A New Mechanism of Carbon Metabolism and Acetic Acid Balance Regulated by CcpA
Abstract
:1. Introduction
2. Results
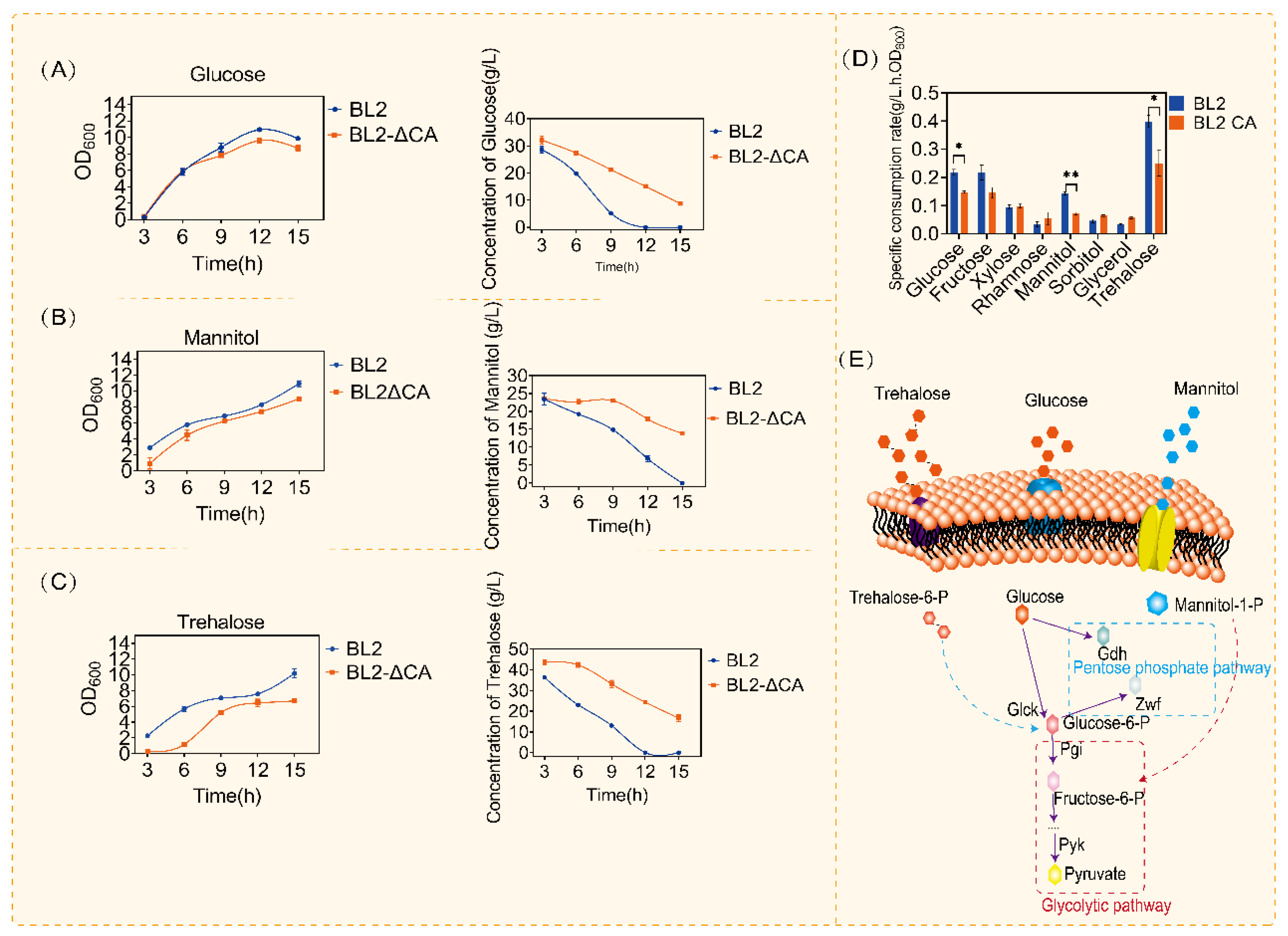
2.1. The Metabolism of Single Carbon Source Containing Six Carbon Sugars Is Obviously Regulated by CcpA
2.2. CcpA Not Only Activates Glycolysis but Also Inhibits Pentose Phosphate Pathway
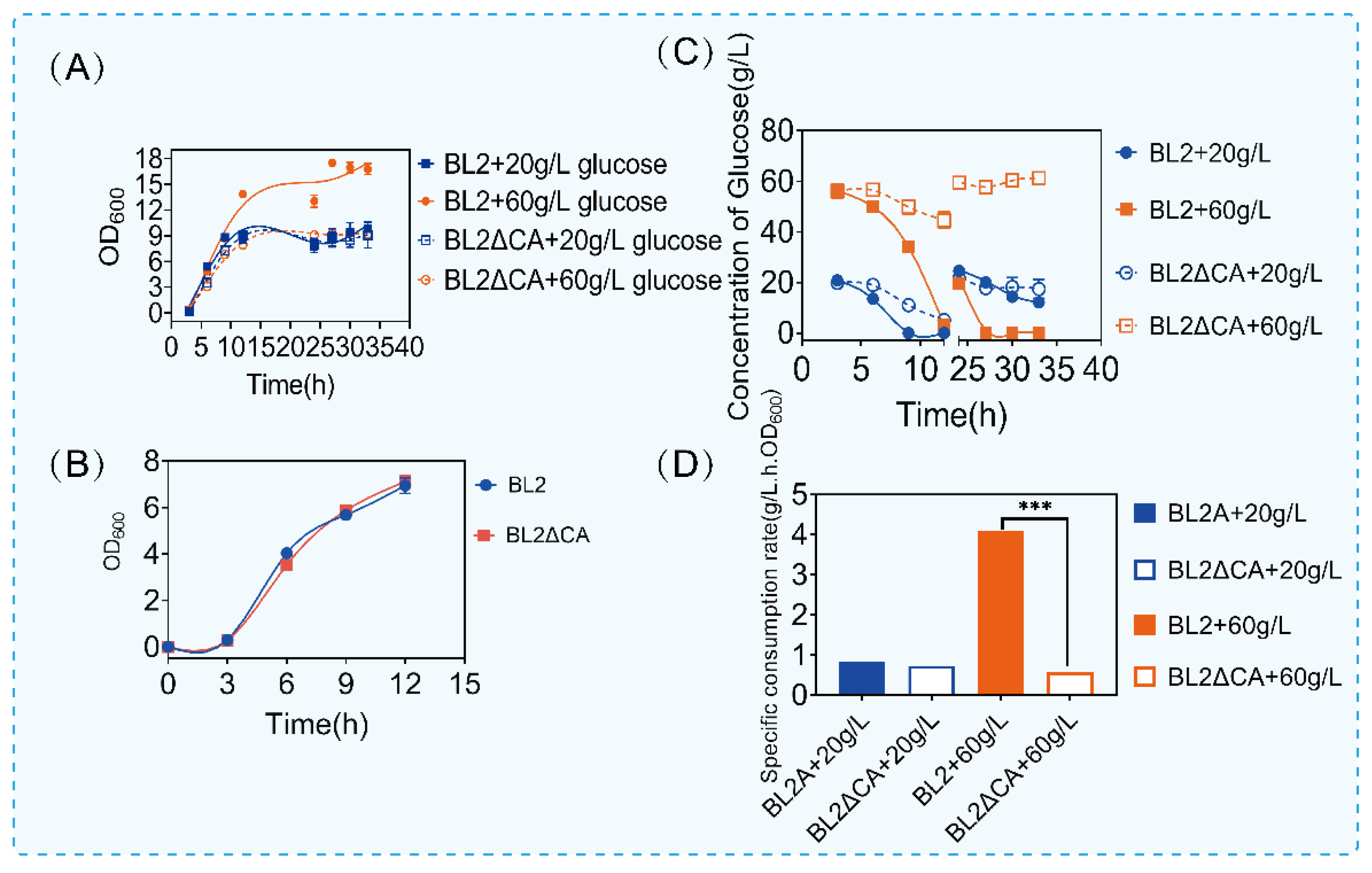
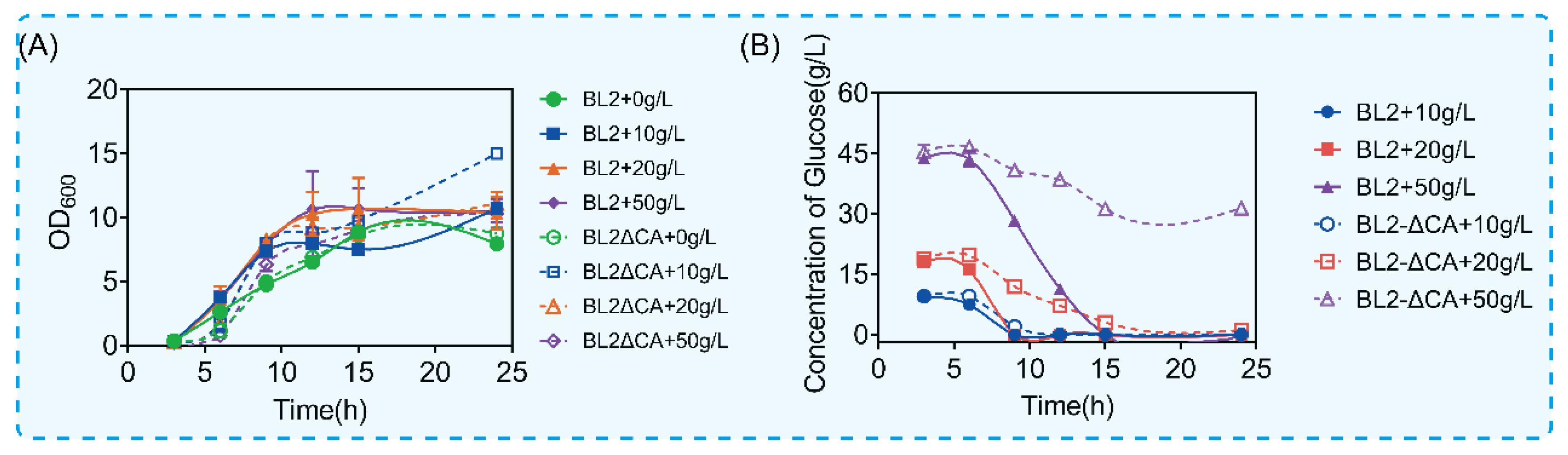
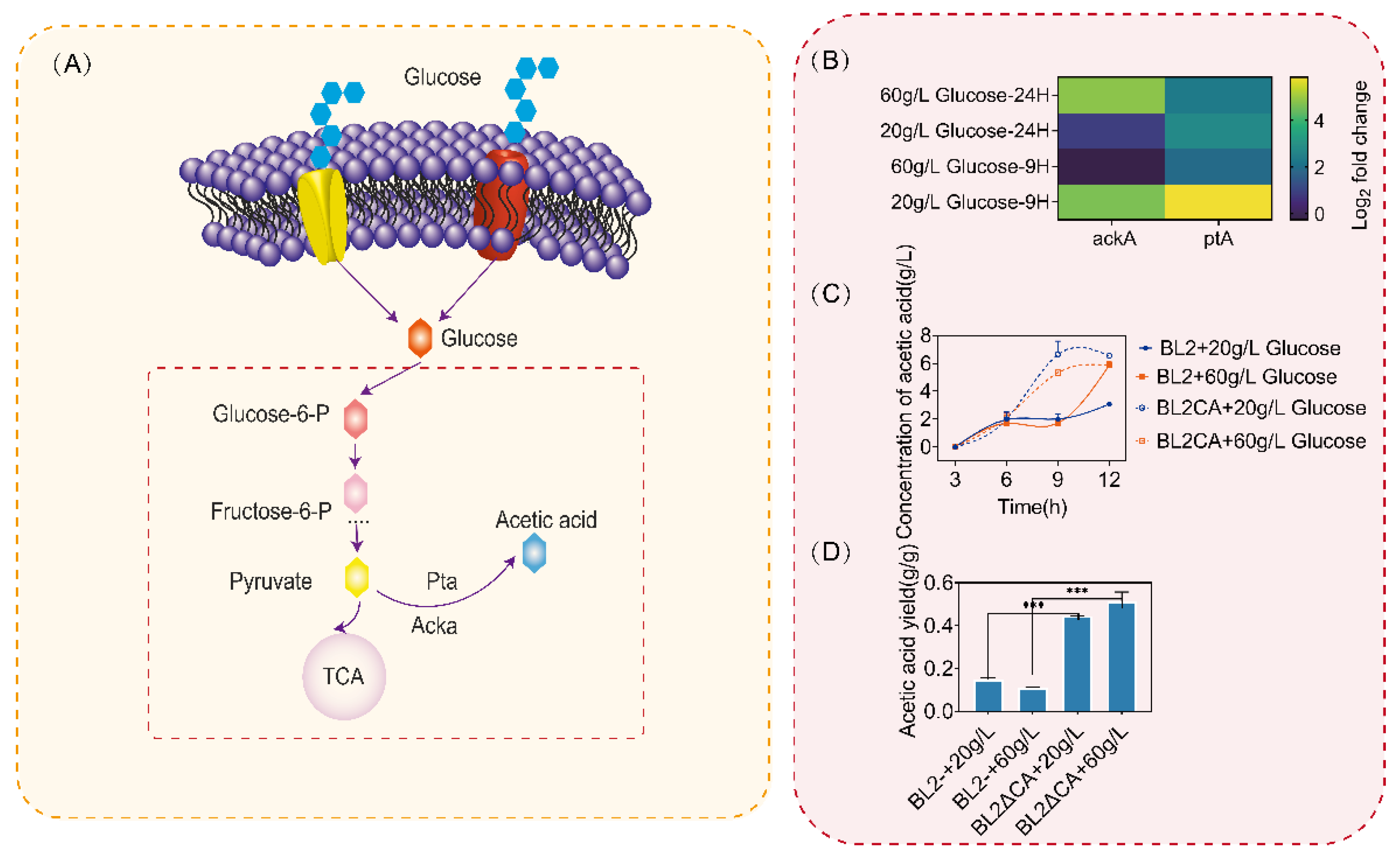
2.3. The Presence of CcpA Can Significantly Inhibit the Metabolism and Synthesis of Acetic Acid
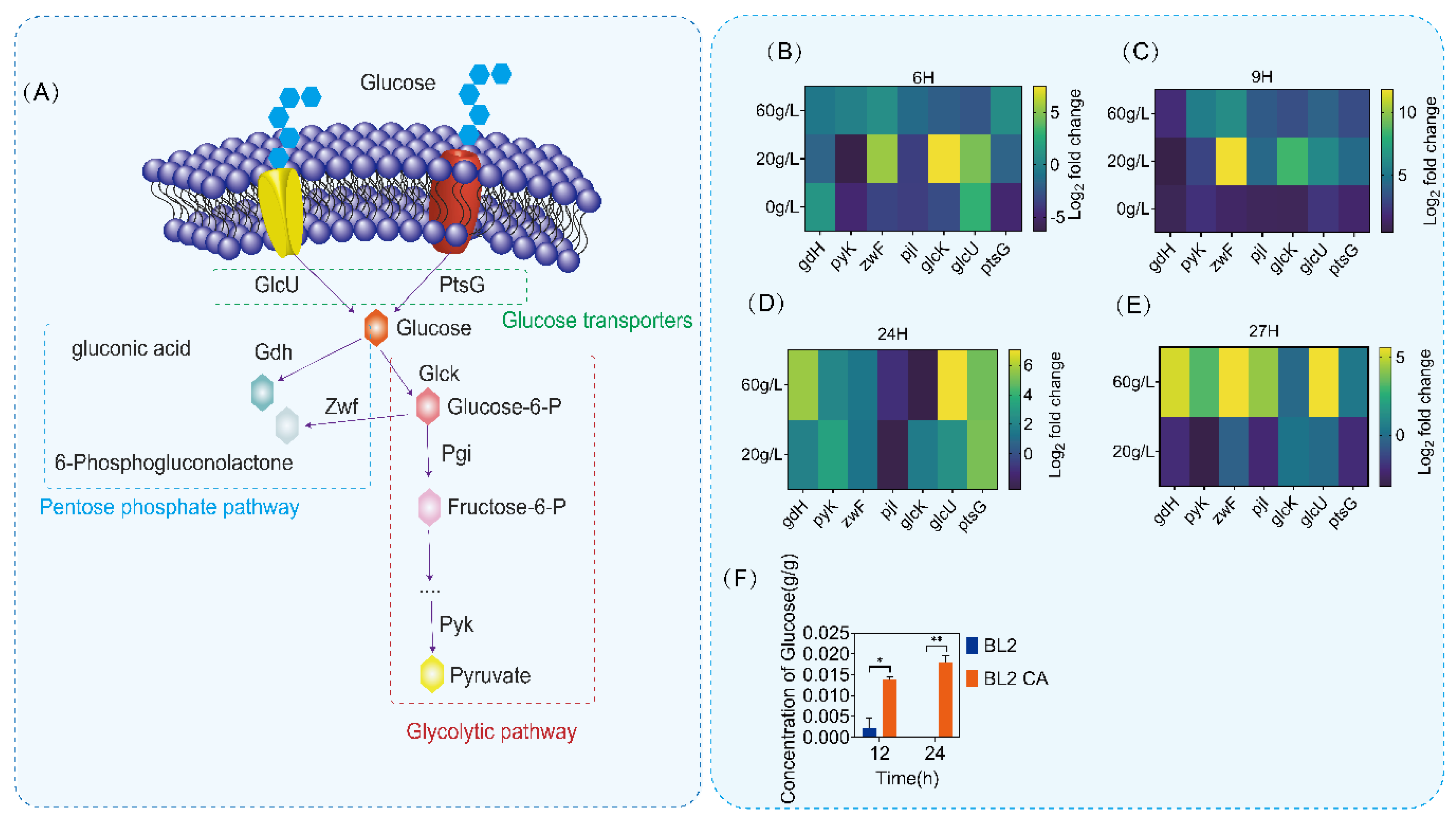
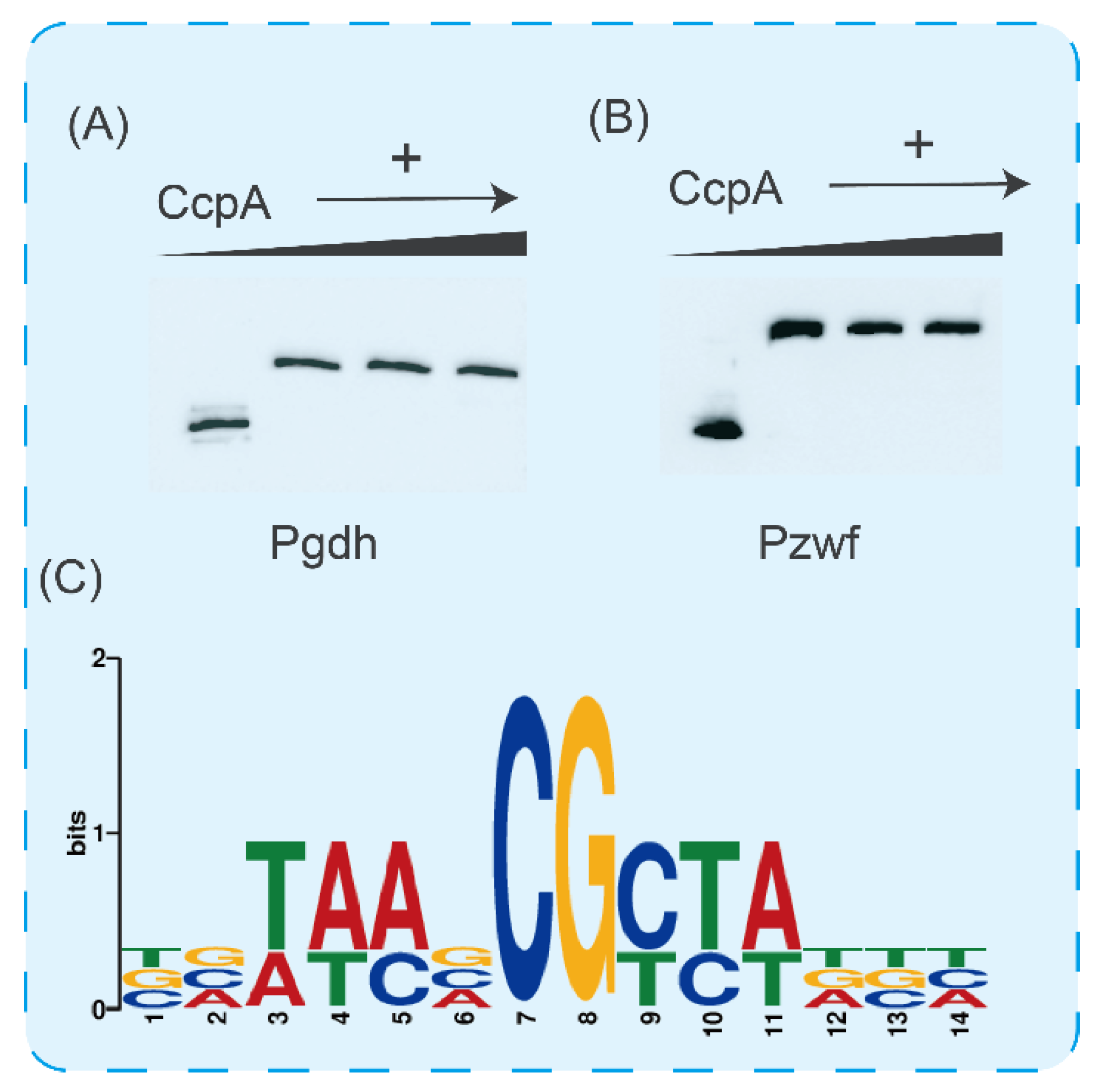
2.4. Molecular Mechanism of Glucose Metabolic Flux Regulated by CcpA
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bosdriesz, E.; Wortel, M.T.; Haanstra, J.R.; Wagner, M.J.; de la Torre Cortés, P.; Teusink, B. Low affinity uniporter carrier proteins can increase net substrate uptake rate by reducing efflux. Sci. Rep. 2018, 8, 5576. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Buskirk, A.R. A ribosome profiling study of mRNA cleavage by the endonuclease RelE. Nucleic Acids Res. 2017, 45, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Schilling, O.; Langbein, I.; Müller, M.; Schmalisch, M.H.; Stülke, J. A protein-dependent riboswitch controlling ptsGHI operon expression in Bacillus subtilis: RNA structure rather than sequence provides interaction specificity. Nucleic Acids Res. 2004, 32, 2853–2864. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Ho, D.H.; Roe, D.G.; Choi, Y.J.; Kim, M.J.; Kim, U.J.; Le, M.L.; Kim, J.; Kim, S.H.; et al. Neurorobotic approaches to emulate human motor control with the integration of artificial synapse. Sci. Adv. 2022, 8, eabo3326. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Sasaki, D.; Kobayashi, S.; Yamaguchi, A.; Uchikura, H.; Shirai, T.; Sasaki, K.; Kondo, A.; Tsuge, Y. Optimal Ratio of Carbon Flux between Glycolysis and the Pentose Phosphate Pathway for Amino Acid Accumulation in Corynebacterium glutamicum. ACS Synth. Biol. 2020, 9, 1615–1622. [Google Scholar] [CrossRef]
- Barajas, J.M.; Reyes, R.; Guerrero, M.J.; Jacob, S.T.; Motiwala, T.; Ghoshal, K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci. Rep. 2018, 8, 9105. [Google Scholar] [CrossRef]
- Jun, H.S.; Weinstein, D.A.; Lee, Y.M.; Mansfield, B.C.; Chou, J.Y. Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood 2014, 123, 2843–2853. [Google Scholar] [CrossRef]
- Moon, J.-S.; Hisata, S.; Park, M.-A.; DeNicola, G.M.; Ryter, S.W.; Nakahira, K.; Choi, A.M.K. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015, 12, 102–115. [Google Scholar] [CrossRef]
- Lee, J.-H.; Liu, R.; Li, J.; Zhang, C.; Wang, Y.; Cai, Q.; Qian, X.; Xia, Y.; Zheng, Y.; Piao, Y.; et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 2017, 8, 949. [Google Scholar] [CrossRef]
- Ruegg, T.L.; Pereira, J.H.; Chen, J.C.; DeGiovanni, A.; Novichkov, P.; Mutalik, V.K.; Tomaleri, G.P.; Singer, S.W.; Hillson, N.J.; Simmons, B.A.; et al. Jungle Express is a versatile repressor system for tight transcriptional control. Nat. Commun. 2018, 9, 3617. [Google Scholar] [CrossRef]
- Wang, X.; Xia, K.; Yang, X.; Tang, C. Growth strategy of microbes on mixed carbon sources. Nat. Commun. 2019, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Hong, C.P.; Kang, H.A.; Hahn, J.-S. Differential activation mechanisms of two isoforms of Gcr1 transcription factor generated from spliced and un-spliced transcripts in Saccharomyces cerevisiae. Nucleic Acids Res. 2021, 49, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Hong, S. Gene-regulatory interactions in embryonic stem cells represent cell-type specific gene regulatory programs. Nucleic Acids Res. 2017, 45, 10428–10435. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, A.; Aguilar, D.; Aviles, F.X.; Querol, E. TrSDB: A Proteome Database of Transcription Factors. Nucleic Acids Res. 2004, 32, D171–D173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.; Che, H.; Sun, Y.; Wang, S.; Zhan, Y.; Cai, D.; Chen, S. Metabolic Engineering of Bacillus licheniformis for the Bioproduction of Nicotinamide Riboside from Nicotinamide and Glucose. ACS Sustain. Chem. Eng. 2023, 11, 6201–6210. [Google Scholar] [CrossRef]
- Shiying, H.; He, P.; Zhang, Y.; Jiang, M.; Wang, Q.; Yang, S.; Chen, S. Transcription Factor Degu-Mediated Multi-Pathway Regulation on Lichenysin Biosynthesis in Bacillus licheniformis. Metab. Eng. 2022, 74, 108–120. [Google Scholar]
- Sprehe, M.; Seidel, G.; Diel, M.; Hillen, W. CcpA Mutants with Differential Activities in Bacillus subtilis. Microb. Physiol. 2006, 12, 96–105. [Google Scholar] [CrossRef]
- Sadykov, M.R.; Hartmann, T.; Mattes, T.A.; Hiatt, M.; Jann, N.J.; Zhu, Y.; Ledala, N.; Landmann, R.; Herrmann, M.; Rohde, H.; et al. CcpA coordinates central metabolism and biofilm formation in Staphylococcus epidermidis. Microbiology 2011, 157, 3458–3468. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Itzek, A.; Herzberg, M.; Kreth, J. CcpA regulates biofilm formation and competence in Streptococcus gordonii. Mol. Oral Microbiol. 2011, 27, 83–94. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Wang, L.; Yu, H.; Tian, H. CcpA-Dependent Carbon Catabolite Repression Regulates Fructooligosaccharides Metabolism in Lactobacillus plantarum. Front. Microbiol. 2018, 9, 1114. [Google Scholar] [CrossRef]
- Bauer, R.; Mauerer, S.; Spellerberg, B. Regulation of the β-Hemolysin Gene Cluster of Streptococcus anginosus by CcpA. Sci. Rep. 2018, 8, 9028. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.B.; Lolkema, J.S. CcpA-Dependent Carbon Catabolite Repression in Bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y. Directional enhancement of fermentative coproduction of hydrogen and acetic acid from glucose via control of headspace pressure. Int. J. Hydrogen Energy 2017, 42, 4095–4101. [Google Scholar] [CrossRef]
- Fragoso-Jiménez, J.C.; Gutierrez-Rios, R.M.; Flores, N.; Martinez, A.; Lara, A.R.; Delvigne, F.; Gosset, G. Glucose consumption rate-dependent transcriptome profiling of Escherichia coli provides insight on performance as microbial factories. Microb. Cell Fact. 2022, 21, 189. [Google Scholar] [CrossRef]
- Sonenshein, A.L. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 2007, 5, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Grundy, F.J.; Turinsky, A.J.; Henkin, T.M. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 1994, 176, 4527–4533. [Google Scholar] [CrossRef]
- Kim, J.N.; Burne, R.A. CcpA and CodY Coordinate Acetate Metabolism in Streptococcus mutans. Appl. Environ. Microbiol. 2017, 83, e03274-16. [Google Scholar] [CrossRef]
- Wang, J.-T.; Shi, T.-T.; Ding, L.; Xie, J.; Zhao, P.-J. Multifunctional Enzymes in Microbial Secondary Metabolic Processes. Catalysts 2023, 13, 581. [Google Scholar] [CrossRef]
- Mira, N.P.; Henriques, S.F.; Keller, G.; Teixeira, M.C.; Matos, R.G.; Arraiano, C.M.; Winge, D.R.; Sa-Correia, I. Identification of a DNA-Binding Site for the Transcription Factor Haa1, Required for Saccharomyces cerevisiae Response to Acetic Acid Stress. Nucleic Acids Res. 2011, 39, 6896–6907. [Google Scholar] [CrossRef]
- Blencke, H.-M.; Homuth, G.; Ludwig, H.; Mäder, U.; Hecker, M.; Stülke, J. Transcriptional Profiling of Gene Expression in Response to Glucose in Bacillus subtilis: Regulation of the Central Metabolic Pathways. Metab. Eng. 2003, 5, 133–149. [Google Scholar] [CrossRef]
- García-Depraect, O.; Valdez-Vázquez, I.; Rene, E.R.; Gómez-Romero, J.; López-López, A.; León-Becerril, E. Lactate- and acetate-based biohydrogen production through dark co-fermentation of tequila vinasse and nixtamalization wastewater: Metabolic and microbial community dynamics. Bioresour. Technol. 2019, 282, 236–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xiao, F.; Wang, H.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Engineering of a Biosensor in Response to Malate in Bacillus licheniformis. ACS Synth. Biol. 2021, 10, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Zhang, Y.; Wang, H.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. A new CcpA binding site plays a bidirectional role in carbon catabolism in Bacillus licheniformis. iScience 2021, 24, 102400. [Google Scholar] [CrossRef] [PubMed]
- Tobisch, S.; Zühlke, D.; Bernhardt, J.; Stülke, J.; Hecker, M. Role of CcpA in Regulation of the Central Pathways of Carbon Catabolism in Bacillus subtilis. J. Bacteriol. 1999, 181, 6996–7004. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xiao, F.; Wang, H.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. CcpA mutants influence selective carbon source utilization by changing interactions with target genes in Bacillus licheniformis. Syst. Microbiol. Biomanufacturing 2021, 2, 193–207. [Google Scholar] [CrossRef]
- Chen, C.; Huang, K.; Li, X.; Tian, H.; Yu, H.; Huang, J.; Yuan, H.; Zhao, S.; Shao, L. Effects of CcpA against salt stress in Lactiplantibacillus plantarum as assessed by comparative transcriptional analysis. Appl. Microbiol. Biotechnol. 2021, 105, 3691–3704. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Ren, C.; Yang, C.; Yang, S.; Gu, Y.; Jiang, W. Molecular modulation of pleiotropic regulator CcpA for glucose and xylose coutilization by solvent-producing Clostridium acetobutylicum. Metab. Eng. 2015, 28, 169–179. [Google Scholar] [CrossRef]
- Yu, W.; Chen, Z.; Ye, H.; Liu, P.; Li, Z.; Wang, Y.; Li, Q.; Yan, S.; Zhong, C.-J.; He, N. Effect of Glucose on Poly-γ-Glutamic Acid Metabolism in Bacillus licheniformis. Microb. Cell Fact. 2017, 16, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Yang, Y.; Jiang, W.; Gu, Y. A Novel Dual-cre Motif Enables Two-Way Autoregulation of CcpA in Clostridium acetobutylicum. Appl. Environ. Microbiol. 2018, 84, e00114-18. [Google Scholar] [CrossRef]
- Kim, J.-H.; Chambliss, G.H. Contacts between Bacillus subtilis Catabolite Regulatory Protein CcpA and amyO Target Site. Nucleic Acids Res. 1997, 25, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Funk, I.; Sieber, V.; Schmid, J. Effects of glucose concentration on 1,18-cis-octadec-9-enedioic acid biotransformation efficiency and lipid body formation in Candida tropicalis. Sci. Rep. 2017, 7, 13842. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Jose, S.; Mulakkal, J.; Karpinsky-Semper, D.; Swick, A.G.; Krishnakumar, I.M. Water-soluble polyphenol-rich clove extract lowers pre- and post-prandial blood glucose levels in healthy and prediabetic volunteers: An open label pilot study. BMC Complement. Altern. Med. 2019, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Tikariha, H.; Dafale, N.A.; Purohit, H.J. Exploring the rearrangement of sensory intelligence in proteobacteria: Insight of Pho regulon. World J. Microbiol. Biotechnol. 2018, 34, 172. [Google Scholar] [CrossRef]
- Gerstmeir, R.; Wendisch, V.F.; Schnicke, S.; Ruan, H.; Farwick, M.; Reinscheid, D.; Eikmanns, B.J. Acetate metabolism and its regulation in Corynebacterium glutamicum. J. Biotechnol. 2003, 104, 99–122. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Yin, H.; Bai, X.; Chang, Y.; Xia, M.; Wang, M. Acetobacter pasteurianus metabolic change induced by initial acetic acid to adapt to acetic acid fermentation conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7007–7016. [Google Scholar] [CrossRef]
- Chen, H.; Liu, K.; Yang, E.; Chen, J.; Gu, Y.; Wu, S.; Yang, M.; Wang, H.; Wang, D.; Li, H. A critical review on microbial ecology in the novel biological nitrogen removal process: Dynamic balance of complex functional microbes for nitrogen removal. Sci. Total Environ. 2023, 857, 159462. [Google Scholar] [CrossRef]
- Bernal, V.; Castaño-Cerezo, S.; Cánovas, M. Acetate Metabolism Regulation in Escherichia coli: Carbon Overflow, Pathogenicity, and Beyond. Appl. Microbiol. Biotechnol. 2016, 100, 8985–9001. [Google Scholar] [CrossRef]
- Gerstmeir, R.; Cramer, A.; Dangel, P.; Schaffer, S.; Eikmanns, B.J. RamB, a Novel Transcriptional Regulator of Genes Involved in Acetate Metabolism of Corynebacterium glutamicum. J. Bacteriol. 2004, 186, 2798–2809. [Google Scholar] [CrossRef]
| Strain | Description | Reference |
|---|---|---|
| Strains | ||
| Escherichia coli JM109 | F′, traD36, proAB + lacIq, Δ(lacZ), M15/Δ (lac-proAB), gln V44, e14−, gyrA96, recA1, relA1, endA1, thi, hsdR17 (CICIM B0012) | CICIM-CU |
| E. coli BL21(DE3) | F-ompT gal dcm lon hsdSB (rB-mB) λ(DE3) | CICIM-CU |
| Bacillus licheniformis CICIM B1391 | wild-type (CICIM B1391) | CICIM-CU |
| Bacillus licheniformis CA | B. licheniformis CICIM B1391, ΔccpA | CICIM-CU |
| BL21pETPA | BL21, harboring pET28aPA | CICIM-CU |
| Primers | Sequence |
|---|---|
| PtsG-Q-F | tacgaatcaggcgggagaaattgtc |
| PtsG-Q-R | aacccataataccggcaacaagc |
| GlcU-Q-F | aatcagctgaaaagcatcaagttgatcg |
| GlcU-Q-R | ttccagcgatgtcagcacgatt |
| GlcK-Q-F | gcggcattattgtaaacggagaaatc |
| GlcK-Q-R | cgcaagtctgtatctagatgaccgg |
| Pji-Q-F | gcgaaacgccgcatttatgc |
| Pji-Q-R | tcatttcatcgatatcggctccgc |
| ZwF-Q-F | aacaggggatttggcaaaacgaa |
| ZwF-Q-R | gtgagatgtaaactcgtccacatcct |
| Pyk-Q-F | tccttcttgatacgaaaggtccgg |
| Pyk-Q-R | ccatcgtcaagcaaaatcgttgaacc |
| Gdh-Q-F | gtaccttctgaggatttgtctctggaag |
| Gdh-Q-R | ttgcttgccgcataatggacaaaat |
| RpsE-F | tggtcgtcgtttccgcttcg |
| RpsE-R | tcgcttctggtacttcttgtgctt |
| ackA-Q-F | acaccgcgtcgtacacg |
| ackA-Q-R | gcattgtttggtggaatgccg |
| pta-Q-F | cgtatttgttttcccaagccttgaag |
| pta-Q-R | caaaaacaaattacagcgcttgaactgc |
| Pgdh-F-Bio | ttcttatgtactccctccataaccgct |
| Pgdh-R | aaaagaaggcggggtgccttc |
| Pzwf-Bio-F | atatcctttcctcctgctaaaaacttcatctatc |
| Pzwf-R | attaaacgtacctcactttattcgaagctaagt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiao, F.; Zhang, L.; Ding, Z.; Shi, G.; Li, Y. A New Mechanism of Carbon Metabolism and Acetic Acid Balance Regulated by CcpA. Microorganisms 2023, 11, 2303. https://doi.org/10.3390/microorganisms11092303
Zhang Y, Xiao F, Zhang L, Ding Z, Shi G, Li Y. A New Mechanism of Carbon Metabolism and Acetic Acid Balance Regulated by CcpA. Microorganisms. 2023; 11(9):2303. https://doi.org/10.3390/microorganisms11092303
Chicago/Turabian StyleZhang, Yupeng, Fengxu Xiao, Liang Zhang, Zhongyang Ding, Guiyang Shi, and Youran Li. 2023. "A New Mechanism of Carbon Metabolism and Acetic Acid Balance Regulated by CcpA" Microorganisms 11, no. 9: 2303. https://doi.org/10.3390/microorganisms11092303





