Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades?
Abstract
:1. Introduction and Historical Perspective
2. Epidemiology
3. Pathophysiology
4. Screening for Sepsis
5. Management
5.1. Initial Resuscitation: The Era of Early Goal-Directed Therapy and the Years After
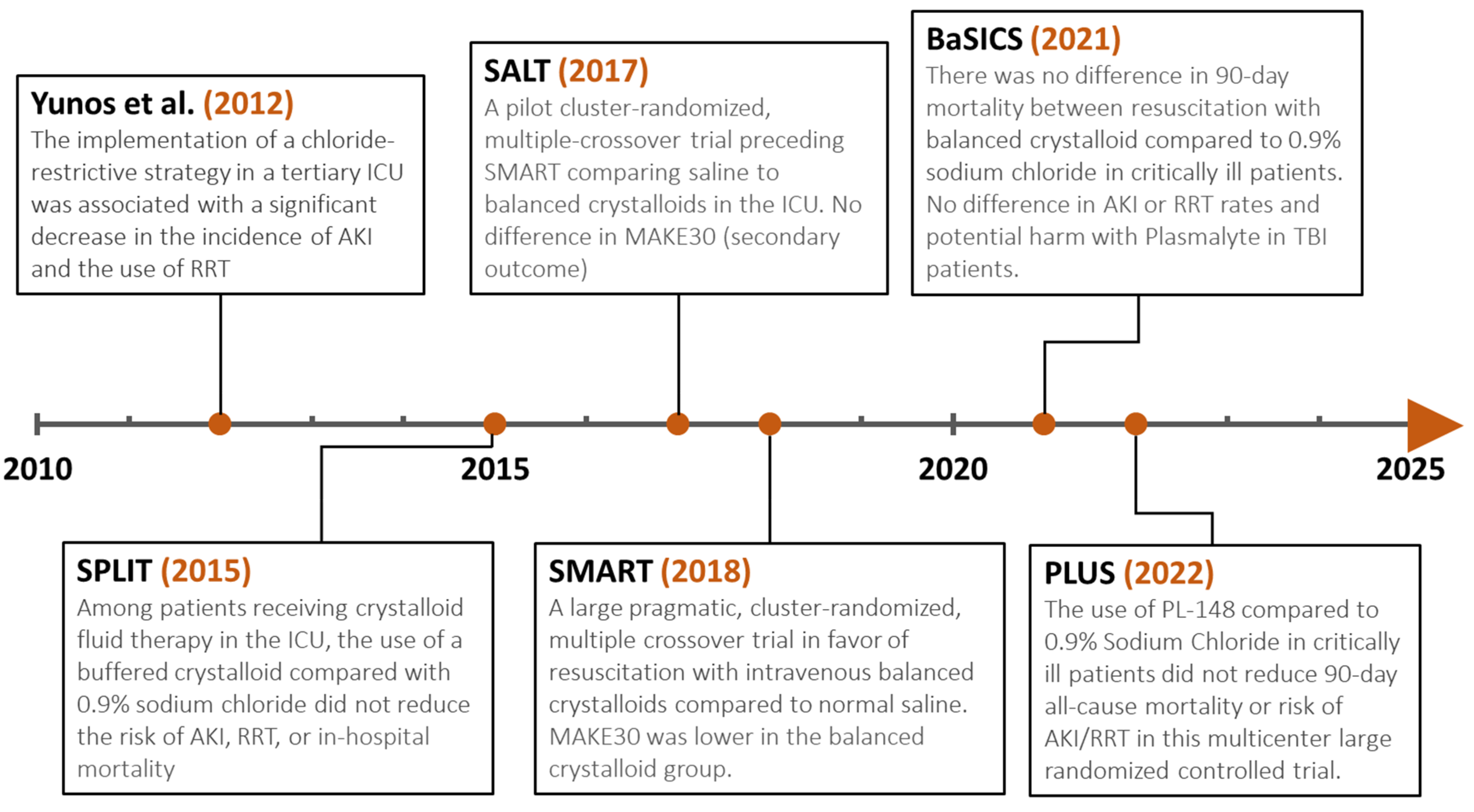
5.2. Intravenous Fluids: Is There an Ideal Fluid, and How Much to Give?
5.2.1. Colloids versus Crystalloids
5.2.2. Balanced Crystalloids versus Saline
5.2.3. Liberal vs. Restrictive Fluid Resuscitation Strategies
5.3. Vasopressors
5.3.1. Choice of Vasopressor Agents
5.3.2. Dopamine versus Norepinephrine
5.3.3. Vasopressin versus Norepinephrine
5.3.4. Epinephrine versus Norepinephrine
5.3.5. Phenylephrine versus Norepinephrine
5.3.6. Angiotensin II versus Norepinephrine
5.3.7. Selepressin versus Norepinephrine
5.3.8. Central vs. Peripheral Administration
5.4. Anti-Infective Therapy
5.4.1. Antibiotic Timing
5.4.2. Antibiotic Choice
5.4.3. Antifungal Coverage
5.4.4. Antibiotic Duration and Role of Sepsis Biomarkers
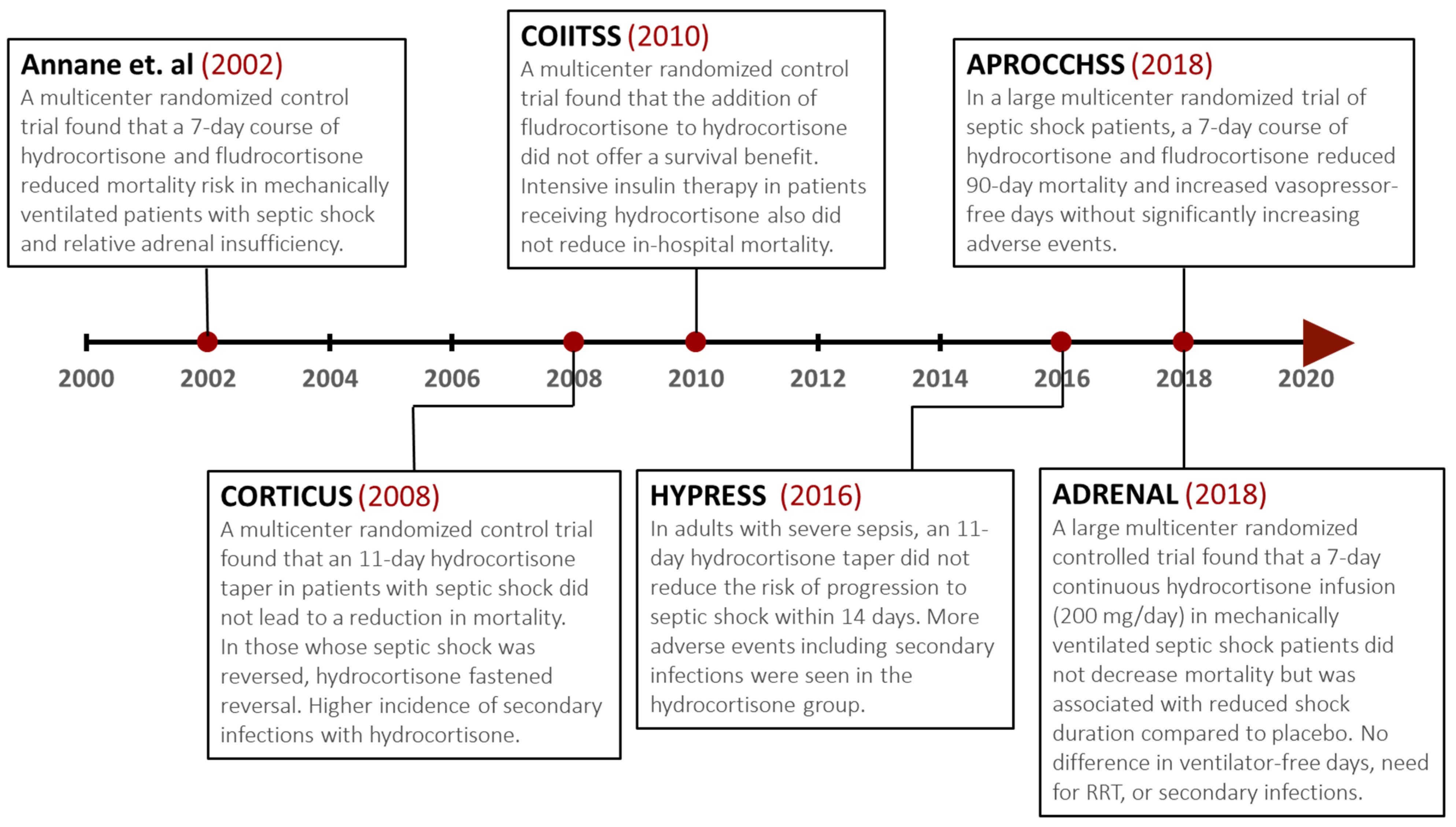
5.5. Adjunctive Glucocorticoid Therapy
5.6. Administration of Red Blood Cells and Transfusion Threshold
5.7. Glucose Control
5.8. The Vitamin C Controversy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funk, D.J.; Parrillo, J.E.; Kumar, A. Sepsis and Septic Shock: A History. Crit. Care Clin. 2009, 25, 83–101. [Google Scholar] [CrossRef]
- Gul, F.; Arslantas, M.K.; Cinel, I.; Kumar, A. Changing Definitions of Sepsis. Turk. J. Anaesthesiol. Reanim. 2017, 45, 129–138. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Cárdenas, C.L.; Yébenes, J.C.; Vela, E.; Clèries, M.; Sirvent, J.M.; Fuster-Bertolín, C.; Reina, C.; Rodríguez, A.; Ruiz-Rodríguez, J.C.; Trenado, J.; et al. Trends in mortality in septic patients according to the different organ failure during 15 years. Crit. Care 2022, 26, 302. [Google Scholar] [CrossRef]
- Silberberg, B.; Aston, S.; Boztepe, S.; Jacob, S.; Rylance, J. Recommendations for fluid management of adults with sepsis in sub-Saharan Africa: A systematic review of guidelines. Crit. Care 2020, 24, 286. [Google Scholar] [CrossRef]
- Andrews, B.; Semler, M.W.; Muchemwa, L.; Kelly, P.; Lakhi, S.; Heimburger, D.C.; Mabula, C.; Bwalya, M.; Bernard, G.R. Effect of an Early Resuscitation Protocol on In-hospital Mortality among Adults with Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017, 318, 1233–1240. [Google Scholar] [CrossRef]
- Bone, R.C.; Grodzin, C.J.; Balk, R.A. Sepsis: A New Hypothesis for Pathogenesis of the Disease Process. Chest 1997, 112, 235–243. [Google Scholar] [CrossRef]
- van der Poll, T.; Opal, S.M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis—Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.-F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin Alfa (Activated) in Adults with Septic Shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Lemachatti, N.; Krastinova, E.; Van Laer, M.; Claessens, Y.-E.; Avondo, A.; Occelli, C.; Feral-Pierssens, A.-L.; Truchot, J.; Ortega, M.; et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality among Patients with Suspected Infection Presenting to the Emergency Department. JAMA 2017, 317, 301–308. [Google Scholar] [CrossRef]
- Serafim, R.; Gomes, J.A.; Salluh, J.; Póvoa, P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest 2018, 153, 646–655. [Google Scholar] [CrossRef]
- Song, J.-U.; Sin, C.K.; Park, H.K.; Shim, S.R.; Lee, J. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: A systematic review and meta-analysis. Crit. Care 2018, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.X.; Lu, Y.; Carey, K.A.; Gilbert, E.R.; Afshar, M.; Akel, M.; Shah, N.S.; Dolan, J.; Winslow, C.; Kipnis, P.; et al. Comparison of Early Warning Scoring Systems for Hospitalized Patients with and Without Infection at Risk for In-Hospital Mortality and Transfer to the Intensive Care Unit. JAMA Netw. Open 2020, 3, e205191. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Snyder, A.; Han, X.; Sokol, S.; Pettit, N.; Howell, M.D.; Edelson, D.P. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2017, 195, 906–911. [Google Scholar] [CrossRef]
- Herwanto, V.; Shetty, A.; Nalos, M.; Chakraborty, M.; McLean, A.; Eslick, G.D.; Tang, B. Accuracy of Quick Sequential Organ Failure Assessment Score to Predict Sepsis Mortality in 121 Studies Including 1,716,017 Individuals: A Systematic Review and Meta-Analysis. Crit. Care Explor. 2019, 1, e0043. [Google Scholar] [CrossRef]
- Damiani, E.; Donati, A.; Serafini, G.; Rinaldi, L.; Adrario, E.; Pelaia, P.; Busani, S.; Girardis, M. Effect of Performance Improvement Programs on Compliance with Sepsis Bundles and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0125827. [Google Scholar] [CrossRef]
- Gattinoni, L.; Brazzi, L.; Pelosi, P.; Latini, R.; Tognoni, G.; Pesenti, A.; Fumagalli, R. A Trial of Goal-Oriented Hemodynamic Therapy in Critically Ill Patients. SvO2 Collaborative Group. N. Engl. J. Med. 1995, 333, 1025–1032. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M.; Early Goal-Directed Therapy Collaborative, G. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004, 32, 858–873. [Google Scholar] [CrossRef]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Investigators, A.; Group, A.C.T.; Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Investigators, P.; Rowan, K.M.; Angus, D.C.; Bailey, M.; Barnato, A.E.; Bellomo, R.; Canter, R.R.; Coats, T.J.; Delaney, A.; Gimbel, E.; et al. Early, Goal-Directed Therapy for Septic Shock-A Patient-Level Meta-Analysis. N. Engl. J. Med. 2017, 376, 2223–2234. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Pepper, D.J.; Sun, J.; Cui, X.; Welsh, J.; Natanson, C.; Eichacker, P.Q. Antibiotic- and Fluid-Focused Bundles Potentially Improve Sepsis Management, but High-Quality Evidence Is Lacking for the Specificity Required in the Centers for Medicare and Medicaid Service’s Sepsis Bundle (SEP-1)*. Crit. Care Med. 2019, 47, 1290–1300. [Google Scholar] [CrossRef]
- Pepper, D.J.; Jaswal, D.; Sun, J.; Welsh, J.; Natanson, C.; Eichacker, P.Q. Evidence Underpinning the Centers for Medicare & Medicaid Services’ Severe Sepsis and Septic Shock Management Bundle (SEP-1): A Systematic Review. Ann. Intern. Med. 2018, 168, 558–568. [Google Scholar] [CrossRef]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Spiegel, R.; Farkas, J.D.; Rola, P.; Kenny, J.-E.; Olusanya, S.; Marik, P.E.; Weingart, S.D. The 2018 Surviving Sepsis Campaign’s Treatment Bundle: When Guidelines Outpace the Evidence Supporting Their Use. Ann. Emerg. Med. 2019, 73, 356–358. [Google Scholar] [CrossRef]
- Kahn, J.M.; Davis, B.S.; Yabes, J.G.; Chang, C.-C.H.; Chong, D.H.; Hershey, T.B.; Martsolf, G.R.; Angus, D.C. Association Between State-Mandated Protocolized Sepsis Care and In-hospital Mortality among Adults with Sepsis. JAMA 2019, 322, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Yu, T.; Wang, R.; Kadri, S.S.; Fram, D.; Chen, H.-C.; Klompas, M.; Program, C.P.E. Association between Implementation of the Severe Sepsis and Septic Shock Early Management Bundle Performance Measure and Outcomes in Patients with Suspected Sepsis in US Hospitals. JAMA Netw. Open 2021, 4, e2138596. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.R.; Phillips, G.S.; Duseja, R.; Tefera, L.; Cruikshank, D.; Dickerson, R.; Nguyen, H.B.; Schorr, C.A.; Levy, M.M.; Dellinger, R.P.; et al. Effects of Compliance with the Early Management Bundle (SEP-1) on Mortality Changes among Medicare Beneficiaries with Sepsis: A Propensity Score Matched Cohort Study. Chest 2022, 161, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Schortgen, F.; Lacherade, J.-C.; Bruneel, F.; Cattaneo, I.; Hemery, F.; Lemaire, F.; Brochard, L. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: A multicentre randomised study. Lancet 2001, 357, 911–916. [Google Scholar] [CrossRef]
- Haase, N.; Perner, A.; Hennings, L.I.; Siegemund, M.; Lauridsen, B.; Wetterslev, M.; Wetterslev, J. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: Systematic review with meta-analysis and trial sequential analysis. BMJ 2013, 346, f839. [Google Scholar] [CrossRef]
- Perner, A.; Haase, N.; Guttormsen, A.B.; Tenhunen, J.; Klemenzson, G.; Åneman, A.; Madsen, K.R.; Møller, M.H.; Elkjær, J.M.; Poulsen, L.M.; et al. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N. Engl. J. Med. 2012, 367, 124–134. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C.; et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N. Engl. J. Med. 2012, 367, 1901–1911. [Google Scholar] [CrossRef]
- Rochwerg, B.; Alhazzani, W.; Sindi, A.; Heels-Ansdell, D.; Thabane, L.; Fox-Robichaud, A.; Mbuagbaw, L.; Szczeklik, W.; Alshamsi, F.; Altayyar, S.; et al. Fluid Resuscitation in Sepsis: A systematic review and network meta-analysis. Ann. Intern. Med. 2014, 161, 347–355. [Google Scholar] [CrossRef]
- Annane, D.; Siami, S.; Jaber, S.; Martin, C.; Elatrous, S.; Declère, A.D.; Preiser, J.C.; Outin, H.; Troché, G.; Charpentier, C.; et al. Effects of Fluid Resuscitation with Colloids vs Crystalloids on Mortality in Critically Ill Patients Presenting with Hypovolemic Shock: The CRISTAL randomized trial. JAMA 2013, 310, 1809–1817. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R.; SAFE Study Investigators. A Comparison of Albumin and Saline for Fluid Resuscitation in the Intensive Care Unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.-J.; Orellana-Jimenez, C.; Melot, C.; De Backer, D.; Berre, J.; Leeman, M.; Brimioulle, S.; Appoloni, O.; Creteur, J.; Vincent, J.-L. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study*. Crit. Care Med. 2006, 34, 2536–2540. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; on behalf of SepNet-Critical Care Trials Group; Bauer, M.; Nierhaus, A.; Kluge, S.; Schumacher, U.; Putensen, C.; Fichtner, F.; Petros, S.; Scheer, C.; et al. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): Protocol for a randomized controlled trial. Trials 2020, 21, 1002. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; Bagshaw, S.M.; Goldstein, S.L.; Scherer, L.A.; Duan, M.; Schermer, C.R.; Kellum, J.A. Major Complications, Mortality, and Resource Utilization After Open Abdominal Surgery. Ann. Surg. 2012, 255, 821–829. [Google Scholar] [CrossRef]
- Yunos, N.M.; Bellomo, R.; Hegarty, C.; Story, D.; Ho, L.; Bailey, M. Association Between a Chloride-Liberal vs Chloride-Restrictive Intravenous Fluid Administration Strategy and Kidney Injury in Critically Ill Adults. JAMA 2012, 308, 1566–1572. [Google Scholar] [CrossRef]
- Finfer, S.; Micallef, S.; Hammond, N.; Navarra, L.; Bellomo, R.; Billot, L.; Delaney, A.; Gallagher, M.; Gattas, D.; Li, Q.; et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N. Engl. J. Med. 2022, 386, 815–826. [Google Scholar] [CrossRef]
- Kellum, J.A. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: Improved short-term survival and acid-base balance with Hextend compared with saline. Crit. Care Med. 2002, 30, 300–305. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Effects of Hyperchloremic Acidosis on Arterial Pressure and Circulating Inflammatory Molecules in Experimental Sepsis. Chest 2004, 125, 243–248. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Cox, E.F.; Francis, S.T.; Lobo, D.N. A Randomized, Controlled, Double-Blind Crossover Study on the Effects of 2-L Infusions of 0.9% Saline and Plasma-Lyte® 148 on Renal Blood Flow Velocity and Renal Cortical Tissue Perfusion in Healthy Volunteers. Ann. Surg. 2012, 256, 18–24. [Google Scholar] [CrossRef]
- Young, P.; Bailey, M.; Beasley, R.; Henderson, S.; Mackle, D.; McArthur, C.; McGuinness, S.; Mehrtens, J.; Myburgh, J.; Psirides, A.; et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 2015, 314, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Wanderer, J.P.; Ehrenfeld, J.M.; Stollings, J.L.; Self, W.H.; Siew, E.D.; Wang, L.; Byrne, D.W.; Shaw, A.D.; Bernard, G.R.; et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Semler, M.W.; Wanderer, J.P.; Wang, L.; Byrne, D.W.; Collins, S.P.; Slovis, C.M.; Lindsell, C.J.; Ehrenfeld, J.M.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N. Engl. J. Med. 2018, 378, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Brown, R.M.; Wang, L.; Coston, T.D.; Krishnan, N.I.; Casey, J.D.; Wanderer, J.P.; Ehrenfeld, J.M.; Byrne, D.W.; Stollings, J.L.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1487–1495. [Google Scholar] [CrossRef]
- Hammond, D.A.; Lam, S.W.; Rech, M.A.; Smith, M.N.; Westrick, J.; Trivedi, A.P.; Balk, R.A. Balanced Crystalloids versus Saline in Critically Ill Adults: A Systematic Review and Meta-Analysis. Ann. Pharmacother. 2020, 54, 5–13. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Serpa-Neto, A.; Paranhos, J.L.R.; Guedes, M.A.V.; et al. Effect of Intravenous Fluid Treatment with a Balanced Solution vs. 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021, 326, 818–829. [Google Scholar] [CrossRef]
- Hammond, N.E.; Zampieri, F.G.; Di Tanna, G.L.; Garside, T.; Adigbli, D.; Cavalcanti, A.B.; Machado, F.R.; Micallef, S.; Myburgh, J.; Ramanan, M.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults—A Systematic Review with Meta-Analysis. NEJM Evid. 2022, 1, EVIDoa2100010. [Google Scholar] [CrossRef]
- Ramanan, M.; Attokaran, A.; Murray, L.; Bhadange, N.; Stewart, D.; Rajendran, G.; Pusapati, R.; Petty, M.; Garrett, P.; Kruger, P.; et al. Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): A cluster, crossover, randomized, controlled trial. Intensive Care Med. 2021, 47, 1248–1257. [Google Scholar] [CrossRef]
- Alghamdi, N.A.; Major, P.B.; Chaudhuri, D.; Tsui, J.P.; Brown, B.; Self, W.H.; Semler, M.W.M.; Ramanan, M.; Rochwerg, B.M. Saline Compared to Balanced Crystalloid in Patients with Diabetic Ketoacidosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Explor. 2022, 4, e0613. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Eskesen, T.G.; Wetterslev, M.; Perner, A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016, 42, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.; Muchemwa, L.M.; Kelly, P.M.; Lakhi, S.M.; Heimburger, D.C.M.; Bernard, G.R. Simplified Severe Sepsis Protocol: A randomized controlled trial of modified early goal-directed therapy in Zambia. Crit. Care Med. 2014, 42, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Baelani, I.; Jochberger, S.; Laimer, T.; Otieno, D.; Kabutu, J.; Wilson, I.; Baker, T.; Dünser, M.W. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: A self-reported, continent-wide survey of anaesthesia providers. Crit. Care 2011, 15, R10. [Google Scholar] [CrossRef] [PubMed]
- Taj, M.; Brenner, M.; Sulaiman, Z.; Pandian, V. Sepsis protocols to reduce mortality in resource-restricted settings: A systematic review. Intensive Crit. Care Nurs. 2022, 72, 103255. [Google Scholar] [CrossRef] [PubMed]
- Meyhoff, T.S.; Møller, M.H.; Hjortrup, P.B.; Cronhjort, M.; Perner, A.; Wetterslev, J. Lower vs Higher Fluid Volumes During Initial Management of Sepsis: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Chest 2020, 157, 1478–1496. [Google Scholar] [CrossRef]
- Meyhoff, T.S.; Hjortrup, P.B.; Wetterslev, J.; Sivapalan, P.; Laake, J.H.; Cronhjort, M.; Jakob, S.M.; Cecconi, M.; Nalos, M.; Ostermann, M.; et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N. Engl. J. Med. 2022, 386, 2459–2470. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network; Shapiro, N.I.; Douglas, I.S.; Brower, R.G.; Brown, S.M.; Exline, M.C.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Hayden, D.; et al. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N. Engl. J. Med. 2023, 388, 499–510. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Avni, T.; Lador, A.; Lev, S.; Leibovici, L.; Paul, M.; Grossman, A. Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0129305. [Google Scholar] [CrossRef]
- Landry, D.W.; Oliver, J.A. The Pathogenesis of Vasodilatory Shock. N. Engl. J. Med. 2001, 345, 588–595. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Factor, P. Role of vasopressin in the management of septic shock. Intensive Care Med. 2004, 30, 1276–1291. [Google Scholar] [CrossRef]
- Patel, B.M.; Chittock, D.R.; Russell, J.A.; Walley, K.R. Beneficial Effects of Short-term Vasopressin Infusion during Severe Septic Shock. Anesthesiology 2002, 96, 576–582. [Google Scholar] [CrossRef]
- Holmes, C.L.; Walley, K.R.; Chittock, D.R.; Lehman, T.; Russell, J.A. The effects of vasopressin on hemodynamics and renal function in severe septic shock: A case series. Intensive Care Med. 2001, 27, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef]
- Polito, A.; Parisini, E.; Ricci, Z.; Picardo, S.; Annane, D. Vasopressin for treatment of vasodilatory shock: An ESICM systematic review and meta-analysis. Intensive Care Med. 2012, 38, 9–19. [Google Scholar] [CrossRef]
- Neto, A.S.; Nassar, A.P.; Cardoso, S.O.; Manetta, J.A.; Pereira, V.G.; Espósito, D.C.; Damasceno, M.C.; Russell, J.A. Vasopressin and terlipressin in adult vasodilatory shock: A systematic review and meta-analysis of nine randomized controlled trials. Crit. Care 2012, 16, R154. [Google Scholar] [CrossRef]
- Belletti, A.; Musu, M.; Silvetti, S.; Saleh, O.; Pasin, L.; Monaco, F.; Hajjar, L.A.; Fominskiy, E.; Finco, G.; Zangrillo, A.; et al. Non-Adrenergic Vasopressors in Patients with or at Risk for Vasodilatory Shock. A Systematic Review and Meta-Analysis of Randomized Trials. PLoS ONE 2015, 10, e0142605. [Google Scholar] [CrossRef]
- Gordon, A.C.; Russell, J.A.; Walley, K.R.; Singer, J.; Ayers, D.; Storms, M.M.; Holmes, C.L.; Hébert, P.C.; Cooper, D.J.; Mehta, S.; et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010, 36, 83–91. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs. Norepinephrine on Kidney Failure in Patients with Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, W.F.; Um, K.J.; Alhazzani, W.; Lengyel, A.P.; Hajjar, L.; Gordon, A.C.; Lamontagne, F.; Healey, J.S.; Whitlock, R.P.; Belley-Côté, E.P. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone with Atrial Fibrillation in Patients with Distributive Shock: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, M.; Russell, J.A.; Walley, K.R.; Brett, S.J.; Perkins, G.D.; Hajjar, L.; Mason, A.J.; Ashby, D.; Gordon, A.C. Vasopressin in septic shock: An individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019, 45, 844–855. [Google Scholar] [CrossRef]
- Ammar, M.A.; Ammar, A.A.; Wieruszewski, P.M.; Bissell, B.D.; Long, M.T.; Albert, L.; Khanna, A.K.; Sacha, G.L. Timing of vasoactive agents and corticosteroid initiation in septic shock. Ann. Intensive Care 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Wieruszewski, P.M.; Khanna, A.K. Vasopressor Choice and Timing in Vasodilatory Shock. Crit. Care 2022, 26, 76. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, J.A.; Higgins, A.; Jovanovska, A.; Lipman, J.; Ramakrishnan, N.; Santamaria, J.; The CAT Study investigators. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008, 34, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Vignon, P.; Renault, A.; Bollaert, P.-E.; Charpentier, C.; Martin, C.; Troché, G.; Ricard, J.-D.; Nitenberg, G.; Papazian, L.; et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: A randomised trial. Lancet 2007, 370, 676–684. [Google Scholar] [CrossRef]
- De Backer, D.; Creteur, J.; Silva, E.; Vincent, J.-L. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: Which is best?*. Crit. Care Med. 2003, 31, 1659–1667. [Google Scholar] [CrossRef]
- Morelli, A.; Ertmer, C.; Rehberg, S.; Lange, M.; Orecchioni, A.; Laderchi, A.; Bachetoni, A.; D’Alessandro, M.; Van Aken, H.; Pietropaoli, P.; et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: A randomized, controlled trial. Crit. Care 2008, 12, R143. [Google Scholar] [CrossRef]
- Vail, E.; Gershengorn, H.B.; Hua, M.; Walkey, A.J.; Rubenfeld, G.; Wunsch, H. Association Between US Norepinephrine Shortage and Mortality among Patients with Septic Shock. JAMA 2017, 317, 1433–1442. [Google Scholar] [CrossRef]
- Law, A.C.; Bosch, N.A.; Peterson, D.; Walkey, A.J. Comparison of Heart Rate After Phenylephrine vs Norepinephrine Initiation in Patients with Septic Shock and Atrial Fibrillation. Chest 2022, 162, 796–803. [Google Scholar] [CrossRef]
- Chawla, L.S.; Busse, L.; Brasha-Mitchell, E.; Davison, D.; Honiq, J.; Alotaibi, Z.; Seneff, M.G. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): A pilot study. Crit. Care 2014, 18, 534. [Google Scholar] [CrossRef]
- Khanna, A.; Ostermann, M.; Bellomo, R. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017, 377, 2601–2604. [Google Scholar] [CrossRef]
- Wieruszewski, P.M.; Bellomo, R.; Busse, L.W.; Ham, K.R.; Zarbock, A.; Khanna, A.K.; Deane, A.M.; Ostermann, M.; Wunderink, R.G.; Boldt, D.W.; et al. Initiating angiotensin II at lower vasopressor doses in vasodilatory shock: An exploratory post-hoc analysis of the ATHOS-3 clinical trial. Crit. Care 2023, 27, 175. [Google Scholar] [CrossRef]
- Tumlin, J.A.; Murugan, R.; Deane, A.M.; Ostermann, M.; Busse, L.W.; Ham, K.R.; Kashani, K.; Szerlip, H.M.; Prowle, J.R.; Bihorac, A.; et al. Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II. Crit. Care Med. 2018, 46, 949–957. [Google Scholar] [CrossRef]
- Smith, S.E.; Newsome, A.S.; Guo, Y.; Hecht, J.; McCurdy, M.T.; Mazzeffi, M.A.; Chow, J.H.; Kethireddy, S. A Multicenter Observational Cohort Study of Angiotensin II in Shock. J. Intensive Care Med. 2022, 37, 75–82. [Google Scholar] [CrossRef]
- Wieruszewski, P.M.; Wittwer, E.D.; Kashani, K.B.; Brown, D.R.; Butler, S.O.; Clark, A.M.; Cooper, C.J.; Davison, D.L.; Gajic, O.; Gunnerson, K.J.; et al. Angiotensin II Infusion for Shock: A Multicenter Study of Postmarketing Use. Chest 2021, 159, 596–605. [Google Scholar] [CrossRef]
- Russell, J.A.; Vincent, J.-L.; Kjølbye, A.L.; Olsson, H.; Blemings, A.; Spapen, H.; Carl, P.; Laterre, P.-F.; Grundemar, L. Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit. Care 2017, 21, 213. [Google Scholar] [CrossRef]
- Laterre, P.-F.; Berry, S.M.; Blemings, A.; Carlsen, J.E.; François, B.; Graves, T.; Jacobsen, K.; Lewis, R.J.; Opal, S.M.; Perner, A.; et al. Effect of Selepressin vs. Placebo on Ventilator- and Vasopressor-Free Days in Patients with Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 2019, 322, 1476. [Google Scholar] [CrossRef]
- Loubani, O.M.; Green, R.S. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J. Crit. Care 2015, 30, 653.e9–653.e17. [Google Scholar] [CrossRef]
- Tian, D.H.; Smyth, C.; Keijzers, G.; Macdonald, S.P.; Peake, S.; Udy, A.; Delaney, A. Safety of peripheral administration of vasopressor medications: A systematic review. Emerg. Med. Australas 2020, 32, 220–227. [Google Scholar] [CrossRef]
- Cardenas-Garcia, J.; Schaub, K.F.; Belchikov, Y.G.; Narasimhan, M.; Koenig, S.J.; Mayo, P.H. Safety of peripheral intravenous administration of vasoactive medication. J. Hosp. Med. 2015, 10, 581–585. [Google Scholar] [CrossRef]
- Ricard, J.-D.; Salomon, L.; Boyer, A.; Thiery, G.; Meybeck, A.; Roy, C.; Pasquet, B.; Le Mière, E.; Dreyfuss, D. Central or Peripheral Catheters for Initial Venous Access of ICU Patients: A randomized controlled trial. Crit. Care Med. 2013, 41, 2108–2115. [Google Scholar] [CrossRef]
- Bima, P.; Orlotti, C.; Smart, O.G.; Morello, F.; Trunfio, M.; Brazzi, L.; Montrucchio, G. Norepinephrine may improve survival of septic shock patients in a low-resource setting: A proof-of-concept study on feasibility and efficacy outside the Intensive Care Unit. Pathog Glob Health 2022, 116, 389–394. [Google Scholar] [CrossRef]
- Alam, N.; Oskam, E.; Stassen, P.M.; van Exter, P.; van de Ven, P.M.; Haak, H.R.; Holleman, F.; van Zanten, A.; van Leeuwen-Nguyen, H.; Bon, V.; et al. Prehospital antibiotics in the ambulance for sepsis: A multicentre, open label, randomised trial. Lancet Respir. Med. 2018, 6, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, T.; Rosenberger, L.H.; Swenson, B.; Metzger, R.; Flohr, T.R.; Politano, A.D.; Riccio, L.M.; Popovsky, K.A.; Sawyer, R.G. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: A quasi-experimental, before and after observational cohort study. Lancet Infect. Dis. 2012, 12, 774–780. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, C.L.; Lundeg, G.; Dondorp, A.M. Sepsis in resource-limited settings–expert consensus recommendations group of the European Society of Intensive Care Medicine (ESICM); The Mahidol-Oxford Research Unit (MORU) in Bangkok, Thailand Recommendations for infection management in patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. 2016, 42, 2040–2042. [Google Scholar] [CrossRef]
- Im, Y.; Kang, D.; Ko, R.-E.; Lee, Y.J.; Lim, S.Y.; Park, S.; Na, S.J.; Chung, C.R.; Park, M.H.; Oh, D.K.; et al. Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: A prospective nationwide multicenter cohort study. Crit. Care 2022, 26, 19. [Google Scholar] [CrossRef]
- Bisarya, R.; Song, X.; Salle, J.; Liu, M.; Patel, A.; Simpson, S.Q. Antibiotic Timing and Progression to Septic Shock among Patients in the ED with Suspected Infection. Chest 2022, 161, 112–120. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of Inappropriate Antimicrobial Therapy Results in a Fivefold Reduction of Survival in Human Septic Shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef]
- Paul, M.; Kariv, G.; Goldberg, E.; Raskin, M.; Shaked, H.; Hazzan, R.; Samra, Z.; Paghis, D.; Bishara, J.; Leibovici, L. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 2010, 65, 2658–2665. [Google Scholar] [CrossRef]
- Lodise, T.P.; McKinnon, P.S.; Swiderski, L.; Rybak, M.J. Outcomes Analysis of Delayed Antibiotic Treatment for Hospital-Acquired Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2003, 36, 1418–1423. [Google Scholar] [CrossRef]
- Webb, B.J.; Sorensen, J.; Jephson, A.; Mecham, I.; Dean, N.C. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: A cohort study. Eur. Respir. J. 2019, 54, 1900057. [Google Scholar] [CrossRef]
- Jones, B.E.; Ying, J.; Stevens, V.; Haroldsen, C.; He, T.; Nevers, M.; Christensen, M.A.; Nelson, R.E.; Stoddard, G.J.; Sauer, B.C.; et al. Empirical Anti-MRSA vs Standard Antibiotic Therapy and Risk of 30-Day Mortality in Patients Hospitalized for Pneumonia. JAMA Intern. Med. 2020, 180, 552–560. [Google Scholar] [CrossRef]
- Baby, N.; Faust, A.C.; Smith, T.; Sheperd, L.A.; Knoll, L.; Goodman, E.L. Nasal Methicillin-Resistant Staphylococcus aureus (MRSA) PCR Testing Reduces the Duration of MRSA-Targeted Therapy in Patients with Suspected MRSA Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e02432-16. [Google Scholar] [CrossRef]
- Cowley, M.C.; Ritchie, D.J.; Hampton, N.; Kollef, M.H.; Micek, S.T. Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest 2019, 155, 53–59. [Google Scholar] [CrossRef]
- Sjövall, F.; Perner, A.; Møller, M.H. Empirical mono-versus combination antibiotic therapy in adult intensive care patients with severe sepsis–A systematic review with meta-analysis and trial sequential analysis. J. Infect. 2017, 74, 331–344. [Google Scholar] [CrossRef]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic Shock Attributed to Candida Infection: Importance of Empiric Therapy and Source Control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Marriott, D.J.; Playford, E.G.; Chen, S.; Slavin, M.; Nguyen, Q.; Ellis, D.; Sorrell, T.C.; the Australian Candidaemia Study. Determinants of mortality in non-neutropenic ICU patients with Candidaemia. Crit. Care 2009, 13, R115. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Cecilia, T.; De Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.-F.; Azoulay, E.; Schwebel, C.; Charles, P.E.; Cornet, M.; Souweine, B.; Klouche, K.; Jaber, S.; Trouillet, J.-L.; Bruneel, F.; et al. Empirical Micafungin Treatment and Survival without Invasive Fungal Infection in Adults with ICU-Acquired Sepsis, Candida Colonization, and Multiple Organ Failure: The EMPIRICUS Randomized Clinical Trial. JAMA 2016, 316, 1555–1564. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.-R.; Paiva, J.-A.; Timsit, J.-F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [CrossRef]
- Klompas, M.; Calandra, T.; Singer, M. Antibiotics for Sepsis—Finding the Equilibrium. JAMA 2018, 320, 1433–1434. [Google Scholar] [CrossRef]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of Short-Course Antimicrobial Therapy for Intraabdominal Infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- Burnham, J.P.; Olsen, M.A.; Stwalley, D.; Kwon, J.H.; Babcock, H.M.; Kollef, M.H. Infectious Diseases Consultation Reduces 30-Day and 1-Year All-Cause Mortality for Multidrug-Resistant Organism Infections. Open Forum Infect. Dis. 2018, 5, ofy026. [Google Scholar] [CrossRef] [PubMed]
- Madaline, T.; Montagne, F.W.; Eisenberg, R.; Mowrey, W.; Kaur, J.; Malik, M.; Gendlina, I.; Guo, Y.; White, D.; Pirofski, L.-A.; et al. Early Infectious Disease Consultation Is Associated with Lower Mortality in Patients with Severe Sepsis or Septic Shock Who Complete the 3-Hour Sepsis Treatment Bundle. Open Forum Infect. Dis. 2019, 6, ofz408. [Google Scholar] [CrossRef]
- van Engelen, T.S.; Wiersinga, W.J.; Scicluna, B.P.; van der Poll, T. Biomarkers in Sepsis. Crit. Care Clin. 2018, 34, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Daubin, C.; for the BPCTrea Study Group; Valette, X.; Thiollière, F.; Mira, J.-P.; Hazera, P.; Annane, D.; Labbe, V.; Floccard, B.; Fournel, F.; et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: A randomized multicenter study. Intensive Care Med. 2018, 44, 428–437. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Nagavci, B.; Aliberti, S.; Antonelli, M.; Bassetti, M.; Bos, L.; Chalmers, J.D.; Derde, L.; de Waele, J.; et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur. Respir. J. 2023, 61, 2200735. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.U.; Hein, L.; Lundgren, B.; Bestle, M.H.; Mohr, T.T.; Andersen, M.H.; Thornberg, K.J.; Løken, J.; Steensen, M.; Fox, Z.; et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial*. Crit. Care Med. 2011, 39, 2048–2058. [Google Scholar] [CrossRef]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients with Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1266–1276. [Google Scholar] [CrossRef]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Nobre, V.; Harbarth, S.; Graf, J.-D.; Rohner, P.; Pugin, J. Use of Procalcitonin to Shorten Antibiotic Treatment Duration in Septic Patients: A randomized trial. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef]
- Lamrous, A.; Repetto, E.; Depp, T.; Jimenez, C.; Chua, A.C.; Kanapathipillai, R.; Jensen, T.O. C-reactive protein and procalcitonin use in adults in low- and middle-income countries: A narrative review. JAC-Antimicrobial. Resist. 2023, 5, dlad057. [Google Scholar] [CrossRef] [PubMed]
- Malinovska, A.; Hernried, B.; Lin, A.; Badaki-Makun, O.; Fenstermacher, K.; Ervin, A.M.; Ehrhardt, S.; Levin, S.; Hinson, J.S. Monocyte Distribution Width as a Diagnostic Marker for Infection: A Systematic Review and Meta-analysis. Chest 2023, 164, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Chen, C.-J.; Shao, S.-C.; Li, C.; Hsiao, C.-H.; Niu, K.-Y.; Yen, C.-C. Comparison of the Diagnostic Accuracies of Monocyte Distribution Width, Procalcitonin, and C-Reactive Protein for Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2023, 51, e106–e114. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, S.; Berger, S. Clinical Trial Design in Studies of Corticosteroids for Bacterial Infections. Ann. Intern. Med. 1974, 81, 36–42. [Google Scholar] [CrossRef]
- Hahn, E.O.; Houser, H.B.; Rammelkamp, C.H., Jr.; Denny, F.W.; Wannamaker, L.W. Effect of cortisone on acute streptococcal infections and post-streptococcal complications 1. J. Clin. Investig. 1951, 30, 274–281. [Google Scholar] [CrossRef]
- Bone, R.C.; Fisher, C.J., Jr.; Clemmer, T.P.; Slotman, G.J.; Metz, C.A.; Balk, R.A.; The Methylprednisolone Severe Sepsis Study Group. A Controlled Clinical Trial of High-Dose Methylprednisolone in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 1987, 317, 653–658. [Google Scholar] [CrossRef]
- The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of High-Dose Glucocorticoid Therapy on Mortality in Patients with Clinical Signs of Systemic Sepsis. N. Engl. J. Med. 1987, 317, 659–665. [Google Scholar] [CrossRef]
- McGowan, J.E., Jr.; Chesney, P.J.; Crossley, K.B.; LaForce, F.M. Guidelines for the use of systemic glucocorticosteroids in the management of selected infections. Working Group on Steroid Use, Antimicrobial Agents Committee, Infectious Diseases Society of America. J. Infect. Dis. 1992, 165, 1–13. [Google Scholar] [CrossRef]
- Lefering, R.; Neugebauer, E.A. Steroid controversy in sepsis and septic shock: A meta-analysis. Crit. Care Med. 1995, 23, 1294–1303. [Google Scholar] [CrossRef]
- Cronin, L.; Cook, D.J.; Carlet, J.; Heyland, D.K.; King, D.B.M.; Lansang, M.A.D.; Fisher, J. Corticosteroid treatment for sepsis: A critical appraisal and meta-analysis of the literature. Crit. Care Med. 1995, 23, 1430–1439. [Google Scholar] [CrossRef]
- Annane, D.; Sébille, V.; Troché, G.; Raphaël, J.-C.; Gajdos, P.; Bellissant, E. A 3-Level Prognostic Classification in Septic Shock Based on Cortisol Levels and Cortisol Response to Corticotropin. JAMA 2000, 283, 1038–1045. [Google Scholar] [CrossRef]
- Meduri, G.U. An Historical Review of Glucocorticoid Treatment in Sepsis. Disease Pathophysiology and the Design of Treatment Investigation. Sepsis 1999, 3, 21–38. [Google Scholar] [CrossRef]
- Bollaert, P.-E.; Charpentier, C.; Levy, B.; Debouverie, M.; Audibert, G.; Larcan, A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit. Care Med. 1998, 26, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Sébille, V.; Charpentier, C.; Bollaert, P.-E.; François, B.; Korach, J.-M.; Capellier, G.; Cohen, Y.; Azoulay, E.; Troché, G.; et al. Effect of Treatment with Low Doses of Hydrocortisone and Fludrocortisone on Mortality in Patients with Septic Shock. JAMA 2002, 288, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone Therapy for Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst. Rev. 2015, 2015, CD002243. [Google Scholar] [CrossRef]
- Volbeda, M.; Wetterslev, J.; Gluud, C.; Zijlstra, J.G.; van der Horst, I.C.C.; Keus, F. Glucocorticosteroids for sepsis: Systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015, 41, 1220–1234. [Google Scholar] [CrossRef]
- Sligl, W.I.; Milner, J.D.A., Jr.; Sundar, S.; Mphatswe, W.; Majumdar, S.R. Safety and Efficacy of Corticosteroids for the Treatment of Septic Shock: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2009, 49, 93–101. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Bollaert, P.-E.; Briegel, J.; Confalonieri, M.; De Gaudio, R.; Keh, D.; Kupfer, Y.; Oppert, M.; Meduri, G.U. Corticosteroids in the Treatment of Severe Sepsis and Septic Shock in Adults: A systematic review. JAMA 2009, 301, 2362–2375. [Google Scholar] [CrossRef]
- Keh, D.; Trips, E.; Marx, G.; Wirtz, S.P.; Abduljawwad, E.; Bercker, S.; Bogatsch, H.; Briegel, J.; Engel, C.; Gerlach, H.; et al. Effect of Hydrocortisone on Development of Shock among Patients with Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA 2016, 316, 1775–1785. [Google Scholar] [CrossRef]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.-P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Investigators, C.S.; Annane, D.; Cariou, A.; Maxime, V.; Azoulay, E.; D’Honneur, G.; Timsit, J.F.; Cohen, Y.; Wolf, M.; Fartoukh, M.; et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: A randomized controlled trial. JAMA 2010, 303, 341–348. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, Y.; Tang, J.; Lunsford, L.D.; Li, T.; Tang, R.; He, J.; Xu, P.; Faramand, A.; Xu, J.; et al. Association of Corticosteroid Treatment with Outcomes in Adult Patients with Sepsis: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y.; Pirracchio, R.; Rochwerg, B. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst. Rev. 2019, 2019, CD002243. [Google Scholar] [CrossRef]
- Rygård, S.L.; Butler, E.; Granholm, A.; Møller, M.H.; Cohen, J.; Finfer, S.; Perner, A.; Myburgh, J.; Venkatesh, B.; Delaney, A. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018, 44, 1003–1016. [Google Scholar] [CrossRef]
- Bosch, N.A.; Teja, B.; Law, A.C.; Pang, B.; Jafarzadeh, S.R.; Walkey, A.J. Comparative Effectiveness of Fludrocortisone and Hydrocortisone vs. Hydrocortisone Alone among Patients with Septic Shock. JAMA Intern. Med. 2023, 183, 451–459. [Google Scholar] [CrossRef]
- Pirracchio, R.; Annane, D.; Waschka, A.K.; Lamontagne, F.; Arabi, Y.M.; Bollaert, P.-E.; Billot, L.; Du, B.; Briegel, J.; Cohen, J.; et al. Patient-Level Meta-Analysis of Low-Dose Hydrocortisone in Adults with Septic Shock. NEJM Evid. 2023, 2, EVIDoa2300034. [Google Scholar] [CrossRef]
- Hebert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef]
- Holst, L.B.; Haase, N.; Wetterslev, J.; Wernerman, J.; Guttormsen, A.B.; Karlsson, S.; Johansson, P.I.; Åneman, A.; Vang, M.L.; Winding, R.; et al. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. N. Engl. J. Med. 2014, 371, 1381–1391. [Google Scholar] [CrossRef]
- Bergamin, F.S.; Almeida, J.P.; Landoni, G.; Galas, F.R.B.G.; Fukushima, J.T.; Fominskiy, E.; Park, C.H.L.; Osawa, E.A.; Diz, M.P.E.; Oliveira, G.Q.; et al. Liberal Versus Restrictive Transfusion Strategy in Critically Ill Oncologic Patients: The Transfusion Requirements in Critically Ill Oncologic Patients Randomized Controlled Trial*. Crit. Care Med. 2017, 45, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Miyoshi, Y.; Kondo, Y.; Okamoto, K.; Tanaka, H. Liberal versus restrictive red blood cell transfusion strategy in sepsis or septic shock: A systematic review and meta-analysis of randomized trials. Crit. Care 2019, 23, 262. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Vincent, J.-L. The Surviving Sepsis Campaign sepsis change bundles and clinical practice. Crit. Care 2005, 9, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive Insulin Therapy in Critically Ill Patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Wilmer, A.; Hermans, G.; Meersseman, W.; Wouters, P.J.; Milants, I.; Van Wijngaerden, E.; Bobbaers, H.; Bouillon, R. Intensive Insulin Therapy in the Medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef]
- NICE-SUGAR Study Investigators; Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Yamada, T.; Shojima, N.; Noma, H.; Yamauchi, T.; Kadowaki, T. Glycemic control, mortality, and hypoglycemia in critically ill patients: A systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med. 2016, 43, 1–15. [Google Scholar] [CrossRef]
- American Diabetes Association. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S144–S151. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Ittoop, R. Pro and Con: Vitamin C and Sepsis–Con. Soc. Crit. Care Anesthesiol. Newsl. 2018, 29, 2. [Google Scholar]
- Harris, R. Doctor Turns Up Possible Treatment for Deadly Sepsis. Available online: https://www.npr.org/sections/health-shots/2017/03/23/521096488/doctor-turns-up-possible-treatment-for-deadly-sepsis (accessed on 15 June 2023).
- Zimick, N.C. Pro and Con: Vitamin C and Sepsis–Pro. Soc. Crit. Care Anesthesiol. Newsl. 2018, 29, 1. [Google Scholar]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support among Patients with Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Moskowitz, A.; Huang, D.T.; Hou, P.C.; Gong, J.; Doshi, P.B.; Grossestreuer, A.V.; Andersen, L.W.; Ngo, L.; Sherwin, R.L.; Berg, K.M.; et al. Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial. JAMA 2020, 324, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Masse, M.-H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.-C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef]
- Sevransky, J.E.; Rothman, R.E.; Hager, D.N.; Bernard, G.R.; Brown, S.M.; Buchman, T.G.; Busse, L.W.; Coopersmith, C.M.; DeWilde, C.; Ely, E.W.; et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients with Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021, 325, 742–750. [Google Scholar] [CrossRef]
- Steinhagen, F.; Schmidt, S.V.; Schewe, J.-C.; Peukert, K.; Klinman, D.M.; Bode, C. Immunotherapy in sepsis-brake or accelerate? Pharmacol. Ther. 2020, 208, 107476. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 2062–2063. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Xu, P.; Wang, Q.; Zhang, J.; Chen, K.; Clements, C.M.; Celi, L.A.; Herasevich, V.; Hong, Y. Effectiveness of automated alerting system compared to usual care for the management of sepsis. NPJ Digit. Med. 2022, 5, 101. [Google Scholar] [CrossRef]
- Adams, R.; Henry, K.E.; Sridharan, A.; Soleimani, H.; Zhan, A.; Rawat, N.; Johnson, L.; Hager, D.N.; Cosgrove, S.E.; Markowski, A.; et al. Prospective, multi-site study of patient outcomes after implementation of the TREWS machine learning-based early warning system for sepsis. Nat. Med. 2022, 28, 1455–1460. [Google Scholar] [CrossRef] [PubMed]

| 1992 SEPSIS—1 Bone et al. [3] | Sepsis Systemic inflammatory response to infection | Severe Sepsis Sepsis associated with organ dysfunction, hypoperfusion, or hypotension | Septic Shock Sepsis-induced hypotension despite adequate fluid resuscitation along with the presence of perfusion abnormalities |
| 2001 SEPSIS—2 Levy et al. [4] | Sepsis A clinical syndrome defined by the presence of both infection and a systemic inflammatory response | Severe Sepsis Sepsis associated with organ dysfunction, hypoperfusion, or hypotension | Septic Shock State of acute circulatory failure characterized by persistent arterial hypotension unexplained by other causes |
| 2016 SEPSIS—3 Singer et al. [5] | Sepsis A life-threatening organ dysfunction caused by a dysregulated host response to infection (SOFA score of ≥2). In-hospital mortality >10% | Septic Shock A subset of sepsis with profound circulatory, cellular, and metabolic abnormalities with higher mortality. Vasopressor requirement (MAP ≥ 65 mmHg) and serum lactate level > 2 mmol/L in the absence of hypovolemia. In-hospital mortality > 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamath, S.; Hammad Altaq, H.; Abdo, T. Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms 2023, 11, 2231. https://doi.org/10.3390/microorganisms11092231
Kamath S, Hammad Altaq H, Abdo T. Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms. 2023; 11(9):2231. https://doi.org/10.3390/microorganisms11092231
Chicago/Turabian StyleKamath, Shiwani, Hiba Hammad Altaq, and Tony Abdo. 2023. "Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades?" Microorganisms 11, no. 9: 2231. https://doi.org/10.3390/microorganisms11092231
APA StyleKamath, S., Hammad Altaq, H., & Abdo, T. (2023). Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms, 11(9), 2231. https://doi.org/10.3390/microorganisms11092231





