycerol as an additional carbon source. Int. J. Food Eng. 2012, 8, 17. [Google Scholar] [CrossRef]
- Xiberras, J.; Klein, M.; Nevoigt, E. Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses—Knowledge gaps regarding the central carbon catabolism of this ‘non-fermentable’ carbon source. Biotechnol. Adv. 2019, 37, 107378. [Google Scholar] [CrossRef] [PubMed]
- Kurylenko, O.; Ruchala, J.; Kruk, B.; Vasylyshyn, R.; Szczepaniak, J.; Dmytruk, K.; Sibirny, A. The role of Mig1, Mig2, Tup1 and Hap4 transcription factors in regulation of xylose and glucose fermentation in the thermotolerant yeast Ogataea polymorpha. FEMS Yeast Res. 2021, 21, foab029. [Google Scholar] [CrossRef]
- Thomsson, E.; Larsson, C.; Albers, E.; Nilsson, A.; Franzén, C.J.; Gustafsson, L. Carbon starvation can induce energy deprivation and loss of fermentative capacity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2003, 69, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, N.; Jacques, P.-E.; Klimova, N.; Guo, X.; Ricciardi, A.; Robert, F.; Turcotte, B. The switch from fermentation to respiration in Saccharomyces cerevisiae is regulated by the Ert1 transcriptional activator/repressor. Genetics 2014, 198, 547–560. [Google Scholar] [CrossRef]
- Turcotte, B.; Liang, X.B.; Robert, F.; Soontorngun, N. Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Res. 2010, 10, 2–13. [Google Scholar] [CrossRef]
- Burphan, T.; Tatip, S.; Limcharoensuk, T.; Kangboonruang, K.; Boonchird, C.; Auesukaree, C. Enhancement of ethanol production in very high gravity fermentation by reducing fermentation-induced oxidative stress in Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 13069. [Google Scholar] [CrossRef]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1217–1235. [Google Scholar] [CrossRef]
- Maslanka, R.; Zadrag-Tecza, R.; Kwolek-Mirek, M. Linkage between carbon metabolism, redox status and cellular physiology in the yeast Saccharomyces cerevisiae devoid of SOD1 or SOD2 gene. Genes 2020, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shen, H.M. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019, 25, 101154. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Tsuzuno, T.; Nishida, S.; Pattanakittivorakul, S.; Murata, M.; Miyakawa, I.; Lertwattanasakul, N.; Limtong, S.; Yamada, M. Distinct metabolic flow in response to temperature in thermotolerant Kluyveromyces marxianus. Appl. Environ. Microbiol. 2022, 88, e02006-21. [Google Scholar] [CrossRef]
- Garcia-Rios, E.; Ramos-Alonso, L.; Guillamon, J.M. Correlation between low temperature adaptation and oxidative stress in Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Cai, W.; Zeng, J.; Liu, N.; Wan, Y.; Fu, G. Research progress of anti-environmental factor stress mechanism and anti-stress tolerance way of Saccharomyces cerevisiae during the brewing process. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Feng, J.; Yao, S.; Dong, Y.; Hu, J.; Whiteway, M.; Feng, J. Nucleotide excision repair protein Rad23 regulates cell virulence independent of Rad4 in Candida albicans. mSphere 2020, 5, e00062-20. [Google Scholar] [CrossRef] [PubMed]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Gessler, N.N.; Aver’yanov, A.A.; Belozerskaya, T.A. Reactive oxygen species in regulation of fungal development. Biochemistry 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Carnecka, M.; Halienova, A.; Breierová, E.; Kočí, R. Production of carotenoid-/ergosterol-supplemented biomass by red yeast Rhodotorula glutinis grown under external stress. Food Technol. Biotechnol. 2010, 48, 56–61. [Google Scholar]
- Zhang, J.; Li, Q.; Lu, Y.; Guan, X.; Liu, J.; Xu, N.; Cai, C.; Li, X.; Nan, B.; Liu, J.; et al. Astaxanthin overproduction of Phaffia rhodozyma PR106 under titanium dioxide stress by transcriptomics and metabolic regulation analysis. Bioresour. Technol. 2021, 342, 125957. [Google Scholar] [CrossRef]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar] [CrossRef]
- Flegler, A.; Lipski, A. The C50 carotenoid bacterioruberin regulates membrane fluidity in pink-pigmented Arthrobacter species. Arch. Microbiol. 2021, 204, 70. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Jawed, A.; Paul, D.; Bandyopadhyay, K.K.; Kumari, A.; Haque, S. Concomitant production of lipids and carotenoids in Rhodosporidium toruloides under osmotic stress using response surface methodology. Front. Microbiol. 2016, 7, 1686. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, B.; Gerken, H.G.; Lu, Y.; Ling, X. Proteomic analysis of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous in response to low carbon levels. Bioprocess Biosyst. Eng. 2017, 40, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.; Mai, T.; Vu, H.; Van, D.; Dao, H.; Tran, P.; Nguyen, N.; Nguyen, P.; Nguyen, N.C. Effect of osmotic stress and nutrient starvation on the growth, carotenoid and lipid accumulation in Dunaliella salina A9. Res. Plant Sci. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Yan, P.; Guo, J.-S.; Zhang, P.; Xiao, Y.; Li, Z.; Zhang, S.-Q.; Zhang, Y.-X.; He, S.-X. The role of morphological changes in algae adaptation to nutrient stress at the single-cell level. Sci. Total Environ. 2021, 754, 142076. [Google Scholar] [CrossRef]
- Sun, H.; Mao, X.; Wu, T.; Ren, Y.; Chen, F.; Liu, B. Novel insight of carotenoid and lipid biosynthesis and their roles in storage carbon metabolism in Chlamydomonas reinhardtii. Bioresour. Technol. 2018, 263, 450–457. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Y.; Chi, S.; Yan, J.; Guan, G.; Hui, B.; Li, Y. Reduction of fatty acid flux results in enhancement of astaxanthin synthesis in a mutant strain of Phaffia rhodozyma. J. Ind. Microbiol. Biotechnol. 2010, 37, 595–602. [Google Scholar] [CrossRef]
- Naz, T.; Nazir, Y.; Nosheen, S.; Ullah, S.; Halim, H.; Fazili, A.B.A.; Li, S.; Mustafa, K.; Mohamed, H.; Yang, W.; et al. Redirecting metabolic flux towards the mevalonate pathway for enhanced beta-carotene production in M. circinelloides CBS 277.49. BioMed Res. Int. 2020, 2020, 8890269. [Google Scholar] [CrossRef]
- Nielsen, J. Synthetic biology for engineering acetyl coenzyme A metabolism in yeast. mBio 2014, 5, e02153. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Matsuda, F.; Okamoto, K.; Ishii, J.; Kondo, A.; Shimizu, H. Repression of mitochondrial metabolism for cytosolic pyruvate-derived chemical production in Saccharomyces cerevisiae. Microb. Cell Fact. 2019, 18, 177. [Google Scholar] [CrossRef]
- Chen, Y.; Siewers, V.; Nielsen, J. Profiling of cytosolic and peroxisomal acetyl-CoA metabolism in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e42475. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Mursula, A.M.; Rottensteiner, H.; Wierenga, R.K.; Kastaniotis, A.J.; Gurvitz, A. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2003, 27, 35–64. [Google Scholar] [CrossRef]
- Harris, R.A.; Joshi, M.; Jeoung, N.H.; Obayashi, M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J. Nutr. 2005, 135, 1527S–1530S. [Google Scholar] [CrossRef]
- Toyokawa, Y.; Koonthongkaew, J.; Takagi, H. An overview of branched-chain amino acid aminotransferases: Functional differences between mitochondrial and cytosolic isozymes in yeast and human. Appl. Microbiol. Biotechnol. 2021, 105, 8059–8072. [Google Scholar] [CrossRef] [PubMed]
- Koonthongkaew, J.; Toyokawa, Y.; Ohashi, M.; Large, C.R.L.; Dunham, M.J.; Takagi, H. Effect of the Ala234Asp replacement in mitochondrial branched-chain amino acid aminotransferase on the production of BCAAs and fusel alcohols in yeast. Appl. Microbiol. Biotechnol. 2020, 104, 7915–7925. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Uprety, B.K.; Dalli, S.S.; Rakshit, S.K. Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Convers. Manag. 2017, 135, 117–128. [Google Scholar] [CrossRef]
- Shakeri, S.; Khoshbasirat, F.; Maleki, M. Rhodosporidium sp. DR37: A novel strain for production of squalene in optimized cultivation conditions. Biotechnol. Biofuels 2021, 14, 95. [Google Scholar] [CrossRef]
- Jagtap, S.S.; Rao, C.V. Production of D-arabitol from D-xylose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Appl. Microbiol. Biotechnol. 2018, 102, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.S.; Bedekar, A.A.; Liu, J.J.; Jin, Y.S.; Rao, C.V. Production of galactitol from galactose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Biotechnol. Biofuels 2019, 12, 250. [Google Scholar] [CrossRef]
- Cui, J.; He, S.; Ji, X.; Lin, L.; Wei, Y.; Zhang, Q. Identification and characterization of a novel bifunctional Δ(12)/Δ(15)-fatty acid desaturase gene from Rhodosporidium kratochvilovae. Biotechnol. Lett. 2016, 38, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Nian, H.; Ji, X.; Lin, L.; Wei, Y.; Zhang, Q. Inhibition of polyunsaturated fatty acids synthesis decreases growth rate and membrane fluidity of Rhodosporidium kratochvilovae at low temperature. Lipids 2017, 52, 729–735. [Google Scholar] [CrossRef]
- He, J.; Cui, Z.; Ji, X.; Luo, Y.; Wei, Y.; Zhang, Q. Novel histidine kinase gene HisK2301 from Rhodosporidium kratochvilovae contributes to cold adaption by promoting biosynthesis of polyunsaturated fatty acids and glycerol. J. Agric. Chem. 2019, 67, 653–660. [Google Scholar] [CrossRef]
- Guo, R.; He, M.; Zhang, X.; Ji, X.; Wei, Y.; Zhang, Q.-L.; Zhang, Q. Genome-wide transcriptional changes of Rhodosporidium kratochvilovae at low temperature. Front.Microbiol. 2021, 12, 727105. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, T.; Guo, C.; Chen, G.; Fan, J.; Zhang, Q. Carotenoid biosynthesis is associated with low-temperature adaptation in Rhodosporidium kratochvilovae. BMC Microbiol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Eiteman, M.A. Nutrient-limited operational strategies for the microbial production of biochemicals. Microorganisms 2022, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, A.; Daran-Lapujade, P.; Fullaondo, A.; Olsthoorn, M.M.; Pronk, J.T.; Slijper, M.; Heck, A.J. Proteome analysis of yeast response to various nutrient limitations. Mol. Syst. Biol. 2006, 2, 2006.0026. [Google Scholar] [CrossRef] [PubMed]
- Boer, V.M.; de Winde, J.H.; Pronk, J.T.; Piper, M.D.W. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 2003, 278, 3265–3274. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, N.; Hayes, A.; Panoutsopoulou, K.; Oliver, S.G. Global analysis of nutrient control of gene expression in Saccharomyces cerevisiae during growth and starvation. Proc. Natl. Acad. Sci. USA 2004, 101, 3148–3153. [Google Scholar] [CrossRef] [PubMed]
- Daran-Lapujade, P.; Jansen, M.L.A.; Daran, J.-M.; van Gulik, W.; de Winde, J.H.; Pronk, J.T. Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae: A chemostat culture study. J. Biol. Chem. 2004, 279, 9125–9138. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Nydahl, F. On the peroxodisulphate oxidation of total nitrogen in waters to nitrate. Water Res. 1978, 12, 1123–1130. [Google Scholar] [CrossRef]
- Iyer, R.; Stepanov, V.G.; Iken, B. Isolation and molecular characterization of a novel pseudomonas putida strain capable of degrading organophosphate and aromatic compounds. Adv. Biol. Chem. 2013, 3, 564–578. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Liu, Y.; Koh, C.M.; Sun, L.; Hlaing, M.M.; Du, M.; Peng, N.; Ji, L. Characterization of glyceraldehyde-3-phosphate dehydrogenase gene RtGPD1 and development of genetic transformation method by dominant selection in oleaginous yeast Rhodosporidium toruloides. Appl. Microbiol. Biotechnol. 2013, 97, 719–729. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, Z.-D.; Liu, Y.; Koh, C.M.J.; Zhang, L.-H. A simplified and efficient method for transformation and gene tagging of Ustilago maydis using frozen cells. Fungal Genet. Biol. 2010, 47, 279–287. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.R.D.; Melo, T.; Maciel, E.A.; Pereira, C.; Morais, C.M.; Santinha, D.R.; Tavares, J.F.; Oliveira, H.; Jurado, A.S.; Costa, V.; et al. Errors in protein synthesis increase the level of saturated fatty acids and affect the overall lipid profiles of yeast. PLoS ONE 2018, 13, e0202402. [Google Scholar] [CrossRef]
- Ranganathan, P.R.; Nawada, N.; Narayanan, A.K.; Rao, D.K.V. Triglyceride deficiency and diacylglycerol kinase1 activity lead to the upregulation of mevalonate pathway in yeast: A study for the development of potential yeast platform for improved production of triterpenoid. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158661. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Hu, B.; Ji, X.; Wei, Y.; Lin, L.; Zhang, Q. Correlation of polyunsaturated fatty acids with the cold adaptation of Rhodotorula glutinis. Yeast 2015, 32, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.; Tucker, D.; Watson, K. Gas chromatography–mass spectrometry analysis of fatty acid profiles of antarctic and non-antarctic yeasts. Antonie van Leeuwenhoek 2014, 106, 381–389. [Google Scholar] [CrossRef]
- Han, M.; He, Q.; Zhang, W.G. Carotenoids production in different culture conditions by Sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 2012, 42, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Liaaen-Jensen, S.; Jensen, A. Quantitative determination of carotenoids in photosynthetic tissues. Methods Enzymol. 1971, 23, 586–602. [Google Scholar]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cowan, K.J.; Storey, K.B. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003, 206, 1107–1115. [Google Scholar] [CrossRef]
- Higa, A.; Chevet, E. Redox signaling loops in the unfolded protein response. Cell Signal 2012, 24, 1548–1555. [Google Scholar] [CrossRef]
- Natvig, D.O.; Sylvester, K.; Dvorachek, W.H.; Baldwin, J.L. Superoxide dismutases and catalases. In Biochemistry and Molecular Biology, Brambl, R., Marzluf, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 3, pp. 191–209. [Google Scholar]
- Zhang, Q.; Zeng, W.; Xu, S.; Zhou, J. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresour. Technol. 2021, 342, 125978. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Meng, D.; Zhao, Y.; Zhang, J. Attenuating the triacylglycerol catabolism enhanced lipid production of Rhodotorula strain U13N3. Appl. Microbiol. Biotechnol. 2023, 107, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shang, F.; Duan, C.-q.; Yan, G.-l. Reduction of fatty acid flux at low temperature led to enhancement of β-carotene biosynthesis in recombinant Saccharomyces cerevisiae. Korean J. Chem. Eng. 2015, 32, 1354–1360. [Google Scholar] [CrossRef]
- Lima Pde, S.; Casaletti, L.; Bailão, A.M.; de Vasconcelos, A.T.; Fernandes Gda, R.; Soares, C.M. Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Neglected Trop. Dis. 2014, 8, e2855. [Google Scholar] [CrossRef]
- Chew, S.Y.; Brown, A.J.P.; Lau, B.Y.C.; Cheah, Y.K.; Ho, K.L.; Sandai, D.; Yahaya, H.; Than, L.T.L. Transcriptomic and proteomic profiling revealed reprogramming of carbon metabolism in acetate-grown human pathogen Candida glabrata. J. Biomed. Sci. 2021, 28, 1. [Google Scholar] [CrossRef]
- Luo, F.; Zhou, H.; Zhou, X.; Xie, X.; Li, Y.; Hu, F.; Huang, B. The intermediates in branched-chain amino acid biosynthesis are indispensable for conidial germination of the insect-pathogenic fungus Metarhizium robertsii. Appl. Environ. Microbiol. 2020, 86, e01682-20. [Google Scholar] [CrossRef]
- Bianchi, F.; Van’t Klooster, J.S.; Ruiz, S.J.; Poolman, B. Regulation of amino acid transport in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2019, 83, e00024-19. [Google Scholar] [CrossRef] [PubMed]
- Starai, V.J.; Escalante-Semerena, J.C. Acetyl-coenzyme A synthetase (AMP forming). Cell. Mol. Life Sci. CMLS 2004, 61, 2020–2030. [Google Scholar] [CrossRef]

| Sample | Raw Reads | Clean Reads | Clean Bases | Total Reads | Total Mapped |
|---|---|---|---|---|---|
| 36_1 | 45,807,814 | 45,289,004 | 6.75 G | 45,289,004 | 40,325,735 (89.04%) |
| 36_2 | 49,754,936 | 49,222,456 | 7.31 G | 49,222,456 | 43,862,890 (89.11%) |
| 36_3 | 48,145,136 | 47,656,900 | 7.09 G | 47,656,900 | 42,238,415 (88.63%) |
| 96_1 | 47,234,546 | 45,888,648 | 6.58 G | 45,888,648 | 40,276,865 (87.77%) |
| 96_2 | 43,789,462 | 43,176,246 | 6.42 G | 43,176,246 | 37,087,034 (85.90%) |
| 96_3 | 48,064,754 | 47,351,146 | 7.05 G | 47,351,146 | 40,812,496 (86.19%) |
| Gene id | log2 Ratio (Treatment/Control) | q-Value | Gene Description | Gene Symbol |
|---|---|---|---|---|
| The top 10 most up-regulated DEGs | ||||
| EVM0002799 | 12.16840050 | 2.64 × 10−212 | OsmC family protein | OsmC |
| EVM0007334 | 12.06697468 | 1.04 × 10−201 | Transcription factor Hsf1 | Hsf1 |
| EVM0000758 | 12.01547883 | 1.57× 10−196 | hypothetical protein | \ |
| EVM0000848 | 11.74144109 | 4.95 × 10−171 | regulator of gluconeogenesis | RGG |
| EVM0000135 | 11.72418730 | 1.55 × 10−169 | leucine-rich repeat domain-containing protein | LRR |
| EVM0001989 | 11.60200485 | 2.60 × 10−159 | hypothetical protein | \ |
| EVM0006562 | 10.57452947 | 7.47 × 10−94 | Serine/threonine-protein phosphatase 2A activator 2 | PP2A |
| EVM0008373 | 10.56314106 | 2.98 × 10−89 | alpha-ketoglutarate dependent 2,4-dichlorophenoxyacetate dioxygenase | TfdA |
| EVM0008165 | 10.07478880 | 2.74 × 10−72 | cleavage and polyadenylation specific factor 5 | Cpsf5 |
| EVM0000497 | 9.601556094 | 2.38 × 10−56 | cell division control protein | CDC |
| The top 10 most down-regulated DEGs | ||||
| EVM0004206 | −11.90660765 | 3.86 × 10−308 | ABC drug exporter AtrF | AtrF |
| EVM0002079 | −11.27993663 | 2.37 × 10−223 | zip-like iron-zinc transporter | ZIP |
| EVM0005177 | −11.05445575 | 1.06 × 10−198 | putative Phospholipid binding protein | Cts1 |
| EVM0000550 | −10.80320920 | 2.71 × 10−174 | acyl-CoA thioester hydrolase | ACOT |
| EVM0002631 | −10.65221864 | 4.94 × 10−161 | ribosomal protein L9 family protein | RPL9 |
| EVM0004367 | −10.64333877 | 2.77 × 10−160 | glutamate-rich WD repeat-containing protein | GRWD |
| EVM0001224 | −10.60837800 | 5.05 × 10−144 | 3-carboxymuconate cyclase | CMC |
| EVM0002253 | −10.44011035 | 1.59 × 10−143 | hypothetical protein | \ |
| EVM0006484 | −10.38925953 | 1.06 × 10−139 | zinc finger, RING-type protein | RZF |
| EVM0003900 | –10.38419536 | 2.24 × 10−111 | macrofage activating glycoprotein | GcMAF |
| Fatty Acid (mg/g DCW) | FFAs | TAGs | ||
|---|---|---|---|---|
| YM25235 | YM25235/pRHRkACOX2 | YM25235 | YM25235/pRHRkACOX2 | |
| C16:0 | 3.47 ± 0.08 | 3.15 ± 0.03 * | 2.43 ± 0.02 | 3.05 ± 0.01 && |
| C16:1 | 0.69 ± 0.06 | 0.98 ± 0.03 * | 0.42 ± 0.02 | 0.64 ± 0.08 |
| C18:0 | 0.57 ± 0.01 | 0.53 ± 0.04 | 0.65 ± 0.07 | 0.73 ± 0.02 |
| C18:1OA | 13.22 ± 0.08 | 13.47 ± 0.33 | 6.47 ± 0.30 | 8.48 ± 0.35 & |
| C18:2LA | 5.40 ± 0.08 | 3.37 ± 0.19 ** | 2.13 ± 0.16 | 1.88 ± 0.26 |
| C18:3ALA | 0.71 ± 0.02 | 0.48 ± 0.03 * | 0.22 ± 0.06 | 0.25 ± 0.03 |
| Strain | Total Carotenoid (mg/g DCW) | Carotenoid Composition (mg/g DCW) | |||
|---|---|---|---|---|---|
| Torularhodin | Torulene | γ-carotene | β-carotene | ||
| YM25235 | 5.68 ± 0.23 | 1.65 ± 0.29 | 0.46 ± 0.09 | 0.40 ± 0.29 | 3.17 ± 0.13 |
| YM25235/pRHRkACOX2 | 7.59 ± 0.35 * | 1.52 ± 0.11 | 0.92 ± 0.11 * | 1.35 ± 0.34 | 3.80 ± 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Guo, R.; Chen, G.; Xiong, C.; Yang, X.; Wei, Y.; Chen, Y.; Qiu, J.; Zhang, Q. Comprehensive Response of Rhodosporidium kratochvilovae to Glucose Starvation: A Transcriptomics-Based Analysis. Microorganisms 2023, 11, 2168. https://doi.org/10.3390/microorganisms11092168
He M, Guo R, Chen G, Xiong C, Yang X, Wei Y, Chen Y, Qiu J, Zhang Q. Comprehensive Response of Rhodosporidium kratochvilovae to Glucose Starvation: A Transcriptomics-Based Analysis. Microorganisms. 2023; 11(9):2168. https://doi.org/10.3390/microorganisms11092168
Chicago/Turabian StyleHe, Meixia, Rui Guo, Gongshui Chen, Chao Xiong, Xiaoxia Yang, Yunlin Wei, Yuan Chen, Jingwen Qiu, and Qi Zhang. 2023. "Comprehensive Response of Rhodosporidium kratochvilovae to Glucose Starvation: A Transcriptomics-Based Analysis" Microorganisms 11, no. 9: 2168. https://doi.org/10.3390/microorganisms11092168





