Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anti-LAM Antibodies
2.2. Ethics Statement
2.3. Bacterial Strains
2.4. Study Design and Participants
2.5. Patient Categories
2.6. ELISA
2.7. Statistics
3. Results
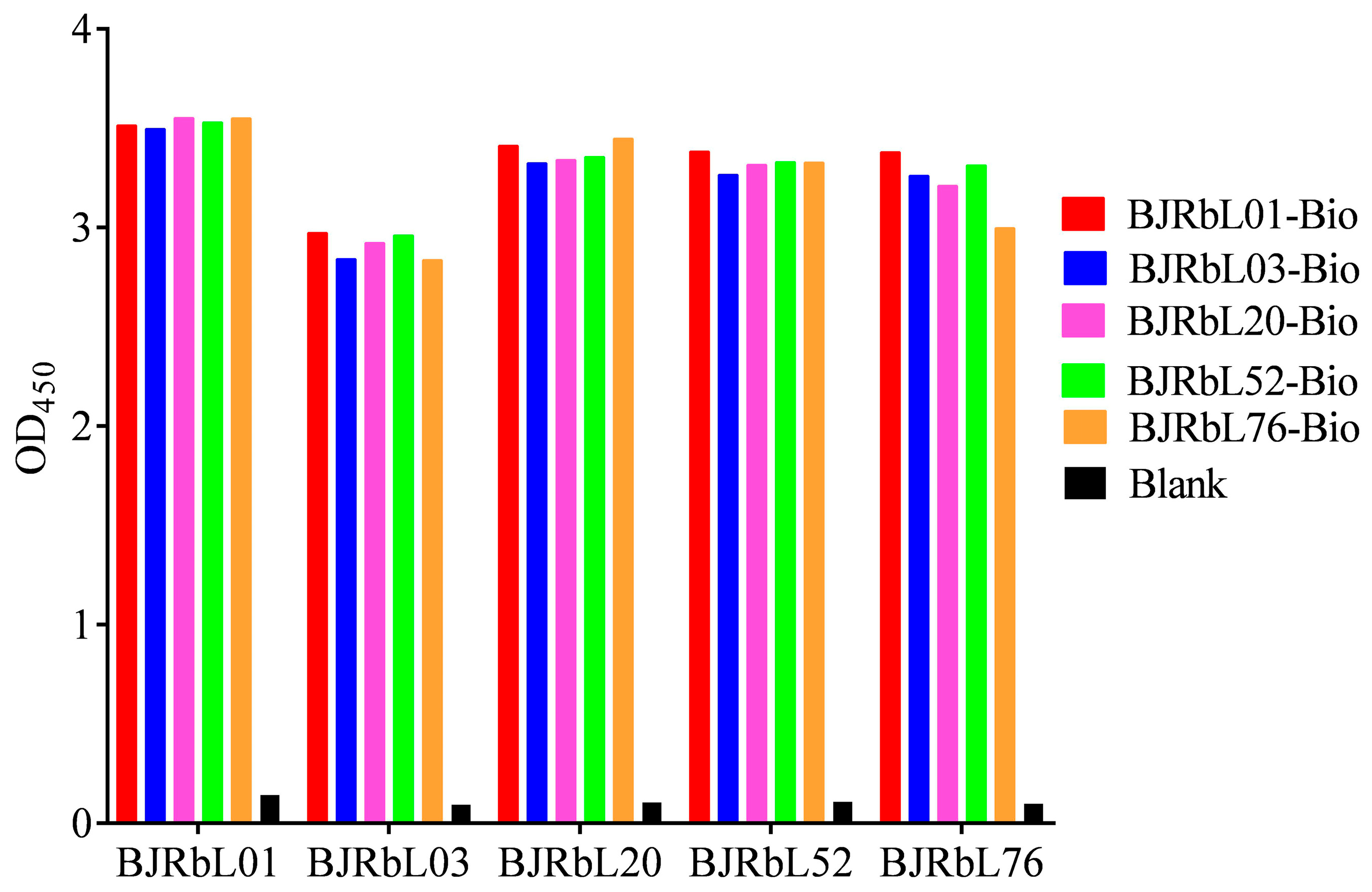
3.1. Screening of Paired Anti-LAM Antibodies by Sandwich ELISA
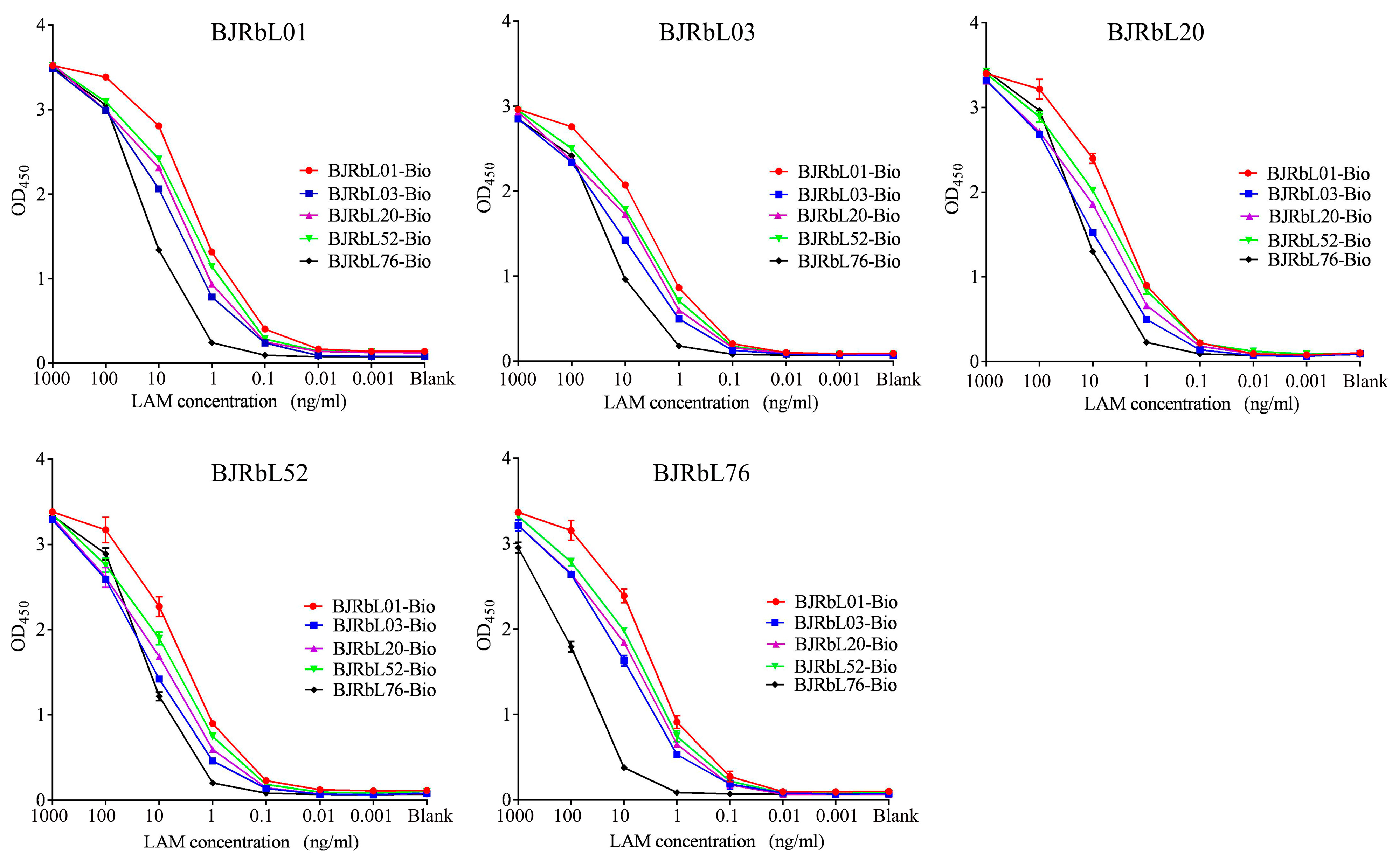
3.2. Sensitivity of the Paired Anti-LAM mAbs for Purified LAM
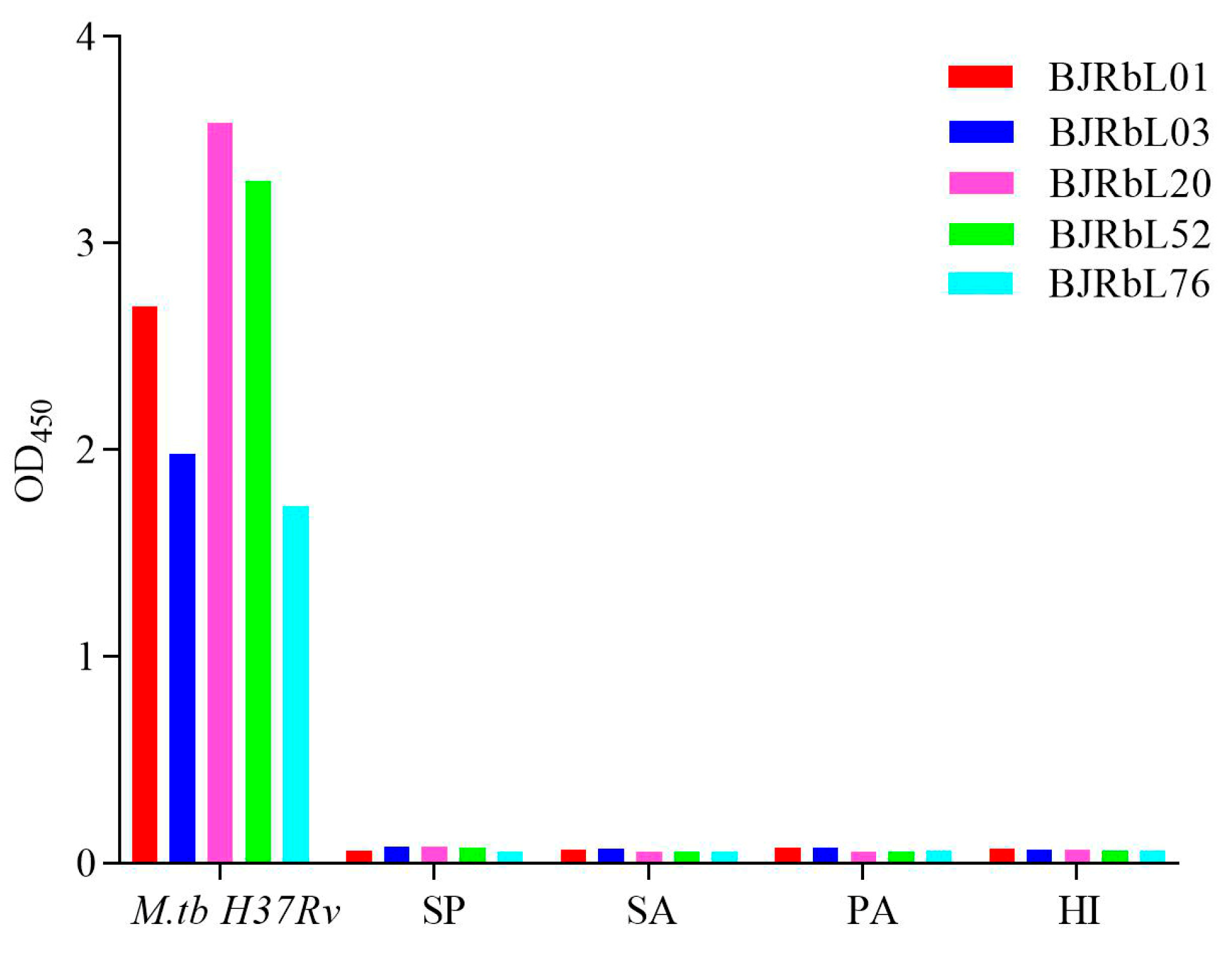
3.3. Reactivity of the Paired Anti-LAM mAbs to Mycobacterial Species
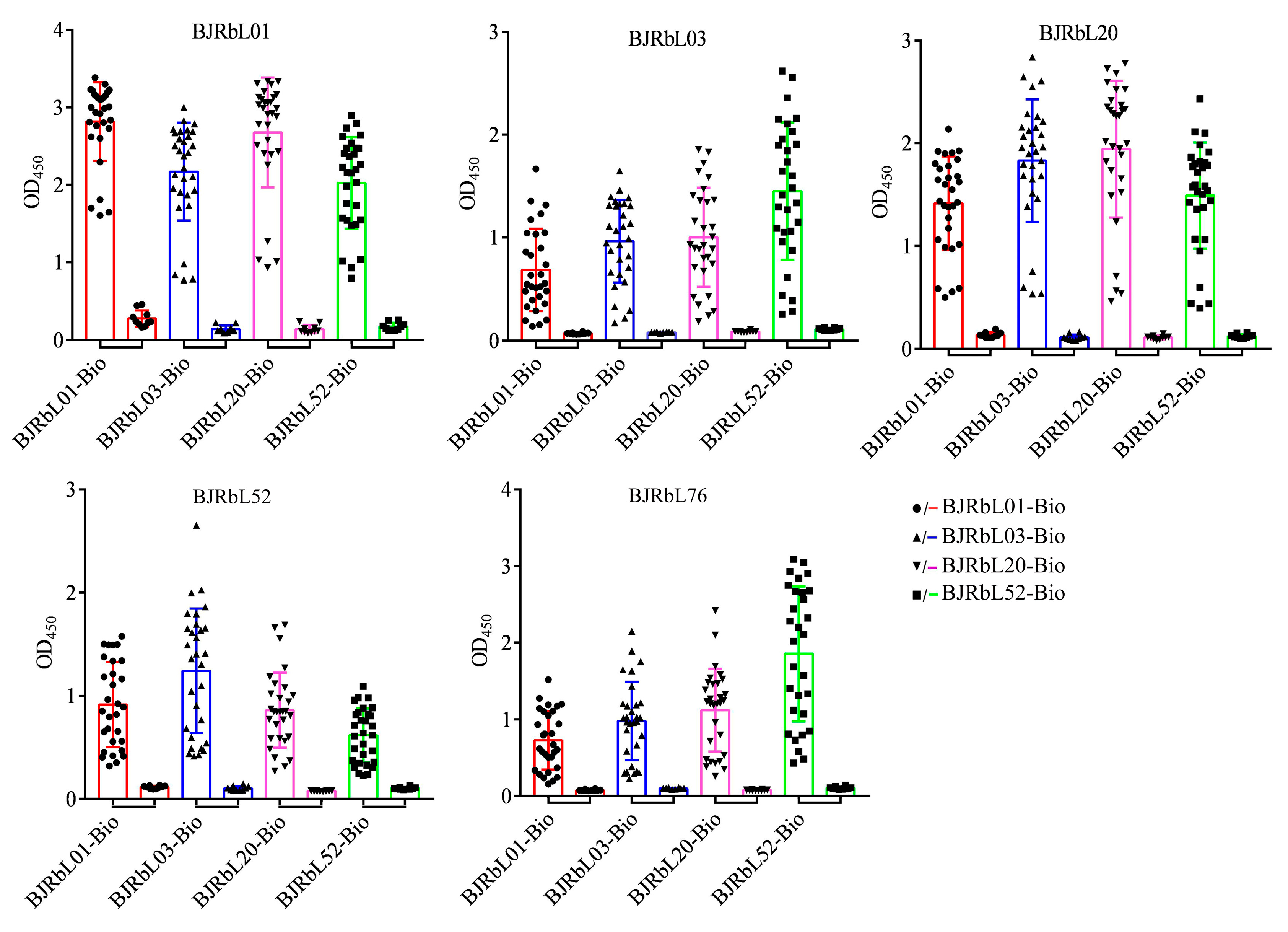
3.4. Reactivity of the Paired Anti-LAM mAbs to Mycobacterial Clinical Isolates
3.5. Reactivity of Anti-LAM mAbs to Common Pneumonia-Causing Pathogenic Bacteria
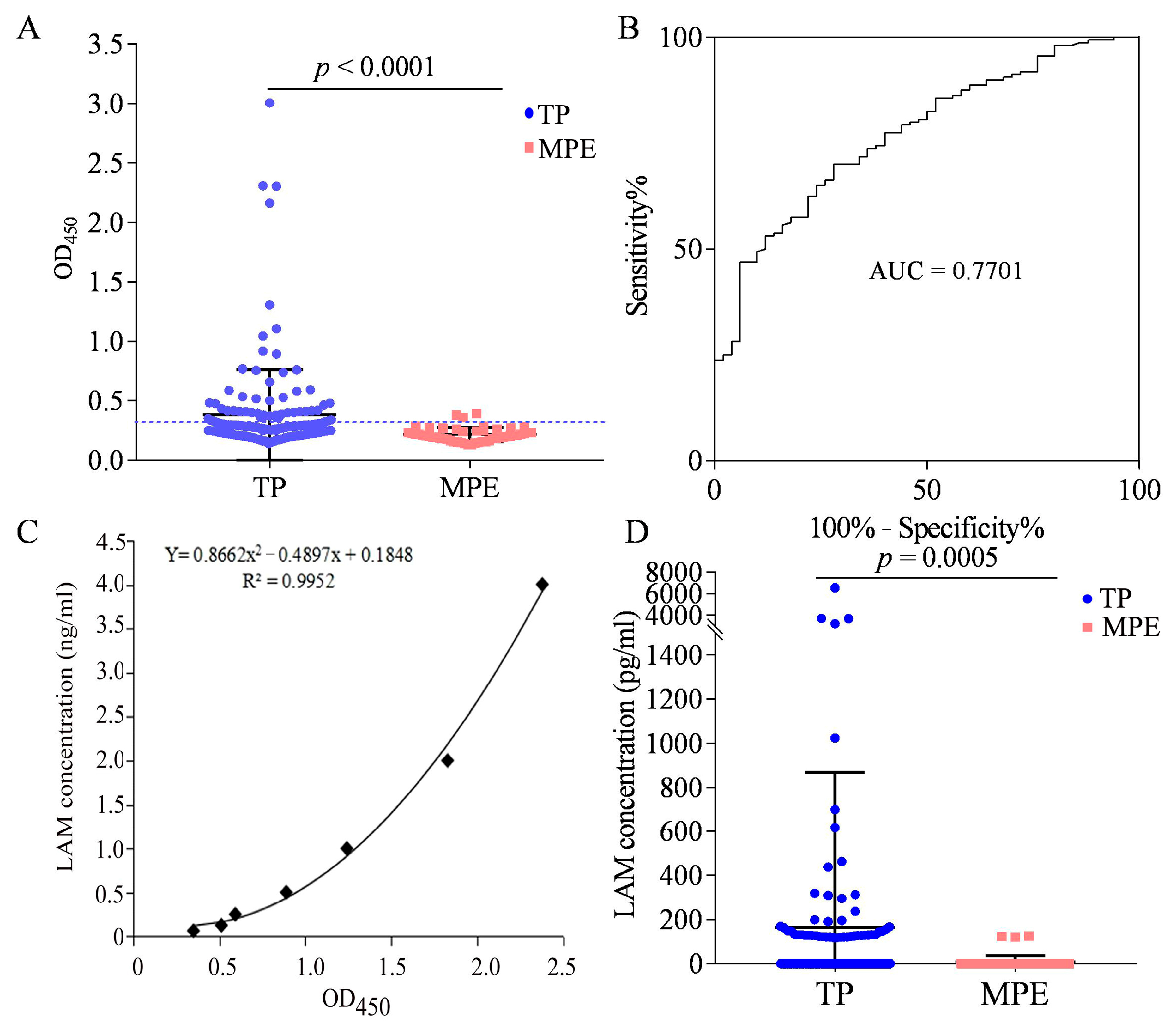
3.6. LAM Detection in the Pleural Fluid of Patients with TP
3.7. LAM Antigen Detection in the Plasma of Patients with TP
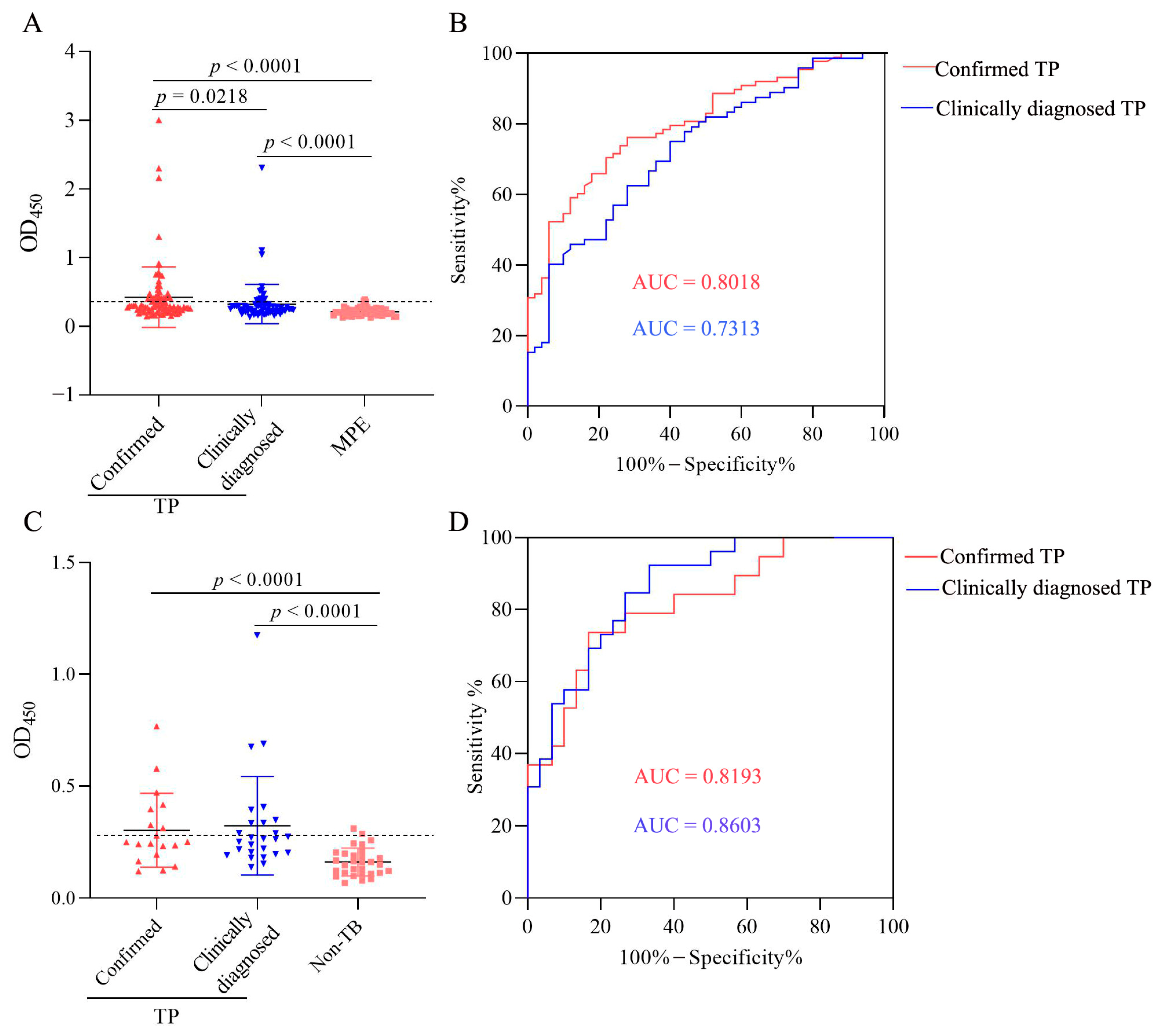
3.8. Characteristics of LAM Level in Confirmed and Clinically Diagnosed TP Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Rodriguez-Takeuchi, S.Y.; Renjifo, M.E.; Medina, F.J. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics 2019, 39, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.; Ravina, M.; Rangan, K.; Dixit, M.; Barai, S.; Bomanji, J.; International Atomic Energy Agency Extra-Pulmonary TBC. Imaging in extrapulmonary tuberculosis. Int. J. Infect. Dis. 2017, 56, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Liu, S.; Du, J.; Tang, P.; Chen, H.; Liu, J.; Ma, J.; Li, M.; Qin, J.; Shu, W.; et al. Epidemiology of concurrent extrapulmonary tuberculosis in inpatients with extrapulmonary tuberculosis lesions in China: A large-scale observational multi-centre investigation. Int. J. Infect. Dis. 2022, 115, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mohan, A.; Kohli, M. Extrapulmonary tuberculosis. Expert. Rev. Respir. Med. 2021, 15, 931–948. [Google Scholar] [CrossRef]
- Ohene, S.A.; Bakker, M.I.; Ojo, J.; Toonstra, A.; Awudi, D.; Klatser, P. Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana. PLoS ONE 2019, 14, e0209650. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, X.; Du, X.; Huang, F.; Wang, N.; Ni, N.; Ren, J.; Zhao, Y.; Jia, Z. Extrapulmonary tuberculosis in China: A national survey. Int. J. Infect. Dis. 2023, 128, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; An, J.; Shu, W.; Huo, F.; Chu, N.; Gao, M.; Qin, S.; Huang, H.; Chen, X.; Xu, S. Epidemiology of Extrapulmonary Tuberculosis among Inpatients, China, 2008–2017. Emerg. Infect. Dis. 2019, 25, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Diacon, A.H.; Koegelenberg, C.F.N. Tuberculous pleural effusion. Respirology 2019, 24, 962–971. [Google Scholar] [CrossRef]
- Shaw, J.A.; Koegelenberg, C.F.N. Pleural Tuberculosis. Clin. Chest Med. 2021, 42, 649–666. [Google Scholar] [CrossRef]
- Gopi, A.; Madhavan, S.M.; Sharma, S.K.; Sahn, S.A. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 2007, 131, 880–889. [Google Scholar] [CrossRef]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, G.R.; Fenton, M.J. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1999, 1, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.; Cancino, J.C.; Chavez-Galan, L. Lipoarabinomannan as a Point-of-Care Assay for Diagnosis of Tuberculosis: How Far Are We to Use It? Front. Microbiol. 2021, 12, 638047. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst. Rev. 2019, 10, CD011420. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Mace, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, A.D.; Sossen, B.; Schutz, C.; Reipold, E.I.; Trollip, A.; Moreau, E.; Schumacher, S.G.; Burton, R.; Ward, A.; Nicol, M.P.; et al. Diagnostic sensitivity of SILVAMP TB-LAM (FujiLAM) point-of-care urine assay for extra-pulmonary tuberculosis in people living with HIV. Eur. Respir. J. 2020, 55, 1901259. [Google Scholar] [CrossRef]
- Muyoyeta, M.; Kerkhoff, A.D.; Chilukutu, L.; Moreau, E.; Schumacher, S.G.; Ruhwald, M. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: A prospective cross-sectional study. Eur. Respir. J. 2021, 58, 2003999. [Google Scholar] [CrossRef]
- Broger, T.; Nicol, M.P.; Sigal, G.B.; Gotuzzo, E.; Zimmer, A.J.; Surtie, S.; Caceres-Nakiche, T.; Mantsoki, A.; Reipold, E.I.; Szekely, R.; et al. Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J. Clin. Investig. 2020, 130, 5756–5764. [Google Scholar] [CrossRef]
- Li, Z.; Tong, X.; Liu, S.; Yue, J.; Fan, H. The Value of FujiLAM in the Diagnosis of Tuberculosis: A Systematic Review and Meta-Analysis. Front. Public. Health. 2021, 9, 757133. [Google Scholar] [CrossRef]
- Yan, Z.H.; Zhao, B.; Pang, Y.; Wang, X.J.; Yi, L.; Wang, H.L.; Yang, B.; Wei, P.J.; Jia, H.Y.; Li, S.P.; et al. Generation of mycobacterial lipoarabinomannan-specific monoclonal antibodies and their ability to identify mycobacterium isolates. J. Microbiol. Immunol. Infect. 2021, 54, 437–446. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Appropriate Course of the Treatment of Tuberculous Pleurisy. ChiCTR-IOR-15006408. Chinese Clinical Trial Register. 2015. Available online: http://www.chictr.org.cn/hvshowproject.aspx?id=12346 (accessed on 26 May 2015).
- Wang, G.; Wang, S.; Yang, X.; Sun, Q.; Jiang, G.; Huang, M.; Huo, F.; Ma, Y.; Chen, X.; Huang, H. Accuracy of Xpert MTB/RIF Ultra for the Diagnosis of Pleural TB in a Multicenter Cohort Study. Chest 2020, 157, 268–275. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission of the People’s Republic of China. Diagnosis for Pulmonary Tuberculosis (WS288-2017). Electron. J. Emerg. Infect. Dis. 2018, 1, 59–61. [Google Scholar]
- Boehme, C.; Molokova, E.; Minja, F.; Geis, S.; Loscher, T.; Maboko, L.; Koulchin, V.; Hoelscher, M. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Meng, Y.; Liu, T.; Peng, W.; Gao, Y.; He, Y.; Qu, R.; Zhang, C.; Hu, W.; Ying, B. Sensitive Urine Immunoassay for Visualization of Lipoarabinomannan for Noninvasive Tuberculosis Diagnosis. ACS Nano 2023, 17, 6998–7006. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.G.; De, P.; Spencer, J.S.; Brennan, P.J.; Daum, J.; Andre, B.G.; Joe, M.; Bai, Y.; Laurentius, L.; Porter, M.D.; et al. Detection of lipoarabinomannan in urine and serum of HIV-positive and HIV-negative TB suspects using an improved capture-enzyme linked immuno absorbent assay and gas chromatography/mass spectrometry. Tuberculosis 2018, 111, 178–187. [Google Scholar] [CrossRef]
- Nigou, J.; Gilleron, M.; Puzo, G. Lipoarabinomannans: From structure to biosynthesis. Biochimie 2003, 85, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Torrelles, J.B. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty026. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Corrigan, D.T.; Ishida, E.; Chatterjee, D.; Lowary, T.L.; Achkar, J.M. Monoclonal antibodies to lipoarabinomannan/arabinomannan—characteristics and implications for tuberculosis research and diagnostics. Trends Microbiol. 2023, 31, 22–35. [Google Scholar] [CrossRef]
- Angala, S.K.; Li, W.; Boot, C.M.; Jackson, M.; McNeil, M.R. Secondary Extended Mannan Side Chains and Attachment of the Arabinan in Mycobacterial Lipoarabinomannan. Commun. Chem. 2020, 3, 101. [Google Scholar] [CrossRef]
- Lisboa, T.; Waterer, G.W.; Lee, Y.C. Pleural infection: Changing bacteriology and its implications. Respirology 2011, 16, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.P.F.; Parrish, J.S. Pleural Space Infections. Life 2023, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Cargill, T.; Harriss, E.; Asciak, R.; Mercer, R.M.; Bedawi, E.O.; McCracken, D.J.; Psallidas, I.; Corcoran, J.P.; Rahman, N.M. The microbiology of pleural infection in adults: A systematic review. Eur. Respir. J. 2019, 54, 1900542. [Google Scholar] [CrossRef] [PubMed]
- Arcos, J.; Sasindran, S.J.; Fujiwara, N.; Turner, J.; Schlesinger, L.S.; Torrelles, J.B. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J. Immunol. 2011, 187, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, T.; Wergeland, I.; Baba, K.; Pathak, S.; Hoosen, A.A.; Dyrhol-Riise, A.M. Mycobacterial antigens in pleural fluid mononuclear cells to diagnose pleural tuberculosis in HIV co-infected patients. BMC Infect. Dis. 2020, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Gayen, S. Malignant Pleural Effusion: Presentation, Diagnosis, and Management. Am. J. Med. 2022, 135, 1188–1192. [Google Scholar] [CrossRef]
- Psallidas, I.; Kalomenidis, I.; Porcel, J.M.; Robinson, B.W.; Stathopoulos, G.T. Malignant pleural effusion: From bench to bedside. Eur. Respir. Rev. 2016, 25, 189–198. [Google Scholar] [CrossRef]


| Detection Antibody | |||||
|---|---|---|---|---|---|
| Capture Antibody | BJRbL01-Bio | BJRbL03-Bio | BJRbL20-Bio | BJRbL52-Bio | BJRbL76-Bio |
| BJRbL01 | 0.1 | 0.1 | 0.1 | 0.1 | 1.0 |
| BJRbL03 | 0.1 | 1.0 | 0.1 | 0.1 | 1.0 |
| BJRbL20 | 0.1 | 1.0 | 0.1 | 0.1 | 1.0 |
| BJRbL52 | 0.1 | 1.0 | 1.0 | 1.0 | 1.0 |
| BJRbL76 | 0.1 | 0.1 | 0.1 | 0.1 | 10.0 |
| Characteristics | TP (n = 160) | MPE (n = 50) | Non-TB (n = 30) |
|---|---|---|---|
| Gender | |||
| Male % (no.) | 69.4% (111) | 50.0% (25) | 56.7% (17) |
| Female % (no.) | 30.6% (49) | 50.0% (25) | 43.3% (13) |
| Age, mean ± SD, years | 36.42 ± 15.91 | 59.82 ± 12.64 | 36.03 ± 10.09 |
| Mycobacterial culture test | |||
| Culture-positive % (no.) | 41.9% (67) | — | — |
| Culture-negative % (no.) | 58.1% (93) | — | — |
| Xpert MTB/RIF assay | |||
| Xpert Mtb positive % (no.) | 41.9% (67) | — | — |
| Xpert Mtb negative % (no.) | 58.1% (93) | — | — |
| Patient subgroups | |||
| Confirmed TP | 55.0% (88) | — | — |
| Clinically diagnosed TP | 45.0% (72) | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Wang, J.; Pang, Y.; Wang, X.; Yi, L.; Wei, P.; Ruan, H.; Gu, M.; Zhang, H.; Yang, X. Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy. Microorganisms 2023, 11, 2259. https://doi.org/10.3390/microorganisms11092259
Yan Z, Wang J, Pang Y, Wang X, Yi L, Wei P, Ruan H, Gu M, Zhang H, Yang X. Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy. Microorganisms. 2023; 11(9):2259. https://doi.org/10.3390/microorganisms11092259
Chicago/Turabian StyleYan, Zhuohong, Jinghui Wang, Yu Pang, Xiaojue Wang, Ling Yi, Panjian Wei, Hongyun Ruan, Meng Gu, Hongtao Zhang, and Xinting Yang. 2023. "Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy" Microorganisms 11, no. 9: 2259. https://doi.org/10.3390/microorganisms11092259
APA StyleYan, Z., Wang, J., Pang, Y., Wang, X., Yi, L., Wei, P., Ruan, H., Gu, M., Zhang, H., & Yang, X. (2023). Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy. Microorganisms, 11(9), 2259. https://doi.org/10.3390/microorganisms11092259







