Development Features of Ixodes ricinus × I. persulcatus Hybrids under Laboratory Conditions
Abstract
:1. Introduction
2. Materials and Methods
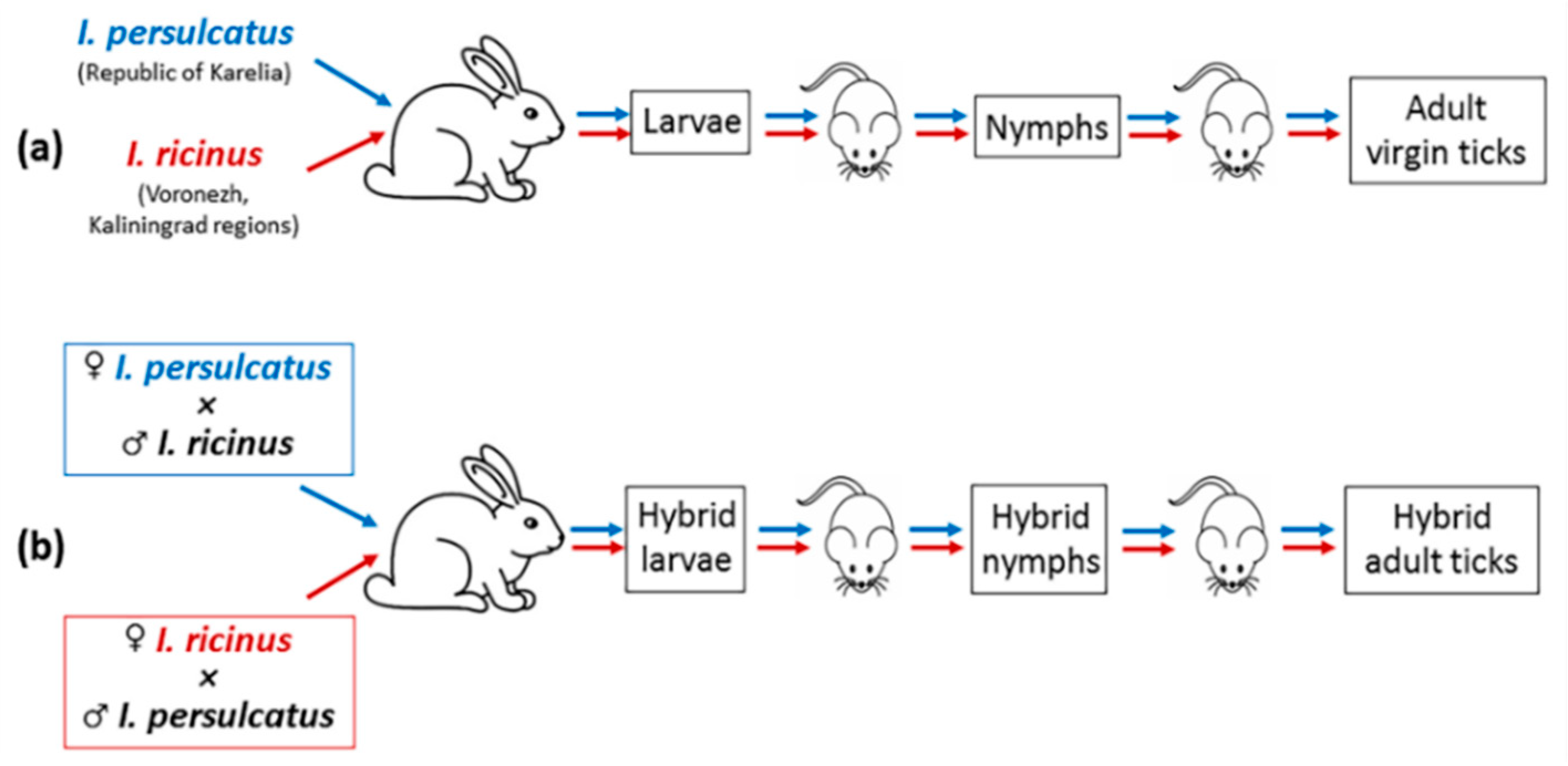
2.1. Ticks
2.2. Study of the Life Cycles of Ticks under Laboratory Conditions
2.3. Statistical Analysis
3. Results
3.1. Feeding and Oviposition of Female Ticks Depending on the Species of the Mating Partner
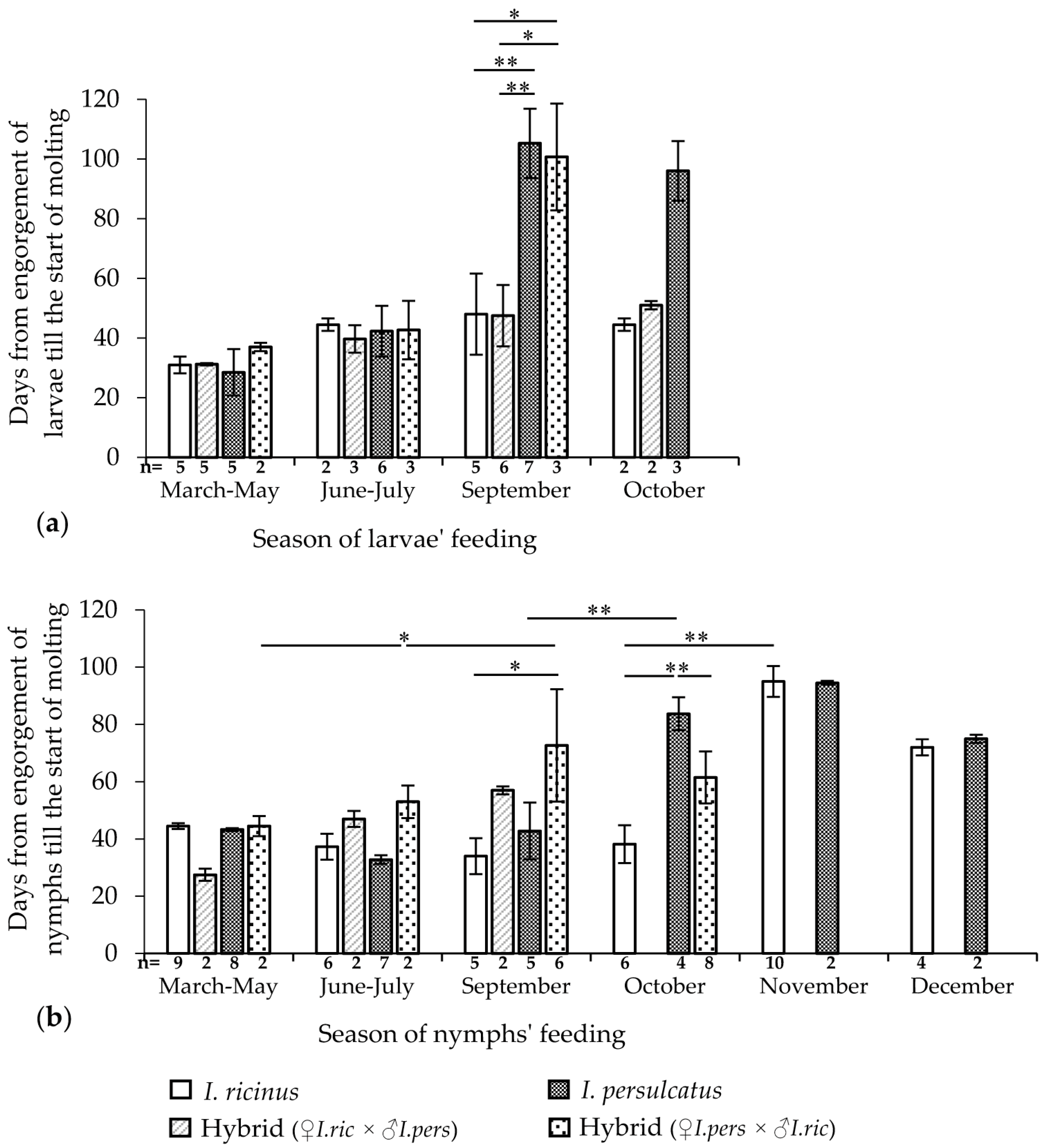
3.2. Development of I. ricinus, I. persulcatus, and Hybrid Larvae and Nymphs under Laboratory Conditions Depending on the Feeding Season
4. Discussion
5. Conclusions
- −
- The mating of I. ricinus and I. persulcatus females with the males of another species leads to a decrease in the engorgement success of the females and a decrease in the number of hatched larvae.
- −
- In our experiments, male and female hybrids were unable to reproduce themselves effectively, even though rare F2 larvae were detected.
- −
- The duration of the feeding of I. ricinus and I. persulcatus larvae significantly differs: the larvae of hybrids from crossing I. ricinus females and I. persulcatus males have a feeding duration similar to that of the mother species, and the feeding time of the larvae of hybrids from another cross is intermediate between the values of the original species.
- −
- Under a natural daylight rhythm, the morphogenetic diapause in the larvae of I. persulcatus and hybrids from crossing I. persulcatus females and I. ricinus males begins in September.
- −
- Under the same experimental conditions, the morphogenetic diapause in I. persulcatus nymphs occurs earlier than in I. ricinus, in October and November, respectively.
- −
- Hybrids generally repeat the features of the life cycle of the mother species.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippova, N.A. Ixodid Ticks of the Subfamily Ixodinae. Fauna SSSR New Ser. 1977, 4, 396. (In Russian) [Google Scholar]
- Kahl, O.; Gray, J.S. The Biology of Ixodes ricinus with Emphasis on Its Ecology. Ticks Tick. Borne. Dis. 2023, 14, 102114. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Liu, J.Y.; Wang, B.Y.; Wang, W.J.; Cui, X.M.; Jiang, J.F.; Sun, Y.; Guo, W.B.; Pan, Y.S.; Zhou, Y.H.; et al. Geographical Distribution of Ixodes persulcatus and Associated Pathogens: Analysis of Integrated Data from a China Field Survey and Global Published Data. One Health 2023, 16, 100508. [Google Scholar] [CrossRef] [PubMed]
- Filippova, N.A. The Sympatry of Closely Related Species of Ixodid Ticks and Its Possible Role in the Parasitic Systems of Natural Foci of Transmissible Diseases. Parazitologiia 1999, 33, 223–241. (In Russian) [Google Scholar] [PubMed]
- Laaksonen, M.; Klemola, T.; Feuth, E.; Sormunen, J.J.; Puisto, A.; Mäkelä, S.; Penttinen, R.; Ruohomäki, K.; Hänninen, J.; Sääksjärvi, I.E.; et al. Tick-Borne Pathogens in Finland: Comparison of Ixodes ricinus and I. persulcatus in Sympatric and Parapatric Areas. Parasites Vectors 2018, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Bormane, A.; Lucenko, I.; Duks, A.; Mavtchoutko, V.; Ranka, R.; Salmina, K.; Baumanis, V. Vectors of Tick-Borne Diseases and Epidemiological Situation in Latvia in 1993–2002. Int. J. Med. Microbiol. Suppl. 2004, 293, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Katargina, O.; Russakova, S.; Geller, J.; Kondrusik, M.; Zajkowska, J.; Zygutiene, M.; Bormane, A.; Trofimova, J.; Golovljova, I. Detection and Characterization of Tick-Borne Encephalitis Virus in Baltic Countries and Eastern Poland. PLoS ONE 2013, 8, e61374. [Google Scholar] [CrossRef] [PubMed]
- Paulauskas, A.; Radzijevskaja, J.; Mardosaite-Busaitiene, D.; Aleksandravičiene, A.; Galdikas, M.; Krikštolaitis, R. New Localities of Dermacentor reticulatus Ticks in the Baltic Countries. Ticks Tick-Borne Dis. 2015, 6, 630–635. [Google Scholar] [CrossRef]
- Filippova, N.A. Forms of Sympatry and Possible Ways of Microevolution of Closely Related Species of The Group Ixodes ricinus—persulcatus (Ixodidae). Acta Zool. Litu. 2002, 12, 215–227. [Google Scholar] [CrossRef]
- Balashov, Y.S. Ixodid Ticks—Parasites and Vectors of Diseases; Nauka: St. Petersburg, Russia, 1998; ISBN 5-02-026082-7. (In Russian) [Google Scholar]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in Ticks of the Medically Important Ixodes kicinus Species Complex. Ticks Tick-Borne Dis. 2016, 7, 992–1003. [Google Scholar] [CrossRef]
- Gray, J.S. The Ecology of Ticks Transmitting Lyme Borreliosis. Exp. Appl. Acarol. 1998, 22, 249–258. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M. (Eds.) Biology of Ticks, 2nd ed.; Oxford University Press: New York, NY, USA, 2013; Volumes 1 and 2. [Google Scholar]
- Kahl, O.; Levin, M.; Beard, C.B. (Eds.) An Annotated Reprint of Yu. S. Balashov’s 1972 Book Bloodsucking Ticks (Ixodoidea)—Vectors of Diseases of Man and Animals; Entomological Society of America: Annapolis, MD, USA, 2023. [Google Scholar] [CrossRef]
- Filippova, N.A. (Ed.) Taiga Tick Ixodes persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, Medical Importance; Nauka: Leningrad, Russia, 1985. (In Russian) [Google Scholar]
- Belozerov, V.N. Diapause and Biological Rhythms in Ticks. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 469–500. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.K.; Jensen, P.M.; et al. Driving Forces for Changes in Geographical Distribution of Ixodes ricinus Ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Belozerov, V.N. Diapause and Quiescence as Two Main Kinds of Dormancy and Their Significance in Life Cycles of Mites and Ticks (Chelicerata: Arachnida: Acari). Part 2. Parasitiformes. Acarina 2009, 17, 3–32. [Google Scholar]
- Korotkov, Y.S. Geographic Variability of Morphogenetic Diapause in Larvae and Nymphs of the Taiga Tick Ixodes persulcatus (Acarina, Ixodidae). Entomol. Rev. 2016, 96, 634–645. [Google Scholar] [CrossRef]
- Balashov, Y.S.; Grigor’eva, L.A.; Oliver, J. Reproductive Isolation and Interspecific Hybridization in Ixodid Ticks of the Ixodes ricinus—I. persulcatus Group (Acarina, Ixodidae). Entomol. Rev. 1998, 78, 500–508. [Google Scholar]
- Tokarevich, N.; Tronin, A.; Gnativ, B.; Revich, B.; Blinova, O.; Evengard, B. Impact of Air Temperature Variation on the Ixodid Ticks Habitat and Tick-Borne Encephalitis Incidence in the Russian Arctic: The Case of the Komi Republic. Int. J. Circumpolar Health 2017, 76, 1298882. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the Geographical Distribution and Abundance of the Tick Ixodes ricinus during the Past 30 Years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef]
- Bugmyrin, S.V.; Bespyatova, L.A.; Korotkov, Y.S.; Burenkova, L.A.; Belova, O.A.; Romanova, L.I.; Kozlovskaya, L.I.; Karganova, G.G.; Ieshko, E.P. Distribution of Ixodes ricinus and I. persulcatus Ticks in Southern Karelia (Russia). Ticks Tick-Borne Dis. 2013, 4, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Soleng, A.; Edgar, K.S.; Paulsen, K.M.; Pedersen, B.N.; Okbaldet, Y.B.; Skjetne, I.E.B.; Gurung, D.; Vikse, R.; Andreassen, K. Distribution of Ixodes ricinus Ticks and Prevalence of Tick-Borne Encephalitis Virus among Questing Ticks in the Arctic Circle Region of Northern Norway. Ticks Tick-Borne Dis. 2018, 9, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slåtsve, A.M.; Stordal, F.; et al. The Distribution Limit of the Common Tick, Ixodes ricinus, and Some Associated Pathogens in North-Western Europe. Ticks Tick-Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef]
- Laaksonen, M.; Sajanti, E.; Sormunen, J.J.; Penttinen, R.; Hänninen, J.; Ruohomäki, K.; Sääksjärvi, I.; Vesterinen, E.J.; Vuorinen, I.; Hytönen, J.; et al. Crowdsourcing-Based Nationwide Tick Collection Reveals the Distribution of Ixodes ricinus and I. persulcatus and Associated Pathogens in Finland. Emerg. Microbes Infect. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kulha, N.; Ruokolainen, K.; Vesterinen, E.J.; Lamppu, M.; Klemola, T.; Sormunen, J.J. Does Environmental Adaptation or Dispersal History Explain the Geographical Distribution of Ixodes ricinus and Ixodes persulcatus Ticks in Finland? Ecol. Evol. 2022, 12, e9538. [Google Scholar] [CrossRef]
- Vikse, R.; Paulsen, K.M.; Edgar, K.S.; Pettersson, J.H.-O.; Ottesen, P.S.; Okbaldet, Y.B.; Kiran, N.; Lamsal, A.; Lindstedt, H.E.H.; Pedersen, B.N.; et al. Geographical Distribution and Prevalence of Tick-Borne Encephalitis Virus in Questing Ixodes ricinus Ticks and Phylogeographic Structure of the Ixodes ricinus Vector in Norway. Zoonoses Public Health 2020, 67, 370–381. [Google Scholar] [CrossRef]
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Nazarova, L.; Katargina, O.; Golovljova, I. Borrelia burgdorferi sensu lato Prevalence in Tick Populations in Estonia. Parasites Vectors 2013, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Kovalevskii, Y.V.; Korenberg, E.I. Differences in Borrelia Infections in Adult Ixodes persulcatus and Ixodes ricinus Ticks (Acari: Ixodidae) in Populations of North-Western Russia. Exp. Appl. Acarol. 1995, 19, 19–29. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Dubinina, H.V.; Van De Pol, I.; Schouls, L.M. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes Ticks in the Baltic Regions of Russia. J. Clin. Microbiol. 2001, 39, 2237–2242. [Google Scholar] [CrossRef]
- Berzina, I.; Capligina, V.; Bormane, A.; Pavulina, A.; Baumanis, V.; Ranka, R.; Granta, R.; Matise, I. Association between Anaplasma phagocytophilum Seroprevalence in Dogs and Distribution of Ixodes ricinus and Ixodes persulcatus Ticks in Latvia. Ticks Tick-Borne Dis. 2013, 4, 83–88. [Google Scholar] [CrossRef]
- Jääskeläinen, A.; Tonteri, E.; Pieninkeroinen, I.; Sironen, T.; Voutilainen, L.; Kuusi, M.; Vaheri, A.; Vapalahti, O. Siberian Subtype Tick-Borne Encephalitis Virus in Ixodes ricinus in a Newly Emerged Focus, Finland. Ticks Tick-Borne Dis. 2016, 7, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, S.Y.; Golovljova, I.V.; Mukhacheva, T.A. Natural Hybridization between Ixodes ricinus and Ixodes persulcatus Ticks Evidenced by Molecular Genetics Methods. Ticks Tick-Borne Dis. 2016, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bugmyrin, S.V.; Belova, O.A.; Ieshko, E.P.; Bespyatova, L.A.; Karganova, G.G. Morphological Differentiation of Ixodes persulcatus and I. ricinus Hybrid Larvae in Experiment and under Natural Conditions. Ticks Tick-Borne Dis. 2015, 6, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Bugmyrin, S.V.; Belova, O.A.; Bespyatova, L.A.; Ieshko, E.P.; Karganova, G.G. Morphological Features of Ixodes persulcatus and I. ricinus Hybrids: Nymphs and Adults. Exp. Appl. Acarol. 2016, 69, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Litov, A.G.; Belova, O.A.; Bugmyrin, S.V.; Kholodilov, I.S.; Romanova, L.I.; Karganova, G.G. Differentiation of Laboratory-Obtained Ixodes ricinus × Ixodes persulcatus Hybrid Ticks: Selection of Suitable Genes. Microorganisms 2022, 10, 1306. [Google Scholar] [CrossRef]
- Fritz, M.L.; Walker, E.D.; Miller, J.R.; Severson, D.W.; Dworkin, I. Divergent Host Preferences of Above- and below-Ground Culex pipiens Mosquitoes and Their Hybrid Offspring. Med. Vet. Entomol. 2015, 29, 115–123. [Google Scholar] [CrossRef]
- Belova, O.A.; Polienko, A.E.; Averianova, A.D.; Karganova, G.G. Hybrids of Ixodes ricinus and Ixodes persulcatus Ticks Effectively Acquire and Transmit Tick-Borne Encephalitis Virus. Front. Cell. Infect. Microbiol. 2023, 13, 1104484. [Google Scholar] [CrossRef]
- Belova, O.A.; Burenkova, L.A.; Karganova, G.G. Different Tick-Borne Encephalitis Virus (TBEV) Prevalences in Unfed versus Partially Engorged Ixodid Ticks—Evidence of Virus Replication and Changes in Tick Behavior. Ticks Tick-Borne Dis. 2012, 3, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Belova, O.A.; Litov, A.G.; Kholodilov, I.S.; Kozlovskaya, L.I.; Bell-Sakyi, L.; Romanova, L.I.; Karganova, G.G. Properties of the Tick-Borne Encephalitis Virus Population during Persistent Infection of Ixodid Ticks and Tick Cell Lines. Ticks Tick-Borne Dis. 2017, 8, 895–906. [Google Scholar] [CrossRef]
- Balashov, Y.S. Bloodsucking Ticks (Ixodoidea)-Vectors of Disease in Man and Animals (English Translation). Misc. Publ. Entomol. Soc. Am. 1972, 8, 161–376. [Google Scholar]
- Voltsit, O.V. Sexual Dimorphism of the Larvae and Nymphs of the Taiga Tick—Ixodes persulcatus. Parazitologiia 1986, 20, 409–412. (In Russian) [Google Scholar] [PubMed]
- Danielová, V.; Daniel, M.; Holubová, J.; Hájková, Z.; Albrecht, V.; Marhoul, Z.; Simonová, V. Influence of Microclimatic Factors on the Development and Virus Infection Rate of Ticks Ixodes ricinus (L.) under Experimental Conditions. Folia Parasitol. 1983, 30, 153–161. [Google Scholar]
- Sirotkin, M.B.; Korenberg, E.I. Thermal Constants of Development of Ixodes persulcatus and Ixodes ricinus Ticks, Which Determine the Duration of Their Life Cycle and Their Distribution. Entomol. Rev. 2022, 102, 257–263. [Google Scholar] [CrossRef]
- Konnai, S.; Saito, Y.; Nishikado, H.; Yamada, S.; Imamura, S.; Mori, A.; Ito, T.; Onuma, M.; Ohashi, K. Establishment of a Laboratory Colony of Taiga Tick Ixodes persulcatus for Tick-Borne Pathogen Transmission Studies. Jpn. J. Vet. Res. 2008, 55, 85–92. [Google Scholar]
- Macleod, J. Ixodes ricinus in Relation to Its Physical Environment: The Influence of Climate on Development. Parasitology 1934, 26, 282–305. [Google Scholar] [CrossRef]
- Honzáková, E. Development of Some Tick Species under Standard Laboratory Conditions. Folia Parasitol. 1971, 18, 357–363. [Google Scholar]
- Babenko, L.V. On the Question of Seasonal Appearances in the Life of the Ticks Ixodes ricinus L. and I. persulcatus P. Sch. Med. Parazitol. Parazit. Bolezn. 1956, 4, 346–352. (In Russian) [Google Scholar]

| Species of Female | Species of Male | Engorgement Success of Females, Abs (%) ** | Mean Weight of Engorged Females, mg ± SD | Number of Females That Laid Eggs, Abs (%) | Number of Batches Where Hatching Occurred, Abs (%) | Success of Larvae Hatching *** (High/Low) |
|---|---|---|---|---|---|---|
| I. persulcatus | I. persulcatus | 54/73 (74.0) a | 339.8 ± 72.1 b | 43/54 (79.6) | 39/43 (90.7) | High |
| I. ricinus | 25/56 (44.6) a,e | 310.5 ± 94.1 c | 23/25 (92.0) | 20/23 (87.0) | Medium | |
| Hybrid (♀I.pers × ♂I.ric) | 2/4 (50) | 267.0 ± 236.2 | 1/2 | 1/1 | Very low (1 LL) | |
| I. ricinus | I. ricinus | 60/82 (73.2) | 284.7 ± 98.6 b | 51/60 (85) | 50/51 (98.0) d | High |
| I. persulcatus | 41/64 (64.1) e | 254.7 ± 81.8 c | 29/41 (70.7) | 26/29 (89.7) | Medium | |
| Hybrid (♀I.pers × ♂I.ric) | 4/7 (57.1) | 217.0 ± 74.6 | 4/4 (100) | 2/4 (50) d | Very low (1–2 LL) | |
| Hybrid (♀I.pers × ♂I.ric) * | I. ricinus | 3/10 (30.0) | 340.0 ± 17.4 | 2/3 (66.7) | 0/2 | - |
| Hybrid (♀I.pers × ♂I.ric) | 14/26 (53.9) | 163.5 ± 89.9 | 9/14 (64.3) | 2/7 (28.6) | Very low (1 LL) | |
| Hybrid (♀I.ric × ♂I.pers) | I. ricinus | 0/4 | - | - | - | - |
| I. persulcatus | 2/4 (50) | 76.5 ± 68.6 | 0/2 | - | - | |
| Hybrid (♀I.ric × ♂I.pers) | 1/4 (25) | 35 | 0/1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belova, O.A.; Polienko, A.E.; Averianova, A.D.; Karganova, G.G. Development Features of Ixodes ricinus × I. persulcatus Hybrids under Laboratory Conditions. Microorganisms 2023, 11, 2252. https://doi.org/10.3390/microorganisms11092252
Belova OA, Polienko AE, Averianova AD, Karganova GG. Development Features of Ixodes ricinus × I. persulcatus Hybrids under Laboratory Conditions. Microorganisms. 2023; 11(9):2252. https://doi.org/10.3390/microorganisms11092252
Chicago/Turabian StyleBelova, Oxana A., Alexandra E. Polienko, Anastasia D. Averianova, and Galina G. Karganova. 2023. "Development Features of Ixodes ricinus × I. persulcatus Hybrids under Laboratory Conditions" Microorganisms 11, no. 9: 2252. https://doi.org/10.3390/microorganisms11092252





