Komagataella phaffii as a Platform for Heterologous Expression of Enzymes Used for Industry
Abstract
:1. Introduction
2. Specific Features of K. phaffii as a Producer of Heterologous Proteins
2.1. Methods and Approaches Used to Create Strains from K. phaffii
2.1.1. Promoters for the Expression of Heterologous Genes in K. phaffii
2.1.2. The Choice of a Signal Sequence
2.1.3. Specifics of Introduction of Foreign DNA into K. phaffii
2.1.4. An Increase in the Copy Number of a Target Gene as a Way to Promote Protein Production
2.2. Bottlenecks of Secretion in K. phaffii: The Unfolded Protein Response (UPR) and Vacuolar Degradation of Heterologous Proteins
2.3. Distinctive Features of Post-Translational Modifications in K. phaffii and Their Impact on the Production of a Heterologous Protein
2.3.1. Introduction of Disulfide Bonds
2.3.2. Glycosylation
3. Enzyme-Based Supplements for Preparation of Feeds
4. K. phaffii as a Producer of Enzymes for Increasing Nutritional Value of Feed
4.1. K. phaffii as a Producer of Phosphohydrolytic Enzymes
Phytase
4.2. K. phaffii as a Producer of Enzymes That Hydrolyze Starch
4.2.1. α-Amylase
4.2.2. Glucoamylase
4.3. K. phaffii as a Producer of Enzymes That Hydrolyze Nonstarch Polysaccharides
4.3.1. Xylanase
4.3.2. Mannanases
4.3.3. Cellulases and Laccases
4.4. K. phaffii as a Protease Producer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pope, B.; Kent, H.M. High efficiency 5 min transformation of Escherichia coli. Nucleic Acids Res. 1996, 24, 536–537. [Google Scholar] [CrossRef]
- Sørensen, H.P.; Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Murray, V.; Cui, C.; Zhang, Y.; Wang, J.; Li, Q. Practical protocols for production of very high yields of recombinant proteins using Escherichia coli. Protein Sci. 2009, 18, 936–948. [Google Scholar] [CrossRef]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Goodarzi, M.T.; Saidijam, M.; Safavieh, S.S. An optimized protocol for overproduction of recombinant protein expression in Escherichia coli. Prep. Biochem. Biotechnol. 2014, 44, 510–528. [Google Scholar] [CrossRef]
- Ahmad, I.; Nawaz, N.; Darwesh, N.M.; ur Rahman, S.; Mustafa, M.Z.; Khan, S.B.; Patching, S.G. Overcoming challenges for amplified expression of recombinant proteins using Escherichia coli. Protein Expr. Purif. 2018, 144, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Y.; Unger, T. Resolving bottlenecks for recombinant protein expression in E. coli. Methods Mol. Biol. 2012, 800, 173–186. [Google Scholar] [CrossRef]
- Lee, S.Y. High cell-density culture of Escherichia coli. Trends Biotechnol. 1996, 14, 98–105. [Google Scholar] [CrossRef]
- Shiloach, J.; Fass, R. Growing E. coli to high cell density—A historical perspective on method development. Biotechnol. Adv. 2005, 23, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeil, M.; Azizi-Dargahlou, S. Factors involved in heterologous expression of proteins in E. coli host. Arch. Microbiol. 2023, 205, 212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tyo, K.E.J.; Martínez, J.L.; Petranovic, D.; Nielsen, J. Different expression systems for production of recombinant proteins in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 1259–1268. [Google Scholar] [CrossRef]
- Gündüz Ergün, B.; Hüccetoğulları, D.; Öztürk, S.; Çelik, E.; Çalık, P. Established and upcoming yeast expression systems. In Recombinant Protein Production in Yeast; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1923, pp. 1–74. [Google Scholar] [CrossRef]
- Delic, M.; Valli, M.; Graf, A.B.; Pfeffer, M.; Mattanovich, D.; Gasser, B. The secretory pathway: Exploring yeast diversity. FEMS Microbiol. Rev. 2013, 37, 872–914. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, X.; Nielsen, J.; Petranovic, D.; Siewers, V. Expression of antibody fragments in Saccharomyces cerevisiae strains evolved for enhanced protein secretion. Microb. Cell Fact. 2021, 20, 134. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Fonseca, C.; Spencer-Martins, I.; Gorwa-Grauslund, M.F. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef]
- Hasunuma, T.; Ishii, J.; Kondo, A. Rational design and evolutional fine tuning of Saccharomyces cerevisiae for biomass breakdown. Curr. Opin. Chem. Biol. 2015, 29, 1–9. [Google Scholar] [CrossRef]
- Morton, C.L.; Potter, P.M. Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda, and COS7 cells for recombinant gene expression. Application to a rabbit liver carboxylesterase. Mol. Biotechnol. 2000, 16, 193–202. [Google Scholar] [CrossRef]
- Çelik, E.; Çalik, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv. 2012, 30, 1108–1118. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, J.; Martínez, J.L.; Petranovic, D.; Nielsen, J. Correlation of cell growth and heterologous protein production by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 8955–8962. [Google Scholar] [CrossRef]
- Nasab, F.P.; Aebi, M.; Bernhard, G.; Frey, A.D. A combined system for engineering glycosylation efficiency and glycan structure in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 997–1007. [Google Scholar] [CrossRef]
- Xie, Y.; Han, X.; Miao, Y. An effective recombinant protein expression and purification system in Saccharomyces cerevisiae. Curr. Protoc. Mol. Biol. 2018, 123, e62. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Guan, B.; Chen, F.; Su, S.; Duan, Z.; Chen, Y.; Li, H.; Jin, J. Effects of co-overexpression of secretion helper factors on the secretion of a HSA fusion protein (IL2-HSA) in Pichia pastoris. Yeast 2016, 33, 587–600. [Google Scholar] [CrossRef]
- Ata, Ö.; Ergün, B.G.; Fickers, P.; Heistinger, L.; Mattanovich, D.; Rebnegger, C.; Gasser, B. What makes Komagataella phaffii non-conventional? FEMS Yeast Res. 2021, 21, foab059. [Google Scholar] [CrossRef]
- Yamada, Y.; Matsuda, M.; Maeda, K.; Mikata, K. The phylogenetic relationships of methanol-assimilating yeasts based on the partial sequences of 18S and 26S ribosomal RNAs: The proposal of Komagataella gen. nov. (Saccharomycetaceae). Biosci. Biotechnol. Biochem. 1995, 59, 439–444. [Google Scholar] [CrossRef]
- Spohner, S.C.; Müller, H.; Quitmann, H.; Czermak, P. Expression of enzymes for the usage in food and feed industry with Pichia pastoris. J. Biotechnol. 2015, 202, 118–134. [Google Scholar] [CrossRef]
- Bustos, C.; Quezada, J.; Veas, R.; Altamirano, C.; Braun-Galleani, S.; Fickers, P.; Berrios, J. Advances in cell engineering of the Komagataella phaffii platform for recombinant protein production. Metabolites 2022, 12, 346. [Google Scholar] [CrossRef]
- Tachioka, M.; Sugimoto, N.; Nakamura, A.; Sunagawa, N.; Ishida, T.; Uchiyama, T.; Igarashi, K.; Samejima, M. Development of simple random mutagenesis protocol for the protein expression system in Pichia pastoris. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Rosenbergová, Z.; Kántorová, K.; Šimkovič, M.; Breier, A.; Rebroš, M. Optimisation of Recombinant Myrosinase Production in Pichia pastoris. Int. J. Mol. Sci. 2021, 22, 3677. [Google Scholar] [CrossRef]
- Jahic, M.; Veide, A.; Charoenrat, T.; Teeri, T.; Enfors, S.O. Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnol. Prog. 2006, 22, 1465–1473. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Karbalaei, M.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A.; Ghazvini, K.; Farsiani, H. Practical Methods for Expression of Recombinant Protein in the Pichia pastoris System. Curr. Protoc. 2021, 1, e155. [Google Scholar] [CrossRef]
- Barone, G.D.; Emmerstorfer-Augustin, A.; Biundo, A.; Pisano, I.; Coccetti, P.; Mapelli, V.; Camattari, A. Industrial production of proteins with Pichia pastoris—Komagataella phaffii. Biomolecules 2023, 13, 441. [Google Scholar] [CrossRef]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Niu, C.; Luo, H.; Shi, P.; Huang, H.; Wang, Y.; Yang, P.; Yao, B. N-Glycosylation improves the pepsin resistance of histidine acid phosphatase phytases by enhancing their stability at acidic pHs and reducing pepsin’s accessibility to its cleavage sites. Appl. Environ. Microbiol. 2015, 82, 1004–1014. [Google Scholar] [CrossRef]
- Gordeeva, T.L.; Borshchevskaya, L.N.; Sineoky, S.P. Biochemical characterisation of glycosylated and deglycosylated forms of phytase from Cronobacter turicensis expressed in Pichia pastoris. Enzyme Microb. Technol. 2023, 162, 110136. [Google Scholar] [CrossRef]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of yeasts as hosts for recombinant protein production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Siripong, W.; Wolf, P.; Kusumoputri, T.P.; Downes, J.J.; Kocharin, K.; Tanapongpipat, S.; Runguphan, W. Metabolic engineering of Pichia pastoris for production of isobutanol and isobutyl acetate. Biotechnol. Biofuels 2018, 11, 1. [Google Scholar] [CrossRef]
- Fischer, J.E.; Glieder, A. Current advances in engineering tools for Pichia pastoris. Curr. Opin. Biotechnol. 2019, 59, 175–181. [Google Scholar] [CrossRef]
- Xiong, A.S.; Yao, Q.H.; Peng, R.H.; Han, P.L.; Cheng, Z.M.; Li, Y. High level expression of a recombinant acid phytase gene in Pichia pastoris. J. Appl. Microbiol. 2005, 98, 418–428. [Google Scholar] [CrossRef]
- Wang, J.R.; Li, Y.Y.; Liu, D.N.; Liu, J.S.; Li, P.; Chen, L.Z.; Xu, S.D. Codon optimization significantly improves the expression level of α -amylase gene from Bacillus licheniformis in Pichia pastoris. Biomed. Res. Int. 2015, 2015, 248680. [Google Scholar] [CrossRef]
- Karaoğlan, M.; Erden-Karaoğlan, F. Effect of codon optimization and promoter choice on recombinant endo-polygalacturonase production in Pichia pastoris. Enzym. Microb. Technol. 2020, 139, 109589. [Google Scholar] [CrossRef]
- Luo, H.Y.; Huang, H.Q.; Bai, Y.G.; Wang, Y.R.; Yang, P.L.; Meng, K.; Yuan, T.Z.; Yao, B. Improving phytase expression by increasing the gene copy number of appA-m in Pichia pastoris. Chin. J. Biotechnol. 2006, 22, 528–533. [Google Scholar] [CrossRef]
- Zhu, T.; Guo, M.; Tang, Z.; Zhang, M.; Zhuang, Y.; Chu, J.; Zhang, S. Efficient generation of multi-copy strains for optimizing secretory expression of porcine insulin precursor in yeast Pichia pastoris. J. Appl. Microbiol. 2009, 107, 954–963. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Z.; Chen, J.; Chu, P.; Cheng, Q.; Liu, J.; Ming, F.; Huang, C.; Xiao, A.; Cai, H.; et al. Effect of cooperation of chaperones and gene dosage on the expression of porcine PGLYRP-1 in Pichia pastoris. Appl. Microbiol. Biotechnol. 2016, 100, 5453–5465. [Google Scholar] [CrossRef]
- Çalik, P.; Ata, Ö.; Güneş, H.; Massahi, A.; Boy, E.; Keskin, A.; Öztürk, S.; Zerze, G.H.; Özdamar, T.H. Recombinant protein production in Pichia pastoris under glyceraldehyde-3-phosphate dehydrogenase promoter: From carbon source metabolism to bioreactor operation parameters. Biochem. Eng. J. 2015, 95, 20–36. [Google Scholar] [CrossRef]
- Parashar, D.; Satyanarayana, T. Enhancing the production of recombinant acidic α-amylase and phytase in Pichia pastoris under dual promoters [constitutive (GAP) and inducible (AOX)] in mixed fed batch high cell density cultivation. Process Biochem. 2016, 51, 1315–1322. [Google Scholar] [CrossRef]
- Vogl, T.; Sturmberger, L.; Kickenweiz, T.; Wasmayer, R.; Schmid, C.; Hatzl, A.M.; Gerstmann, M.A.; Pitzer, J.; Wagner, M.; Thallinger, G.G.; et al. A toolbox of diverse promoters related to methanol utilization: Functionally verified parts for heterologous pathway expression in Pichia pastoris. ACS Synth. Biol. 2016, 5, 172–186. [Google Scholar] [CrossRef]
- Bernat-Camps, N.; Ebner, K.; Schusterbauer, V.; Fischer, J.E.; Nieto-Taype, M.A.; Valero, F.; Glieder, A.; Garcia-Ortega, X. Enabling growth-decoupled Komagataella phaffii recombinant protein production based on the methanol-free PDH promoter. Front. Bioeng. Biotechnol. 2023, 11, 1130583. [Google Scholar] [CrossRef]
- Liu, S.H.; Chou, W.I.; Sheu, C.C.; Chang, M.D.T. Improved secretory production of glucoamylase in Pichia pastoris by combination of genetic manipulations. Biochem. Biophys. Res. Commun. 2005, 326, 817–824. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Zheng, X.; Pang, N.; Liao, X.; Liu, X.; Huang, Y.; Liang, S. Combined strategies for improving expression of Citrobacter amalonaticus phytase in Pichia pastoris. BMC Biotechnol. 2015, 15, 88. [Google Scholar] [CrossRef]
- Barrero, J.J.; Casler, J.C.; Valero, F.; Ferrer, P.; Glick, B.S. An improved secretion signal enhances the secretion of model proteins from Pichia pastoris. Microb. Cell Fact. 2018, 17, 161. [Google Scholar] [CrossRef]
- Ito, Y.; Terai, G.; Ishigami, M.; Hashiba, N.; Nakamura, Y.; Bamba, T.; Kumokita, R.; Hasunuma, T.; Asai, K.; Ishii, J.; et al. Exchange of endogenous and heterogeneous yeast terminators in Pichia pastoris to tune mRNA stability and gene expression. Nucleic Acids Res. 2020, 48, 13000–13012. [Google Scholar] [CrossRef]
- Karaoğlan, M. Alternative secretory signal sequences for recombinant protein production in Pichia pastoris. Enzyme Microb. Technol. 2023, 168, 110256. [Google Scholar] [CrossRef]
- Delic, M.; Göngrich, R.; Mattanovich, D.; Gasser, B. Engineering of protein folding and secretion-strategies to overcome bottlenecks for efficient production of recombinant proteins. Antioxid. Redox Signal. 2014, 21, 414–437. [Google Scholar] [CrossRef]
- Navone, L.; Vogl, T.; Luangthongkam, P.; Blinco, J.A.; Luna-Flores, C.; Chen, X.; von Hellens, J.; Speight, R. Synergistic optimisation of expression, folding, and secretion improves E. coli AppA phytase production in Pichia pastoris. Microb. Cell Fact. 2021, 20, 8. [Google Scholar] [CrossRef]
- Raschmanová, H.; Weninger, A.; Knejzlík, Z.; Melzoch, K.; Kovar, K. Engineering of the unfolded protein response pathway in Pichia pastoris: Enhancing production of secreted recombinant proteins. Appl. Microbiol. Biotechnol. 2021, 105, 4397–4414. [Google Scholar] [CrossRef]
- Peña, D.A.; Gasser, B.; Zanghellini, J.; Steiger, M.G.; Mattanovich, D. Metabolic engineering of Pichia pastoris. Metab. Eng. 2018, 50, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, J.S.; Su, C.; Li, H.; Li, H.; Rao, Z.M.; Xu, Z.H.; Shi, J.S. Pathway engineering facilitates efficient protein expression in Pichia pastoris. Appl. Microbiol. Biotechnol. 2022, 106, 5893–5912. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Shvetsov, A.; Soboleva, E.; Kil, Y.; Sergeev, V.; Surzhik, M. Thermostability improvement of Aspergillus awamori glucoamylase via directed evolution of its gene located on episomal expression vector in Pichia pastoris cells. Protein Eng. Des. Sel. 2019, 32, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.R.; Yu, S.M.; Ho, T.H.D. Improvements of the productivity and saccharification efficiency of the cellulolytic β-glucosidase D2-BGL in Pichia pastoris via directed evolution. Biotechnol. Biofuels 2021, 14, 126. [Google Scholar] [CrossRef]
- Navone, L.; Moffitt, K.; Behrendorff, J.; Sadowski, P.; Hartley, C.; Speight, R. Biosensor-guided rapid screening for improved recombinant protein secretion in Pichia pastoris. Microb. Cell Fact. 2023, 22, 92. [Google Scholar] [CrossRef]
- Offei, B.; Braun-Galleani, S.; Venkatesh, A.; Casey, W.T.; O’Connor, K.E.; Byrne, K.P.; Wolfe, K.H. Identification of genetic variants of the industrial yeast Komagataella phaffii (Pichia pastoris) that contribute to increased yields of secreted heterologous proteins. PLoS Biol. 2022, 20, e3001877. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tan, Z.L.; Lin, Y.; Han, S.; Xing, X.; Zhang, C. Gel microdroplet-based high-throughput screening for directed evolution of xylanase-producing Pichia pastoris. J. Biosci. Bioeng. 2019, 128, 662–668. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Y.; Lin, Y.; Tu, R.; Guo, Y.; Zhang, Y.; Wang, Q. Microfluidic screening and genomic mutation identification for enhancing cellulase production in Pichia pastoris. Biotechnol. Biofuels Bioprod. 2022, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Vogl, T.; Luangthongkam, P.; Blinco, J.A.; Luna-Flores, C.H.; Chen, X.; von Hellens, J.; Mahler, S.; Speight, R. Disulfide bond engineering of AppA phytase for increased thermostability requires co-expression of protein disulfide isomerase in Pichia pastoris. Biotechnol. Biofuels 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, X.; Song, L.; Liu, H.; Zhou, X.; Wang, Q.; Zhang, Y.; Cai, M. CRISPR-Cas9-mediated genomic multiloci integration in Pichia pastoris. Microb. Cell Fact. 2019, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Jin, Z.; Liu, X.; Gao, X.; Guo, S.; Yu, T. A novel CRISPR/Cas9 system with high genomic editing efficiency and recyclable auxotrophic selective marker for multiple-step metabolic rewriting in Pichia pastoris. Synth. Syst. Biotechnol. 2023, 8, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Hartner, F.S.; Glieder, A. New opportunities by synthetic biology for biopharmaceutical production in Pichia pastoris. Curr. Opin. Biotechnol. 2013, 24, 1094–1101. [Google Scholar] [CrossRef]
- Weninger, A.; Hatzl, A.M.; Schmid, C.; Vogl, T.; Glieder, A. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris. J. Biotechnol. 2016, 235, 139–149. [Google Scholar] [CrossRef]
- Cos, O.; Ramón, R.; Montesinos, J.L.; Valero, F. Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: A review. Microb. Cell Fact. 2006, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Türkanoğlu Özçelik, A.; Yılmaz, S.; Inan, M. Pichia pastoris promoters. Methods Mol. Biol. 2019, 1923, 97–112. [Google Scholar] [CrossRef]
- Hu, X.; Chu, J.; Zhang, S.; Zhuang, Y. Comparative performance of S-adenosyl-L-methionine biosynthesis and degradation in Pichia pastoris using different promoters and novel consumption inhibitors. Enzym. Microb. Technol. 2014, 55, 94–99. [Google Scholar] [CrossRef]
- Prabhu, R.R.; Parashar, D.; Satyanarayana, T. Production and characteristics of the recombinant extracellular bifunctional endoglucanase of the polyextremophilic bacterium Bacillus halodurans and its applicability in saccharifying agro-residues. Bioprocess Biosyst. Eng. 2017, 40, 651–662. [Google Scholar] [CrossRef]
- Yadav, D.; Ranjan, B.; Mchunu, N.; Le Roes-Hill, M.; Kudanga, T. Enhancing the expression of recombinant small laccase in Pichia pastoris by a double promoter system and application in antibiotics degradation. Folia Microbiol. 2021, 66, 917–930. [Google Scholar] [CrossRef]
- Shen, S.; Sulter, G.; Jeffries, T.W.; Cregg, J.M. A strong nitrogen source-regulated promoter for controlled expression of foreign genes in the yeast Pichia pastoris. Gene 1998, 216, 93–102. [Google Scholar] [CrossRef]
- Resina, D.; Serrano, A.; Valero, F.; Ferrer, P. Expression of a Rhizopus oryzae lipase in Pichia pastoris under control of the nitrogen source-regulated formaldehyde dehydrogenase promoter. J. Biotechnol. 2004, 109, 103–113. [Google Scholar] [CrossRef]
- Vogl, T.; Glieder, A. Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol. 2013, 30, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zou, C.; Lin, Y.; Zhang, X.; Ye, Y. Identification and characterization of P GCW14: A novel, strong constitutive promoter of Pichia pastoris. Biotechnol. Lett. 2013, 35, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Nong, L.; Zhang, Y.; Duan, Y.; Hu, S.; Lin, Y.; Liang, S. Engineering the regulatory site of the catalase promoter for improved heterologous protein production in Pichia pastoris. Biotechnol. Lett. 2020, 42, 2703–2709. [Google Scholar] [CrossRef]
- Liu, B.; Cong, W.; Zhao, Y.; Zhou, H.; Zhang, J. An inducible Komagataella phaffii system for protein expression using sorbitol dehydrogenase promoter. Biotechnol. Lett. 2023, 45, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villegas, M.; Rebnegger, C.; Kowarz, V.; Prielhofer, R.; Mattanovich, D.; Gasser, B. Systematic sequence engineering enhances the induction strength of the glucose-regulated GTH1 promoter of Komagataella phaffii. Nucleic Acids Res. 2023, 51, 11358–11374. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, V.; Metzger, K.; Schmid, C.; Spadiut, O. A novel bi-directional promoter system allows tunable recombinant protein production in Pichia pastoris. Microb. Cell Fact. 2017, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T. Engineering of promoters for gene expression in Pichia pastoris. Methods Mol. Biol. 2022, 2513, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Sturmberger, L.; Fauland, P.C.; Hyden, P.; Fischer, J.E.; Schmid, C.; Thallinger, G.G.; Geier, M.; Glieder, A. Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors. Biotechnol. Bioeng. 2018, 115, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Hsiung, H.A.; Hong, K.L.; Huang, C.T. Enhancing the efficiency of the Pichia pastoris AOX1 promoter via the synthetic positive feedback circuit of transcription factor Mxr1. BMC Biotechnol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Paifer, E.; Margolles, E.; Cremata, J.; Montesino, R.; Herrera, L.; Delgado, J.M. Efficient expression and secretion of recombinant alpha amylase in Pichia pastoris using two different signal sequences. Yeast 1994, 10, 1415–1419. [Google Scholar] [CrossRef]
- Heimo, H.; Palmu, K.; Suominen, I. Expression in Pichia pastoris and purification of Aspergillus awamori glucoamylase catalytic domain. Protein Expr. Purif. 1997, 10, 70–79. [Google Scholar] [CrossRef]
- Romero, P.A.; Lussier, M.; Sdicu, A.M.; Bussey, H.; Herscovics, A. Ktr1p is an alpha-1,2-mannosyltransferase of Saccharomyces cerevisiae. Comparison of the enzymic properties of soluble recombinant Ktr1p and Kre2p/Mnt1p produced in Pichia pastoris. Biochem. J. 1997, 321 Pt 2, 289–295. [Google Scholar] [CrossRef]
- Kuwae, S.; Ohyama, M.; Ohya, T.; Ohi, H.; Kobayashi, K. Production of recombinant human antithrombin by Pichia pastoris. J. Biosci. Bioeng. 2005, 99, 264–271. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.; Qin, Y.; Liu, Z.; Mo, D.; Cong, P.; Chen, Y. Comparison of alpha-factor preprosequence and a classical mammalian signal peptide for secretion of recombinant xylanase xynB from yeast Pichia pastoris. J. Microbiol. Biotechnol. 2012, 22, 479–483. [Google Scholar] [CrossRef]
- Vadhana, A.K.P.; Samuel, P.; Berin, R.M.; Krishna, J.; Kamatchi, K.; Meenakshisundaram, S. Improved secretion of Candida antarctica lipase B with its native signal peptide in Pichia pastoris. Enzym. Microb. Technol. 2013, 52, 177–183. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, H.; Zhang, Y.; Du, G.; Chen, J. Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications. World J. Microbiol. Biotechnol. 2017, 33, 19. [Google Scholar] [CrossRef]
- Daly, R.; Hearn, M.T.W. Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005, 18, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Brake, A.J.; Merryweather, J.P.; Coit, D.G.; Heberlein, U.A.; Masiarz, F.R.; Mullenbach, G.T.; Urdea, M.S.; Valenzuela, P.; Barr, P.J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1984, 81, 4642. [Google Scholar] [CrossRef] [PubMed]
- Lin-Cereghino, G.P.; Stark, C.M.; Kim, D.; Chang, J.; Shaheen, N.; Poerwanto, H.; Agari, K.; Moua, P.; Low, L.K.; Tran, N.; et al. The effect of α-mating factor secretion signal mutations on recombinant protein expression in Pichia pastoris. Gene 2013, 519, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dalvie, N.C.; Naranjo, C.A.; Rodriguez-Aponte, S.A.; Johnston, R.S.; Christopher Love, J. Steric accessibility of the N-terminus improves the titer and quality of recombinant proteins secreted from Komagataella phaffii. Microb. Cell Fact. 2022, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Rakestraw, J.A.; Sazinsky, S.L.; Piatesi, A.; Antipov, E.; Wittrup, K.D. Directed evolution of a secretory leader for the improved expression of heterologous proteins and full-length antibodies in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2009, 103, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Nurdiani, D.; Hariyatun; Kusharyoto, W. Secretory expression of human insulin precursor in Pichia pastoris employing truncated α-factor leader sequence and a short C-peptide. Indones. J. Biotechnol. 2018, 23, 102–108. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mishra, S. Differential role of segments of α-mating factor secretion signal in Pichia pastoris towards granulocyte colony-stimulating factor emerging from a wild type or codon optimized copy of the gene. Microb. Cell Fact. 2020, 19, 199. [Google Scholar] [CrossRef]
- Utami, N.; Nurdiani, D.; Hariyatun, H.; Putro, E.W.; Patria, F.P.; Kusharyoto, W. Full-length versus truncated α-factor secretory signal sequences for expression of recombinant human insulin precursor in yeast Pichia pastoris: A comparison. J. Genet. Eng. Biotechnol. 2023, 21, 67. [Google Scholar] [CrossRef]
- Plath, K.; Mothes, W.; Wilkinson, B.M.; Stirling, C.J.; Rapoport, T.A. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 1998, 94, 795–807. [Google Scholar] [CrossRef]
- Fitzgerald, I.; Glick, B.S. Secretion of a foreign protein from budding yeasts is enhanced by cotranslational translocation and by suppression of vacuolar targeting. Microb. Cell Fact. 2014, 13, 125. [Google Scholar] [CrossRef]
- Barrero, J.J.; Pagazartaundua, A.; Glick, B.S.; Valero, F.; Ferrer, P. Bioreactor-scale cell performance and protein production can be substantially increased by using a secretion signal that drives co-translational translocation in Pichia pastoris. New Biotechnol. 2021, 60, 85–95. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, X.T.; Guo, Q.; Xue, Y.Z.; Xue, Y.P.; Zheng, Y.G. Potential of the signal peptide derived from the PAS_chr3_0030 gene product for secretory expression of valuable enzymes in Pichia pastoris. Appl. Environ. Microbiol. 2022, 88, e00296-22. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, C.; Ye, Y.; Lin, Y. Endogenous signal peptides efficiently mediate the secretion of recombinant proteins in Pichia pastoris. Biotechnol. Lett. 2013, 35, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Massahi, A.; Çalık, P. Endogenous signal peptides in recombinant protein production by Pichia pastoris: From in-silico analysis to fermentation. J. Theor. Biol. 2016, 408, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Ding, L.; Wei, D.; Zhou, H.; Chu, J.; Zhang, S.; Qian, J. Screening endogenous signal peptides and protein folding factors to promote the secretory expression of heterologous proteins in Pichia pastoris. J. Biotechnol. 2019, 306, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Lu, L.; Wang, S.; Zhang, C.; Chen, X.; Lin, Y.; Huang, Y. The α-mating factor secretion signals and endogenous signal peptides for recombinant protein secretion in Komagataella phaffii. Biotechnol. Biofuels Bioprod. 2022, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Mastropietro, G.; Aw, R.; Polizzi, K.M. Expression of proteins in Pichia pastoris. Methods Enzymol. 2021, 660, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.E.; Venkataraman, K. A Systematic review of the potential of Pichia pastoris (Komagataella phaffii) as an alternative host for biologics production. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Tronche, F.; Casanova, E.; Turiault, M.; Sahly, I.; Kellendonk, C. When reverse genetics meets physiology: The use of site-specific recombinases in mice. FEBS Lett. 2002, 529, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Näätsaari, L.; Mistlberger, B.; Ruth, C.; Hajek, T.; Hartner, F.S.; Glieder, A. Deletion of the Pichia pastoris KU70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS ONE 2012, 7, e39720. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Anumanthan, A.; Gao, X.G.; Ilangovan, K.; Suzara, V.V.; Düzgüneş, N.; Renugopalakrishnan, V. Expression of recombinant proteins in Pichia pastoris. Appl. Biochem. Biotechnol. 2007, 142, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Sunga, A.J.; Tolstorukov, I.; Cregg, J.M. Posttransformational vector amplification in the yeast Pichia pastoris. FEMS Yeast Res. 2008, 8, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Aw, R.; Polizzi, K.M. Liquid PTVA: A faster and cheaper alternative for generating multi-copy clones in Pichia pastoris. Microb. Cell Fact. 2016, 15, 29. [Google Scholar] [CrossRef]
- Seresht, A.K.; Nørgaard, P.; Palmqvist, E.A.; Andersen, A.S.; Olsson, L. Modulating heterologous protein production in yeast: The applicability of truncated auxotrophic markers. Appl. Microbiol. Biotechnol. 2013, 97, 3939–3948. [Google Scholar] [CrossRef]
- Erhart, E.; Hollenberg, C.P. The presence of a defective LEU2 gene on 2 mu DNA recombinant plasmids of Saccharomyces cerevisiae is responsible for curing and high copy number. J. Bacteriol. 1983, 156, 625–635. [Google Scholar] [CrossRef]
- Servienė, E.; Melvydas, V. Effect of the defective leucine gene leu2-d on the properties of recombinant plasmid in yeast Saccharomyces cerevisiae. Biologija 2001, 4, 30–33. [Google Scholar]
- Betancur, M.O.; Reis, V.C.B.; Nicola, A.M.; De Marco, J.L.; de Moraes, L.M.P.; Torres, F.A.G. Multicopy plasmid integration in Komagataella phaffii mediated by a defective auxotrophic marker. Microb. Cell Fact. 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, B.; Li, S.; Zhou, J.; Cao, H.; Huang, Y.; Cui, Z. A novel vector for construction of markerless multicopy overexpression transformants in Pichia pastoris. Front. Microbiol. 2017, 8, 1698. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Puxbaum, V.; Mattanovich, D.; Gasser, B. Quo vadis? The challenges of recombinant protein folding and secretion in Pichia pastoris. Appl. Microbiol. Biotechnol. 2015, 99, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Zahrl, R.J.; Gasser, B.; Mattanovich, D.; Ferrer, P. Detection and elimination of cellular bottlenecks in protein-producing yeasts. Methods Mol. Biol. 2019, 1923, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Mori, K. Signalling pathways in the unfolded protein response: Development from yeast to mammals. J. Biochem. 2009, 146, 743–750. [Google Scholar] [CrossRef]
- Zimmermann, R.; Eyrisch, S.; Ahmad, M.; Helms, V. Protein translocation across the ER membrane. Biochim. Biophys. Acta 2011, 1808, 912–924. [Google Scholar] [CrossRef]
- Hampton, R.Y. ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 2002, 14, 476–482. [Google Scholar] [CrossRef]
- Thibault, G.; Ng, D.T.W. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harb. Perspect. Biol. 2012, 4, a013193. [Google Scholar] [CrossRef]
- Gasser, B.; Maurer, M.; Rautio, J.; Sauer, M.; Bhattacharyya, A.; Saloheimo, M.; Penttilä, M.; Mattanovich, D. Monitoring of transcriptional regulation in Pichia pastoris under protein production conditions. BMC Genom. 2007, 8, 179. [Google Scholar] [CrossRef]
- Graf, A.; Gasser, B.; Dragosits, M.; Sauer, M.; Leparc, G.G.; Tüchler, T.; Kreil, D.P.; Mattanovich, D. Novel insights into the unfolded protein response using Pichia pastoris specific DNA microarrays. BMC Genom. 2008, 9, 390. [Google Scholar] [CrossRef]
- Kohno, K. Stress-sensing mechanisms in the unfolded protein response: Similarities and differences between yeast and mammals. J. Biochem. 2010, 147, 27–33. [Google Scholar] [CrossRef]
- Zahrl, R.J.; Mattanovich, D.; Gasser, B. The impact of ERAD on recombinant protein secretion in Pichia pastoris (syn Komagataella spp.). Microbiology 2018, 164, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Raschmanová, H.; Zamora, I.; Borčinová, M.; Meier, P.; Weninger, A.; Mächler, D.; Glieder, A.; Melzoch, K.; Knejzlík, Z.; Kovar, K. Single-cell approach to monitor the unfolded protein response during biotechnological processes with Pichia pastoris. Front. Microbiol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Sauer, M.; Maurer, M.; Stadlmayr, G.; Mattanovich, D. Transcriptomics-based identification of novel factors enhancing heterologous protein secretion in yeasts. Appl. Environ. Microbiol. 2007, 73, 6499–6507. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhou, Q.; Su, Z.; Yan, Y. Efficient heterologous production of Rhizopus oryzae lipase via optimization of multiple expression-related helper proteins. Int. J. Mol. Sci. 2018, 19, 3372. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Thallinger, G.G.; Zellnig, G.; Drew, D.; Cregg, J.M.; Glieder, A.; Freigassner, M. Towards improved membrane protein production in Pichia pastoris: General and specific transcriptional response to membrane protein overexpression. New Biotechnol. 2014, 31, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Tir, N.; Heistinger, L.; Grünwald-Gruber, C.; Jakob, L.A.; Dickgiesser, S.; Rasche, N.; Mattanovich, D. From strain engineering to process development: Monoclonal antibody production with an unnatural amino acid in Pichia pastoris. Microb. Cell Fact. 2022, 21, 157. [Google Scholar] [CrossRef]
- Guerfal, M.; Ryckaert, S.; Jacobs, P.P.; Ameloot, P.; Van Craenenbroeck, K.; Derycke, R.; Callewaert, N. The HAC1 gene from Pichia pastoris: Characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb. Cell Fact. 2010, 9, 49. [Google Scholar] [CrossRef]
- Damasceno, L.M.; Anderson, K.A.; Ritter, G.; Cregg, J.M.; Old, L.J.; Batt, C.A. Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Appl. Microbiol. Biotechnol. 2007, 74, 381–389. [Google Scholar] [CrossRef]
- Han, M.; Wang, W.; Zhou, J.; Gong, X.; Xu, C.; Li, Y.; Li, Q. Activation of the unfolded protein response via co-expression of the HAC1i gene enhances expression of recombinant elastase in Pichia pastoris. Biotechnol. Bioprocess Eng. 2020, 25, 302–307. [Google Scholar] [CrossRef]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. Biochim. Biophys. Acta 2009, 1793, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Marsalek, L.; Gruber, C.; Altmann, F.; Aleschko, M.; Mattanovich, D.; Gasser, B.; Puxbaum, V. Disruption of genes involved in CORVET complex leads to enhanced secretion of heterologous carboxylesterase only in protease deficient Pichia pastoris. Biotechnol. J. 2017, 12, 1600584. [Google Scholar] [CrossRef] [PubMed]
- Marsalek, L.; Puxbaum, V.; Buchetics, M.; Mattanovich, D.; Gasser, B. Disruption of vacuolar protein sorting components of the HOPS complex leads to enhanced secretion of recombinant proteins in Pichia pastoris. Microb. Cell Fact. 2019, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, G.P.L.; Cereghino, J.L.; Ilgen, C.; Cregg, J.M. Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Curr. Opin. Biotechnol. 2002, 13, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Elvira, M.; Torres-Quiroz, F.; Escamilla-Ayala, A.; Domínguez-Martin, E.; Escalante, R.; Kawasaki, L.; Ongay-Larios, L.; Coria, R. The unfolded protein response pathway in the yeast Kluyveromyces lactis. A comparative view among yeast species. Cells 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Dato, L.; Graf, A.B.; Frascotti, G.; Dragosits, M.; Porro, D.; Mattanovich, D.; Ferrer, P.; Branduardi, P. The impact of oxygen on the transcriptome of recombinant S. cerevisiae and P. pastoris—A comparative analysis. BMC Genom. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Fauzee, Y.N.B.M.; Taniguchi, N.; Ishiwata-Kimata, Y.; Takagi, H.; Kimata, Y. The unfolded protein response in Pichia pastoris without external stressing stimuli. FEMS Yeast Res. 2020, 20, foaa053. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, C.J.; Park, J.S. Strategies for optimizing the production of proteins and peptides with multiple disulfide bonds. Antibiotics 2020, 9, 541. [Google Scholar] [CrossRef]
- Subramanyam, S.; Spies, M. Expression, purification, and biochemical evaluation of human RAD51 Protein. Methods Enzymol. 2018, 600, 157–178. [Google Scholar] [CrossRef]
- Kemmink, J.; Darby, N.J.; Dijkstrat, K.; Nilges, M.; Creighton, T.E. The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr. Biol. 1997, 7, 239–245. [Google Scholar] [CrossRef]
- Irvine, A.G.; Wallis, A.K.; Sanghera, N.; Rowe, M.L.; Ruddock, L.W.; Howard, M.J.; Williamson, R.A.; Blindauer, C.A.; Freedman, R.B. Protein disulfide-isomerase interacts with a substrate protein at all stages along its folding pathway. PLoS ONE 2014, 9, e82511. [Google Scholar] [CrossRef]
- Inan, M.; Aryasomayajula, D.; Sinha, J.; Meagher, M.M. Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase. Biotechnol. Bioeng. 2006, 93, 771–778. [Google Scholar] [CrossRef]
- Tsai, C.W.; Duggan, P.F.; Shimp, R.L.; Miller, L.H.; Narum, D.L. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J. Biotechnol. 2006, 121, 458–470. [Google Scholar] [CrossRef]
- Zhang, S.T.; Fang, H.M.; Zhao, L.; Tian, Q.N.; Qin, Y.F.; Lu, P.; Chen, S.J.; Bao, Z.X.; Liang, F. Co-overexpression of PpPDI enhances secretion of ancrod in Pichia pastoris. Appl. Biochem. Biotechnol. 2011, 164, 1037–1047. [Google Scholar] [CrossRef]
- Yan, P.; Zou, Z.; Zhang, S.; Wang, R.; Niu, T.; Zhang, X.; Liu, D.; Zhou, X.; Chang, A.K.; Milton, N.G.N.; et al. Defining the mechanism of PDI interaction with disulfide-free amyloidogenic proteins: Implications for exogenous protein expression and neurodegenerative disease. Int. J. Biol. Macromol. 2021, 174, 175–184. [Google Scholar] [CrossRef]
- Wang, C.; Tsou, C. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB J. 1993, 7, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, P.; Lu, H.; Wang, H.; Luo, H.; Huang, H.; Yang, P.; Yao, B. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem. 2015, 173, 283–289. [Google Scholar] [CrossRef]
- Chang, X.; Xu, B.; Bai, Y.; Luo, H.; Ma, R.; Shi, P.; Yao, B. Role of N-linked glycosylation in the enzymatic properties of a thermophilic GH 10 xylanase from Aspergillus fumigatus expressed in Pichia pastoris. PLoS ONE 2017, 12, e0171111. [Google Scholar] [CrossRef] [PubMed]
- De Wachter, C.; Van Landuyt, L.; Callewaert, N. Engineering of yeast glycoprotein expression. Adv. Biochem. Eng. Biotechnol. 2021, 175, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Qiu, H. The Mechanistic Impact of N-Glycosylation on stability, pharmacokinetics, and immunogenicity of therapeutic proteins. J. Pharm. Sci. 2019, 108, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Prates, E.T.; Guan, X.; Li, Y.; Wang, X.; Chaffey, P.K.; Skaf, M.S.; Crowley, M.F.; Tan, Z.; Beckham, G.T. The impact of O-glycan chemistry on the stability of intrinsically disordered proteins. Chem. Sci. 2018, 9, 3710–3715. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.R.; Cook, W.J.; Gomathinayagam, S.; Burnina, I.; Bukowski, J.; Hopkins, D.; Schwartz, S.; Du, M.; Sharkey, N.J.; Bobrowicz, P.; et al. Production of sialylated O-linked glycans in Pichia pastoris. Glycobiology 2013, 23, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Callewaert, N. N-glycosylation engineering of biopharmaceutical expression systems. Curr. Mol. Med. 2009, 9, 774–800. [Google Scholar] [CrossRef]
- Thak, E.J.; Kim, J.; Lee, D.J.; Kim, J.Y.; Kang, H.A. Structural analysis of N-/O-glycans assembled on proteins in yeasts. J. Microbiol. 2018, 56, 11–23. [Google Scholar] [CrossRef]
- Bretthauer, R.K.; Castellino, F.J. Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Biochem. 1999, 30, 193–200. [Google Scholar] [CrossRef]
- Nett, J.H.; Cook, W.J.; Chen, M.T.; Davidson, R.C.; Bobrowicz, P.; Kett, W.; Brevnova, E.; Potgieter, T.I.; Mellon, M.T.; Prinz, B.; et al. Characterization of the Pichia pastoris protein-O-mannosyltransferase gene family. PLoS ONE 2013, 8, e68325. [Google Scholar] [CrossRef]
- Gong, B.; Cukan, M.; Fisher, R.; Li, H.; Stadheim, T.A.; Gerngross, T. Characterization of N-linked glycosylation on recombinant glycoproteins produced in Pichia pastoris using ESI-MS and MALDI-TOF. Methods Mol. Biol. 2009, 534, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Laukens, B.; De Wachter, C.; Callewaert, N. Engineering the Pichia pastoris N-glycosylation pathway using the GlycoSwitch technology. Methods Mol. Biol. 2015, 1321, 103–122. [Google Scholar] [CrossRef]
- Wang, B.; Yan, Y.; Xu, J.; Fu, X.; Han, H.; Gao, J.; Li, Z.; Wang, L.; Tian, Y.; Peng, R.; et al. Heterologous Expression and characterization of a laccase from Laccaria bicolor in Pichia pastoris and Arabidopsis thaliana. J. Microbiol. Biotechnol. 2018, 28, 2057–2063. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Peng, H.; Guo, W.; Wang, M.; Li, G.; Liu, D. The introduction of an N-glycosylation site into prochymosin greatly enhances its production and secretion by Pichia pastoris. Microb. Cell Fact. 2022, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Gündüz Ergün, B.; Çalık, P. Lignocellulose degrading extremozymes produced by Pichia pastoris: Current status and future prospects. Bioprocess Biosyst. Eng. 2016, 39, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Duman-Özdamar, Z.E.; Binay, B. Production of industrial enzymes via Pichia pastoris as a cell factory in bioreactor: Current status and future aspects. Protein J. 2021, 40, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, T.; Khan, A.; Ling, Z.; Sharma, M.; Feng, P.; Ali, G.; Saif, I.; Wang, H.; Li, X.; et al. Feed-additive of bioengineering strain with surface-displayed laccase degrades sulfadiazine in broiler manure and maintains intestinal flora structure. J. Hazard. Mater. 2021, 406, 124440. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yuan, M.; Xu, L.; Lio, E.; Zhang, F.; Mou, H.; Secundo, F. Application of enzymes as a feed additive in aquaculture. Mar. Life Sci. Technol. 2022, 4, 208–221. [Google Scholar] [CrossRef]
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carillo, R. The Generally Recognized as Safe (GRAS) Process for Industrial Microbial Enzymes. Ind. Biotechnol. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2014, 95, 878–896. [Google Scholar] [CrossRef]
- Selle, P.H.; Ravindran, V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007, 135, 1–41. [Google Scholar] [CrossRef]
- Ravindran, V.; Cabahug, S.; Ravindran, G.; Bryden, W.L. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 1999, 78, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.H.; Yu, S.; Peron, A.; Cadogan, D.J.; Khoddami, A.; Roberts, T.H.; Liu, S.Y.; Selle, P.H. Phytase supplementation of maize-, sorghum-and wheat-based broiler diets with identified starch pasting properties influences phytate (IP 6) and sodium jejunal and ileal digestibility. Anim. Feed Sci. Technol. 2014, 198, 248–256. [Google Scholar] [CrossRef]
- Truong, H.H.; Bold, R.M.; Liu, S.Y.; Selle, P.H. Standard phytase inclusion in maize-based broiler diets enhances digestibility coefficients of starch, amino acids and sodium in four small intestinal segments and digestive dynamics of starch and protein. Anim. Feed Sci. Technol. 2015, 209, 240–248. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Weaver, J.D.; Mullaney, E.; Ullah, A.H.; Azain, M.J. Phytase, a new life for an “old” enzyme. Annu. Rev. Anim. Biosci. 2013, 1, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Olukosi, O.A. Influence of graded concentrations of phytase in high-phytate diets on growth performance, apparent ileal amino acid digestibility, and phytate concentration in broilers from hatch to 28 D post-hatch. Poult. Sci. 2019, 98, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Jatuwong, K.; Suwannarach, N.; Kumla, J.; Penkhrue, W.; Kakumyan, P.; Lumyong, S. Bioprocess for Production, Characteristics, and biotechnological applications of fungal phytases. Front. Microbiol. 2020, 11, 188. [Google Scholar] [CrossRef]
- Yao, M.Z.; Zhang, Y.H.; Lu, W.L.; Hu, M.Q.; Wang, W.; Liang, A.H. Phytases: Crystal structures, protein engineering and potential biotechnological applications. J. Appl. Microbiol. 2012, 112, 1–14. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Fang, T.J.; Tseng, S.M. Isolation and characterization of a novel phytase from Penicillium simplicissimum. Folia Microbiol. 2000, 45, 121–127. [Google Scholar] [CrossRef]
- Sabu, A.; Sarita, S.; Pandey, A.; Bogar, B.; Szakacs, G.; Soccol, C.R. Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl. Biochem. Biotechnol. 2002, 102–103, 251–260. [Google Scholar] [CrossRef]
- Roopesh, K.; Ramachandran, S.; Nampoothiri, K.M.; Szakacs, G.; Pandey, A. Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresour. Technol. 2006, 97, 506–511. [Google Scholar] [CrossRef]
- Shah, P.; Bhavsar, K.; Soni, S.K.; Khire, J.M. Strain improvement and up scaling of phytase production by Aspergillus niger NCIM 563 under submerged fermentation conditions. J. Ind. Microbiol. Biotechnol. 2009, 36, 373–380. [Google Scholar] [CrossRef]
- Monteiro, P.S.; Guimarães, V.M.; de Melo, R.R.; de Rezende, S.T. Isolation of a thermostable acid phytase from Aspergillus niger UFV-1 with strong proteolysis resistance. Braz. J. Microbiol. 2015, 46, 251–260. [Google Scholar] [CrossRef]
- Coban, H.B.; Demirci, A. Screening of phytase producers and optimization of culture conditions for submerged fermentation. Bioprocess Biosyst. Eng. 2014, 37, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, Y.O.; Lee, J.H.; Kim, K.K.; Kim, Y.J. Isolation and characterization of a phytase with improved properties from Citrobacter braakii. Biotechnol. Lett. 2003, 25, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S.; Han, K.H.; Jang, M.S.; Park, H.; Marshall, V.; Dersjant-Li, Y.; Sewalt, V.J. Safety evaluation of a novel variant of consensus bacterial phytase. Toxicol. Rep. 2020, 7, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, P.; Yan, X.; Yang, Y.; Li, X.; Liu, R.; Zhou, Z. Thermostability enhancement of Escherichia coli phytase by error-prone polymerase chain reaction (epPCR) and site-directed mutagenesis. Front. Bioeng. Biotechnol. 2023, 11, 1167530. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.H.; Wilson, D.B.; Lei, X.G. Comparison of extracellular Escherichia coli AppA phytases expressed in Streptomyces lividans and Pichia pastoris. Biotechnol. Lett. 2003, 25, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wu, P.H.; Huang, C.T.; Cheng, K.J. A Pichia pastoris fermentation strategy for enhancing the heterologous expression of an Escherichia coli phytase. Enzym. Microb. Technol. 2004, 35, 315–320. [Google Scholar] [CrossRef]
- Tai, H.M.; Yin, L.J.; Chen, W.C.; Jiang, S.T. Overexpression of Escherichia coli phytase in Pichia pastoris and its biochemical properties. J. Agric. Food Chem. 2013, 61, 6007–6015. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Stahl, C.H.; Lei, X.G. Expression of Escherichia coli AppA2 phytase in four yeast systems. Biotechnol. Lett. 2005, 27, 327–334. [Google Scholar] [CrossRef]
- Shi, X.W.; Sun, M.L.; Zhou, B.; Wang, X.Y. Identification, characterization, and overexpression of a phytase with potential industrial interest. Can. J. Microbiol. 2009, 55, 599–604. [Google Scholar] [CrossRef]
- Lim, D.; Golovan, S.; Forsberg, C.W.; Jia, Z. Crystal structures of Escherichia coli phytase and its complex with phytate. Nat. Struct. Biol. 2000, 7, 108–113. [Google Scholar] [CrossRef]
- Huang, H.G.; Luo, H.Y.; Bai, Y.G.; Wang, Y.R.; Yao, B.; Meng, K.; Yuan, T.Z.; Yang, P.L. Overexpression of Citrobacter braakii phytase with high specific activity in Pichia pastoris. Wei Sheng Wu Xue Bao 2006, 46, 945–950. [Google Scholar]
- Luo, H.; Huang, H.; Yang, P.; Wang, Y.; Yuan, T.; Wu, N.; Yao, B.; Fan, Y. A novel phytase appA from Citrobacter amalonaticus CGMCC 1696: Gene cloning and overexpression in Pichia pastoris. Curr. Microbiol. 2007, 55, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.A.; Kalinina, A.N.; Borshchevskaya, L.N.; Sineoky, S.P.; Gordeeva, T.L. A novel phytase from Citrobactergillenii: Characterization and expression in Pichia pastoris (Komagataella pastoris). FEMS Microbiol. Lett. 2021, 368, fnaa217. [Google Scholar] [CrossRef] [PubMed]
- Promdonkoy, P.; Tang, K.; Sornlake, W.; Harnpicharnchai, P.; Kobayashi, R.S.; Ruanglek, V.; Upathanpreecha, T.; Vesaratchavest, M.; Eurwilaichitr, L.; Tanapongpipat, S. Expression and characterization of Aspergillus thermostable phytases in Pichia pastoris. FEMS Microbiol. Lett. 2009, 290, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.H.; Gibson, D.M. Extracellular phytase (E.C. 3.1.3.8) from Aspergillus ficuum NRRL 3135: Purification and characterization. Prep. Biochem. 1987, 17, 63–91. [Google Scholar] [CrossRef]
- Xiong, A.S.; Yao, Q.H.; Peng, R.H.; Zhang, Z.; Xu, F.; Liu, J.G.; Han, P.L.; Chen, J.M. High level expression of a synthetic gene encoding Peniophora lycii phytase in methylotrophic yeast Pichia pastoris. Appl. Microbiol. Biotechnol. 2006, 72, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Tsutsumi, N.; Terui, Y.; Kong, X.Y.; Yurimoto, H.; Sakai, Y. Engineering the expression system for Komagataella phaffii (Pichia pastoris): An attempt to develop a methanol-free expression system. FEMS Yeast Res. 2019, 19, foz059. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef]
- Kikani, B.A.; Singh, S.P. Amylases from thermophilic bacteria: Structure and function relationship. Crit. Rev. Biotechnol. 2022, 42, 325–341. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Madhavan, A.; Beevi, U.S.; Mathew, A.K.; Abraham, A.; Pandey, A.; Kumar, V. Molecular improvements in microbial α-amylases for enhanced stability and catalytic efficiency. Bioresour. Technol. 2017, 245, 1740–1748. [Google Scholar] [CrossRef]
- Yao, D.; Su, L.; Li, N.; Wu, J. Enhanced extracellular expression of Bacillus stearothermophilus α-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and α-amylase mutant selection. Microb. Cell Fact. 2019, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Zhang, K.; Su, L.; Liu, Z.; Wu, J. Enhanced extracellular Bacillus stearothermophilus α-amylase production in Bacillus subtilis by balancing the entire secretion process in an optimal strain. Biochem. Eng. J. 2021, 168, 107948. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Jeevarathinam, G.; Kumar, S.K.S.; Muniraj, I.; Uthandi, S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Siddique, F.; Shahid Mahmood, M.; Ahmed, S.I. A review of the microbiological aspect of α-amylase production. Int. J. Agric. Biol. 2013, 15, 1029–1034. [Google Scholar]
- Gopinath, S.C.B.; Anbu, P.; Arshad, M.K.M.; Lakshmipriya, T.; Voon, C.H.; Hashim, U.; Chinni, S.V. Biotechnological processes in microbial amylase production. Biomed. Res. Int. 2017, 2017, 1272193. [Google Scholar] [CrossRef] [PubMed]
- Far, B.E.; Ahmadi, Y.; Khosroushahi, A.Y.; Dilmaghani, A. Microbial Alpha-amylase production: Progress, challenges and perspectives. Adv. Pharm. Bull. 2020, 10, 350–358. [Google Scholar] [CrossRef]
- Ferreira, A.V.F.; Silva, F.F.; Silva, A.A.M.; Azevedo, L.S.; da Fonseca, S.T.D.; Camilo, N.H.; dos Santos, K.P.E.; de Carvalho, L.C.; Tarabal, V.S.; da Silva, J.O.; et al. Recent patents on the industrial application of alpha-amylases. Recent Pat. Biotechnol. 2020, 14, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Chor Leow, T.; Oslan, S.N. Expression and characterization of Geobacillus stearothermophilus SR74 recombinant α-amylase in Pichia pastoris. Biomed. Res. Int. 2015, 2015, 529059. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Gao, Y.; Zhou, X.; Zhang, Y.; Cai, M. Regulating unfolded protein response activator HAC1p for production of thermostable raw-starch hydrolyzing α-amylase in Pichia pastoris. Bioprocess Biosyst. Eng. 2017, 40, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yuan, X.; He, N.; Zhuang, T.Z.; Wu, P.; Zhang, G. Expression of Bacillus licheniformis α-amylase in Pichia pastoris without antibiotics-resistant gene and effects of glycosylation on the enzymic thermostability. 3 Biotech 2019, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhao, N.; Ma, J.W.; Liu, J.; Yan, Q.J.; Jiang, Z.Q. High-level expression of a novel α-amylase from Thermomyces dupontii in Pichia pastoris and its application in maltose syrup production. Int. J. Biol. Macromol. 2019, 127, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, L.; Shin, H.D.; Chen, R.R.; Li, J.; Du, G.; Chen, J. Comparative analysis of heterologous expression, biochemical characterization optimal production of an alkaline α-amylase from alkaliphilic Alkalimonas amylolytica in Escherichia coli and Pichia pastoris. Biotechnol. Prog. 2013, 29, 39–47. [Google Scholar] [CrossRef]
- Nayab, D.E.; Akhtar, S.; Bangash, N.; Nisa, W.U.; Hayat, M.T.; Zulfiqar, A.; Niaz, M.; Qayyum, A.; Syed, A.; Bahkali, A.H.; et al. Production of glucoamylase from novel strain of alternaria alternata under solid state fermentation. Biomed. Res. Int. 2022, 2022, 2943790. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.Q.; Zhao, C.Q.; Li, A.N.; Zhou, Q.X.; Li, D.C. Cloning of a gene encoding thermostable glucoamylase from Chaetomium thermophilum and its expression in Pichia pastoris. J. Appl. Microbiol. 2007, 103, 2277–2284. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Mao, Y.; Gu, J.; Pan, Q.; Zhou, S.; Gao, B.; Wei, D. Cloning of a novel thermostable glucoamylase from thermophilic fungus Rhizomucor pusillus and high-level co-expression with α-amylase in Pichia pastoris. BMC Biotechnol. 2014, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Luo, H.; Bai, Y.; Wang, K.; Niu, C.; Huang, H.; Shi, P.; Wang, C.; Yang, P.; Yao, B. A thermostable glucoamylase from Bispora sp. MEY-1 with stability over a broad pH range and significant starch hydrolysis capacity. PLoS ONE 2014, 9, e113581. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.M.R.; Husaini, A.; Hossain, M.A.; Sing, N.N.; Mohd Sinang, F.; Hussain, M.H.M.; Roslan, H.A. Heterologous, expression, and characterization of thermostable glucoamylase derived from Aspergillus flavus NSH9 in Pichia pastoris. Biomed. Res. Int. 2016, 2016, 5962028. [Google Scholar] [CrossRef]
- Tong, L.; Zheng, J.; Wang, X.; Wang, X.; Huang, H.; Yang, H.; Tu, T.; Wang, Y.; Bai, Y.; Yao, B.; et al. Improvement of thermostability and catalytic efficiency of glucoamylase from Talaromyces leycettanus JCM12802 via site-directed mutagenesis to enhance industrial saccharification applications. Biotechnol. Biofuels 2021, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Yujie, G.; Tao, T.; Jin, Q.; Lige, T.; Huiying, L.; Bin, Y. Characterization and structure of a novel thermostable glucoamylase from Talaromyces leycettanus JCM12802. Sheng Wu Gong Cheng Xue Bao 2019, 35, 616–625. [Google Scholar] [CrossRef]
- Fierobe, H.P.; Mirgorodskaya, E.; Frandsen, T.P.; Roepstorff, P.; Svensson, B. Overexpression and characterization of Aspergillus awamori wild-type and mutant glucoamylase secreted by the methylotrophic yeast Pichia pastoris: Comparison with wild-type recombinant glucoamylase produced using Saccharomyces cerevisiae and Aspergillus niger as hosts. Protein Expr. Purif. 1997, 9, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.R.; Courtin, C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poult. Sci. 2019, 98, 4606–4621. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, G.; Sola-Oriol, D.; Martinez-Mora, M.; Perez, J.F.; Bedford, M.R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poult. Sci. 2017, 96, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.H.; Nguyen, H.T.; Morgan, N.K. Dietary soluble non-starch polysaccharide level and xylanase supplementation influence performance, egg quality and nutrient utilization in laying hens fed wheat-based diets. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2021, 7, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Moita, V.H.C.; Kim, S.W. Nutritional and functional roles of phytase and xylanase enhancing the intestinal health and growth of nursery pigs and broiler chickens. Animals 2022, 12, 3322. [Google Scholar] [CrossRef]
- Basit, A.; Liu, J.; Rahim, K.; Jiang, W.; Lou, H. Thermophilic xylanases: From bench to bottle. Crit. Rev. Biotechnol. 2018, 38, 989–1002. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Guan, L.; Jiang, Z.; Yan, Q.; Yang, S. High-level expression and enzymatic properties of a novel thermostable xylanase with high arabinoxylan degradation ability from Chaetomium sp. suitable for beer mashing. Int. J. Biol. Macromol. 2021, 168, 223–232. [Google Scholar] [CrossRef]
- Wang, X.; Luo, H.; Yu, W.; Ma, R.; You, S.; Liu, W.; Hou, L.; Zheng, F.; Xie, X.; Yao, B. A thermostable Gloeophyllum trabeum xylanase with potential for the brewing industry. Food Chem. 2016, 199, 516–523. [Google Scholar] [CrossRef]
- Basit, A.; Liu, J.; Miao, T.; Zheng, F.; Rahim, K.; Lou, H.; Jiang, W. Characterization of two endo-β-1,4-xylanases from Myceliophthora thermophila and their saccharification efficiencies, synergistic with commercial cellulase. Front. Microbiol. 2018, 9, 304706. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, Y.; Zhang, L.; Xu, H.; Guo, X.; Yang, X.; Dong, B.; Cao, Y. Improved thermostability of an acidic xylanase from Aspergillus sulphureus by combined disulphide bridge introduction and proline residue substitution. Sci. Rep. 2017, 7, 1587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Bao, C.; Dong, B.; Cao, Y. Characterization of a novel GH10 xylanase with a carbohydrate binding module from Aspergillus sulphureus and its synergistic hydrolysis activity with cellulase. Int. J. Biol. Macromol. 2021, 182, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhny, A.V.; Ushakov, V.S.; Rozanov, A.S.; Bogacheva, N.V.; Shlyakhtun, V.N.; Voskoboev, M.E.; Korzhuk, A.V.; Romancev, V.A.; Bannikova, S.V.; Mescheryakova, I.A.; et al. Heterologous Expression of Xylanase xAor from Aspergillus oryzae in Komagataella phaffii T07. Int. J. Mol. Sci. 2022, 23, 8741. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, J.; Yang, J.; Wang, H.; Yang, Y.; Huang, H.; Shi, P.; Yuan, T.; Fan, Y.; Yao, B. A thermophilic and acid stable family-10 xylanase from the acidophilic fungus Bispora sp. MEY-1. Extremophiles 2009, 13, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Basit, A.; Zhuang, H.; Chen, J.; Zhang, J.; Chen, W. Biochemical characterization of a novel acidophilic β-xylanase from Trichoderma asperellum ND-1 and its synergistic hydrolysis of beechwood xylan. Front. Microbiol. 2022, 13, 998160. [Google Scholar] [CrossRef]
- Fan, G.; Katrolia, P.; Jia, H.; Yang, S.; Yan, Q.; Jiang, Z. High-level expression of a xylanase gene from the thermophilic fungus Paecilomyces thermophila in Pichia pastoris. Biotechnol. Lett. 2012, 34, 2043–2048. [Google Scholar] [CrossRef]
- Guo, N.; Zheng, J.; Tian, J.; Wu, L.; Zhou, H. Characterization and constitutive expression of an acidic mesophilic endo-1,4-β-D-xylanohydrolase with high thermotolerance and catalytic efficiency in Pichia pastoris. World J. Microbiol. Biotechnol. 2013, 29, 2095–2103. [Google Scholar] [CrossRef]
- Zhao, L.; Geng, J.; Guo, Y.; Liao, X.; Liu, X.; Wu, R.; Zheng, Z.; Zhang, R. Expression of the Thermobifida fusca xylanase Xyn11A in Pichia pastoris and its characterization. BMC Biotechnol. 2015, 15, 18. [Google Scholar] [CrossRef]
- Xia, W.; Hu, M.; Pan, Y.; Wu, D.; Wu, J. Improved production of Streptomyces sp. FA1 xylanase in a dual-plasmid Pichia pastoris system. Curr. Issues Mol. Biol. 2021, 43, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Fan, G.; Yan, Q.; Liu, Y.; Yan, Y.; Jiang, Z. High-level expression of a hyperthermostable Thermotoga maritima xylanase in Pichia pastoris by codon optimization. J. Mol. Catal. B Enzym. 2012, 78, 72–77. [Google Scholar] [CrossRef]
- Lu, Y.; Fang, C.; Wang, Q.; Zhou, Y.; Zhang, G.; Ma, Y. High-level expression of improved thermo-stable alkaline xylanase variant in Pichia pastoris through codon optimization, multiple gene insertion and high-density fermentation. Sci. Rep. 2016, 6, 37869. [Google Scholar] [CrossRef]
- Kiribayeva, A.; Mukanov, B.; Silayev, D.; Akishev, Z.; Ramankulov, Y.; Khassenov, B. Cloning, expression, and characterization of a recombinant xylanase from Bacillus sonorensis T6. PLoS ONE 2022, 17, e0265647. [Google Scholar] [CrossRef]
- Shang, T.; Si, D.; Zhang, D.; Liu, X.; Zhao, L.; Hu, C.; Fu, Y.; Zhang, R. Enhancement of thermoalkaliphilic xylanase production by Pichia pastoris through novel fed-batch strategy in high cell-density fermentation. BMC Biotechnol. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef]
- Yamabhai, M.; Sak-Ubol, S.; Srila, W.; Haltrich, D. Mannan biotechnology: From biofuels to health. Crit. Rev. Biotechnol. 2016, 36, 32–42. [Google Scholar] [CrossRef]
- Dawood, A.; Ma, K. Applications of microbial β-mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef]
- Rana, M.; Jassal, S.; Yadav, R.; Sharma, A.; Puri, N.; Mazumder, K.; Gupta, N. Functional β-mannooligosaccharides: Sources, enzymatic production and application as prebiotics. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Latham, R.E.; Williams, M.P.; Walters, H.G.; Carter, B.; Lee, J.T. Efficacy of β-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan. Poult. Sci. 2018, 97, 549–556. [Google Scholar] [CrossRef]
- White, D.; Adhikari, R.; Wang, J.; Chen, C.; Lee, J.H.; Kim, W.K. Effects of dietary protein, energy and β-mannanase on laying performance, egg quality, and ileal amino acid digestibility in laying hens. Poult. Sci. 2021, 100, 101312. [Google Scholar] [CrossRef]
- Kiarie, E.G.; Steelman, S.; Martinez, M.; Livingston, K. Significance of single β-mannanase supplementation on performance and energy utilization in broiler chickens, laying hens, turkeys, sows, and nursery-finish pigs: A meta-analysis and systematic review. Transl. Anim. Sci. 2021, 5, txab160. [Google Scholar] [CrossRef]
- David, A.; Singh Chauhan, P.; Kumar, A.; Angural, S.; Kumar, D.; Puri, N.; Gupta, N. Coproduction of protease and mannanase from Bacillus nealsonii PN-11 in solid state fermentation and their combined application as detergent additives. Int. J. Biol. Macromol. 2018, 108, 1176–1184. [Google Scholar] [CrossRef]
- Tuntrakool, P.; Keawsompong, S. Kinetic properties analysis of beta-mannanase from Klebsiella oxytoca KUB-CW2-3 expressed in Escherichia coli. Protein Expr. Purif. 2018, 146, 23–26. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Wang, Y.; Na, J.; Ping, W.; Ge, J. Purification, biochemical and secondary structural characterisation of β-mannanase from Lactobacillus casei HDS-01 and juice clarification potential. Int. J. Biol. Macromol. 2020, 154, 826–834. [Google Scholar] [CrossRef]
- Agrawal, P.; Verma, D.; Daniell, H. Expression of Trichoderma reesei β-Mannanase in tobacco chloroplasts and its utilization in lignocellulosic woody biomass hydrolysis. PLoS ONE 2011, 6, e29302. [Google Scholar] [CrossRef]
- Blibech, M.; Ellouz Ghorbel, R.; Chaari, F.; Dammak, I.; Bhiri, F.; Neifar, M.; Ellouz Chaabouni, S. Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds. ISRN Microbiol. 2011, 2011, 938347. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, G.C.; Cho, S.S.; Choi, Y.H.; Choi, Y.S.; Jee, J.P.; Seong, C.N.; Yoo, J.C. An extremely alkaline mannanase from Streptomyces sp. CS428 hydrolyzes galactomannan producing series of mannooligosaccharides. World J. Microbiol. Biotechnol. 2016, 32, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Wang, Y.; Niu, C.; Ma, R.; Wang, Y.; Bai, Y.; Luo, H.; Yao, B. Biochemical characterization of an acidophilic β-mannanase from Gloeophyllum trabeum CBS900.73 with significant transglycosylation activity and feed digesting ability. Food Chem. 2016, 197, 474–481. [Google Scholar] [CrossRef]
- Xie, J.; He, Z.; Wang, Z.; Wang, B.; Pan, L. Efficient expression of a novel thermophilic fungal β-mannosidase from Lichtheimia ramosa with broad-range pH stability and its synergistic hydrolysis of locust bean gum. J. Biosci. Bioeng. 2019, 128, 416–423. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, C.; Yuan, M.; Yang, S.; Wei, X.; Xiao, M.; Fu, X.; Zhu, C.; Mou, H. High-level expression of a thermophilic and acidophilic β-mannanase from Aspergillus kawachii IFO 4308 with significant potential in mannooligosaccharide preparation. Bioresour. Technol. 2020, 295, 122257. [Google Scholar] [CrossRef]
- Yan, S.; Duan, B.; Liu, C.; Liu, G.; Kang, L.; Sun, L.; Yi, L.; Zhang, Z.; Liu, Z.; Yuan, S. Heterologous expression, purification and characterization of an alkalic thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea. J. Fungi 2023, 9, 378. [Google Scholar] [CrossRef]
- Li, J.F.; Zhao, S.G.; Tang, C.D.; Wang, J.Q.; Wu, M.C. Cloning and functional expression of an acidophilic β-mannanase gene (Anman5A) from Aspergillus niger LW-1 in Pichia pastoris. J. Agric. Food Chem. 2012, 60, 765–773. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, J.; Zhou, H. bo A thermotolerant and cold-active mannan endo-1,4-β-mannosidase from Aspergillus niger CBS 513.88: Constitutive overexpression and high-density fermentation in Pichia pastoris. Bioresour. Technol. 2011, 102, 7538–7547. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, J.; Li, G.; Du, Y.; Yu, X. A Recombinant highly thermostable β-mannanase (ReTMan26) from thermophilic Bacillus subtilis (TBS2) expressed in Pichia pastoris and its pH and temperature stability. Appl. Biochem. Biotechnol. 2017, 182, 1259–1275. [Google Scholar] [CrossRef]
- Bien-Cuong, D.; Thi-Thu, D.; Berrin, J.G.; Haltrich, D.; Kim-Anh, T.; Sigoillot, J.C.; Yamabhai, M. Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mannan endo-1,4-beta-mannosidase from Aspergillus niger BK01. Microb. Cell Fact. 2009, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Fliedrová, B.; Gerstorferová, D.; Křen, V.; Weignerová, L. Production of Aspergillus niger β-mannosidase in Pichia pastoris. Protein Expr. Purif. 2012, 85, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Z.; Wang, Y.; Chen, W.; Fu, L.; Tang, W.; Chen, C.; Liu, Y.; Zhang, X.; Ma, L. High-level expression and characterization of a thermophilic β-mannanase from Aspergillus niger in Pichia pastoris. Biotechnol. Lett. 2015, 37, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, H.; Niu, C.; Shi, P.; Huang, H.; Meng, K.; Bai, Y.; Wang, K.; Hua, H.; Yao, B. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl. Microbiol. Biotechnol. 2015, 99, 1217–1228. [Google Scholar] [CrossRef]
- Wang, K.; Luo, H.; Bai, Y.; Shi, P.; Huang, H.; Xue, X.; Yao, B. A thermophilic endo-1,4-β-glucanase from Talaromyces emersonii CBS394.64 with broad substrate specificity and great application potentials. Appl. Microbiol. Biotechnol. 2014, 98, 7051–7060. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Pandey, A. Thermostable cellulases: Current status and perspectives. Bioresour. Technol. 2019, 279, 385–392. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess 2021, 8, 95. [Google Scholar] [CrossRef]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Thermostable cellulose saccharifying microbial enzymes: Characteristics, recent advances and biotechnological applications. Int. J. Biol. Macromol. 2021, 188, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef]
- Lázaro, R.; García, M.; Araníbar, M.J.; Mateos, G.G. Effect of enzyme addition to wheat-, barley- and rye-based diets on nutrient digestibility and performance of laying hens. Br. Poult. Sci. 2003, 44, 256–265. [Google Scholar] [CrossRef]
- Karunaratne, N.D.; Classen, H.L.; Ames, N.P.; Bedford, M.R.; Newkirk, R.W. Effects of hulless barley and exogenous beta-glucanase levels on ileal digesta soluble beta-glucan molecular weight, digestive tract characteristics, and performance of broiler chickens. Poult. Sci. 2021, 100, 100967. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, N.D.; Classen, H.L.; Ames, N.P.; Bedford, M.R.; Newkirk, R.W. Effects of diet hulless barley and beta-glucanase levels on ileal digesta soluble beta-glucan molecular weight and carbohydrate fermentation in laying hens. Poult. Sci. 2022, 101, 101735. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, N.; Mohamed, M.A.; Larbier, M. Effect of enzyme preparation containing xylanase and beta-glucanase on performance of laying hens fed wheat/barley- or maize/soybean meal-based diets. Br. Poult. Sci. 2003, 44, 60–66. [Google Scholar] [CrossRef]
- Bhat, M.K.; Bhat, S. Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 1997, 15, 583–620. [Google Scholar] [CrossRef]
- Sohail, M.; Barzkar, N.; Michaud, P.; Jahromi, S.T.; Babich, O.; Sukhikh, S.; Das, R.; Nahavandi, R. Cellulolytic and xylanolytic enzymes from yeasts: Properties and industrial applications. Molecules 2022, 27, 3783. [Google Scholar] [CrossRef] [PubMed]
- Šuchová, K.; Fehér, C.; Ravn, J.L.; Bedő, S.; Biely, P.; Geijer, C. Cellulose- and xylan-degrading yeasts: Enzymes, applications and biotechnological potential. Biotechnol. Adv. 2022, 59, 107981. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Yadav, A. Actinomycetes: A source of lignocellulolytic enzymes. Enzym. Res. 2015, 2015, 279381. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Aggarwal, N.K.; Yadav, A. Cellulolytic Potential of actinomycetes isolated from different habitats. Bioeng. Biosci. 2016, 4, 88–94. [Google Scholar] [CrossRef]
- de França Passos, D.; Pereira, N.; de Castro, A.M. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Bayer, E.A.; Laura, A.; Benatti, T.; De, M.; Teixeira, L.; Polizeli, M. Lignocellulolytic biocatalysts: The main players involved in multiple biotechnological processes for biomass valorization. Microorganisms 2023, 11, 162. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15, 106. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P. Genetic engineering of Trichoderma reesei cellulases and their production. Microb. Biotechnol. 2017, 10, 1485–1499. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of action, properties, production, and application of laccase: A review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Shrivastava, B.; Sastry, V.R.B.; Sehgal, N.; Kuhad, R.C. Middle-redox potential laccase from Ganoderma sp.: Its application in improvement of feed for monogastric animals. Sci. Rep. 2013, 3, 1299. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Basit, A.; Wen, J.; Liu, J.; Zheng, F.; Cao, Y.; Jiang, W. High efficient degradation of glucan/glucomannan to cello-/mannan-oligosaccharide by endoglucanase via tetrasaccharide as intermediate. Food Chem. 2021, 350, 129175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Yi, X.N.; Chen, Q.; Zhou, J.B.; Li, S.F.; Cai, X.; Chen, D.S.; Cheng, X.P.; Li, M.; Wang, H.Y.; et al. Improvement of catalytic performance of endoglucanase CgEndo from Colletotrichum graminicola by site-directed mutagenesis. Enzym. Microb. Technol. 2022, 154, 109963. [Google Scholar] [CrossRef] [PubMed]
- Amengual, N.G.; Csarman, F.; Wohlschlager, L.; Ludwig, R. Expression and characterization of a family 45 glycosyl hydrolase from Fomitopsis pinicola and comparison to Phanerochaete chrysosporium Cel45A. Enzym. Microb. Technol. 2022, 156, 110000. [Google Scholar] [CrossRef] [PubMed]
- Ramani, G.; Meera, B.; Vanitha, C.; Rajendhran, J.; Gunasekaran, P. Molecular cloning and expression of thermostable glucose-tolerant β-glucosidase of Penicillium funiculosum NCL1 in Pichia pastoris and its characterization. J. Ind. Microbiol. Biotechnol. 2015, 42, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Woon, J.S.K.; Mackeen, M.M.; Illias, R.M.; Mahadi, N.M.; Broughton, W.J.; Murad, A.M.A.; Bakar, F.D.A. Cellobiohydrolase B of Aspergillus niger over-expressed in Pichia pastoris stimulates hydrolysis of oil palm empty fruit bunches. PeerJ 2017, 5, e3909. [Google Scholar] [CrossRef]
- Li, L.; Qu, M.; Liu, C.; Pan, K.; Xu, L.; OuYang, K.; Song, X.; Li, Y.; Zhao, X. Expression of a recombinant Lentinula edodes cellobiohydrolase by Pichia pastoris and its effects on in vitro ruminal fermentation of agricultural straws. Int. J. Biol. Macromol. 2019, 134, 146–155. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Sunagawa, N.; Tachioka, M.; Igarashi, K.; Samejima, M. Thermostable mutants of glycoside hydrolase family 6 cellobiohydrolase from the basidiomycete Phanerochaete chrysosporium. J. Appl. Glycosci. 2020, 67, 79–86. [Google Scholar] [CrossRef]
- Gutierrez-Gutierrez, D.A.; Fuentes-Garibay, J.A.; Viader-Salvadó, J.M.; Guerrero-Olazarán, M. Biochemical characterization of the β-glucosidase Glu1B from Coptotermes formosanus produced in Pichia pastoris. Enzym. Microb. Technol. 2023, 163, 110155. [Google Scholar] [CrossRef]
- Song, Q.; Deng, X.; Song, R.Q. Expression of Pleurotus ostreatus laccase gene in Pichia pastoris and its degradation of corn stover lignin. Microorganisms 2020, 8, 601. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Yin, Q.; Fang, W.; Xiao, Y.; Fang, Z. Expression of a thermo- and alkali-philic fungal laccase in Pichia pastoris and its application. Protein Expr. Purif. 2019, 154, 16–24. [Google Scholar] [CrossRef]
- Yadav, D.; Ranjan, B.; Mchunu, N.; Le Roes-Hill, M.; Kudanga, T. Secretory expression of recombinant small laccase from Streptomyces coelicolor A3(2) in Pichia pastoris. Int. J. Biol. Macromol. 2018, 108, 642–649. [Google Scholar] [CrossRef]
- Li, Q.; Pei, J.; Zhao, L.; Xie, J.; Cao, F.; Wang, G. Overexpression and characterization of laccase from Trametes versicolor in Pichia pastoris. Prikl. Biokhim. Mikrobiol. 2014, 50, 163–170. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wang, W.; Wang, F.Q.; Wei, D.Z. A comparative study of β-1,4-endoglucanase (possessing β-1,4-exoglucanase activity) from Bacillus subtilis LH expressed in Pichia pastoris GS115 and Escherichia coli Rosetta (DE3). Bioresour. Technol. 2012, 110, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lindenmuth, B.E.; McDonald, K.A. Production and characterization of Acidothermus cellulolyticus endoglucanase in Pichia pastoris. Protein Expr. Purif. 2011, 77, 153–158. [Google Scholar] [CrossRef]
- Boonvitthya, N.; Bozonnet, S.; Burapatana, V.; O’Donohue, M.J.; Chulalaksananukul, W. Comparison of the heterologous expression of Trichoderma reesei endoglucanase II and cellobiohydrolase II in the yeasts Pichia pastoris and Yarrowia lipolytica. Mol. Biotechnol. 2013, 54, 158–169. [Google Scholar] [CrossRef]
- Chen, X.; Ma, W.; Hu, N.; Yan, Y.; Zhu, Y.; Wang, Z.; Jiao, G.; Chen, X. Effects of alkaline protease on the production performance, egg quality, and cecal microbiota of hens during late laying period. Anim. Sci. J. 2021, 92, e13658. [Google Scholar] [CrossRef]
- Poudel, I.; Hodge, V.R.; Wamsley, K.G.S.; Roberson, K.D.; Adhikari, P.A. Effects of protease enzyme supplementation and varying levels of amino acid inclusion on productive performance, egg quality, and amino acid digestibility in laying hens from 30 to 50 weeks of age. Poult. Sci. 2023, 102, 102465. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S.; Rooke, J.A.; Galbraith, H.; Bedford, M.R. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br. Poult. Sci. 2002, 43, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, R.; Gutiérrez-Sánchez, G.; Bergmann, C.W.; Loera-Corral, O.; Rojo-Domínguez, A.; Huerta-Ochoa, S.; Regalado-González, C.; Prado-Barragán, L.A. Purification and characterization of a thermodynamic stable serine protease from Aspergillus fumigatus. Process Biochem. 2011, 46, 2001–2006. [Google Scholar] [CrossRef]
- Song, P.; Xu, W.; Zhang, Y.; Wang, F.; Zhou, X.; Shi, H.; Feng, W. A new carboxypeptidase from Aspergillus niger with good thermostability, pH stability and broad substrate specificity. Sci. Rep. 2021, 11, 18745. [Google Scholar] [CrossRef]
- Wei, M.; Chen, P.; Zheng, P.; Tao, X.; Yu, X.; Wu, D. Purification and characterization of aspartic protease from Aspergillus niger and its efficient hydrolysis applications in soy protein degradation. Microb. Cell Fact. 2023, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, K.; Bhat, S.K.; Singh, S.A.; Marathe, G.K.; Appu Rao, A.R.G. Aspartic protease from Aspergillus niger: Molecular characterization and interaction with pepstatin A. Int. J. Biol. Macromol. 2019, 139, 199–212. [Google Scholar] [CrossRef]
- Guo, Y.; Tu, T.; Zheng, J.; Bai, Y.; Huang, H.; Su, X.; Wang, Y.; Wang, Y.; Yao, B.; Luo, H. Improvement of BsAPA aspartic protease thermostability via autocatalysis-resistant mutation. J. Agric. Food Chem. 2019, 67, 10505–10512. [Google Scholar] [CrossRef]
- Beklemishev, A.B.; Pykhtina, M.B.; Kulikov, Y.M.; Goryachkovskaya, T.N.; Bochkov, D.V.; Sergeeva, S.V.; Vasileva, A.R.; Romanov, V.P.; Novikova, D.S.; Peltek, S.E. Creation of a recombinant Komagataella phaffii strain, a producer of proteinase K from Tritirachium album. Vavilovskii Zhurnal Genet. Sel. 2021, 25, 882–888. [Google Scholar] [CrossRef]
- Zadorozhny, A.V.; Voskoboev, M.E.; Bochkov, D.V.; Rozanov, A.S.; Shedko, E.D.; Mescheryakova, I.A.; Blinov, A.G.; Korzhuk, A.V.; Shlyakhtun, V.N.; Bogacheva, N.V.; et al. Heterologous expression of extracellular proteinase pAsPs of Aspergillus pseudotamarii in Komagataella phaffii. Int. J. Mol. Sci. 2022, 23, 15035. [Google Scholar] [CrossRef]
- Zadorozhny, A.V.; Pavlova, E.Y.; Shipova, V.S.A.; Goryachkovskaya, T.N.; Peltek, S.E. Heterologous expression of the Thermothielavioides terrestris mannanase gene in the Komagataella phaffii genome. Biotechnology 2022, 8, 344–351. [Google Scholar] [CrossRef]

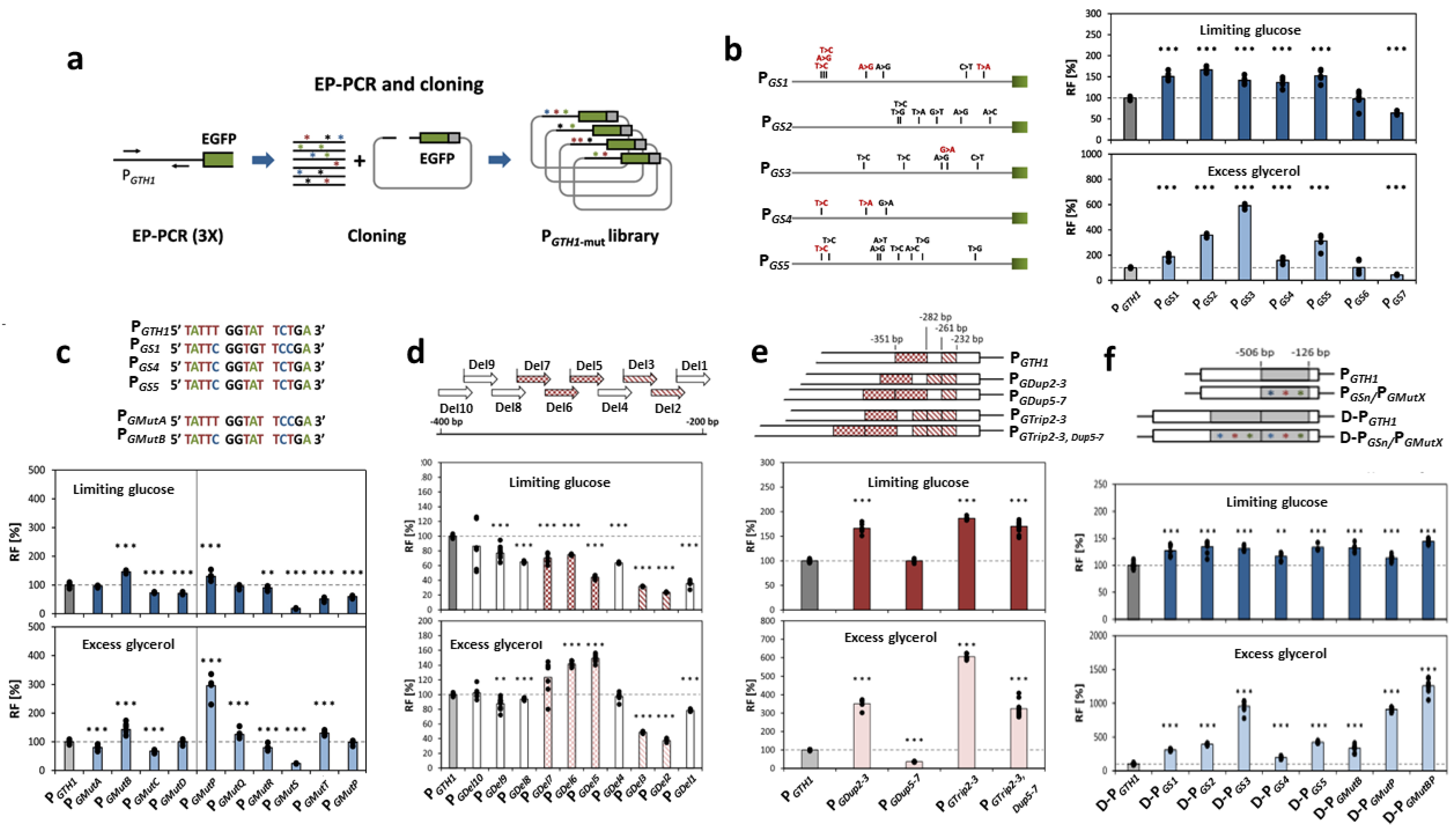


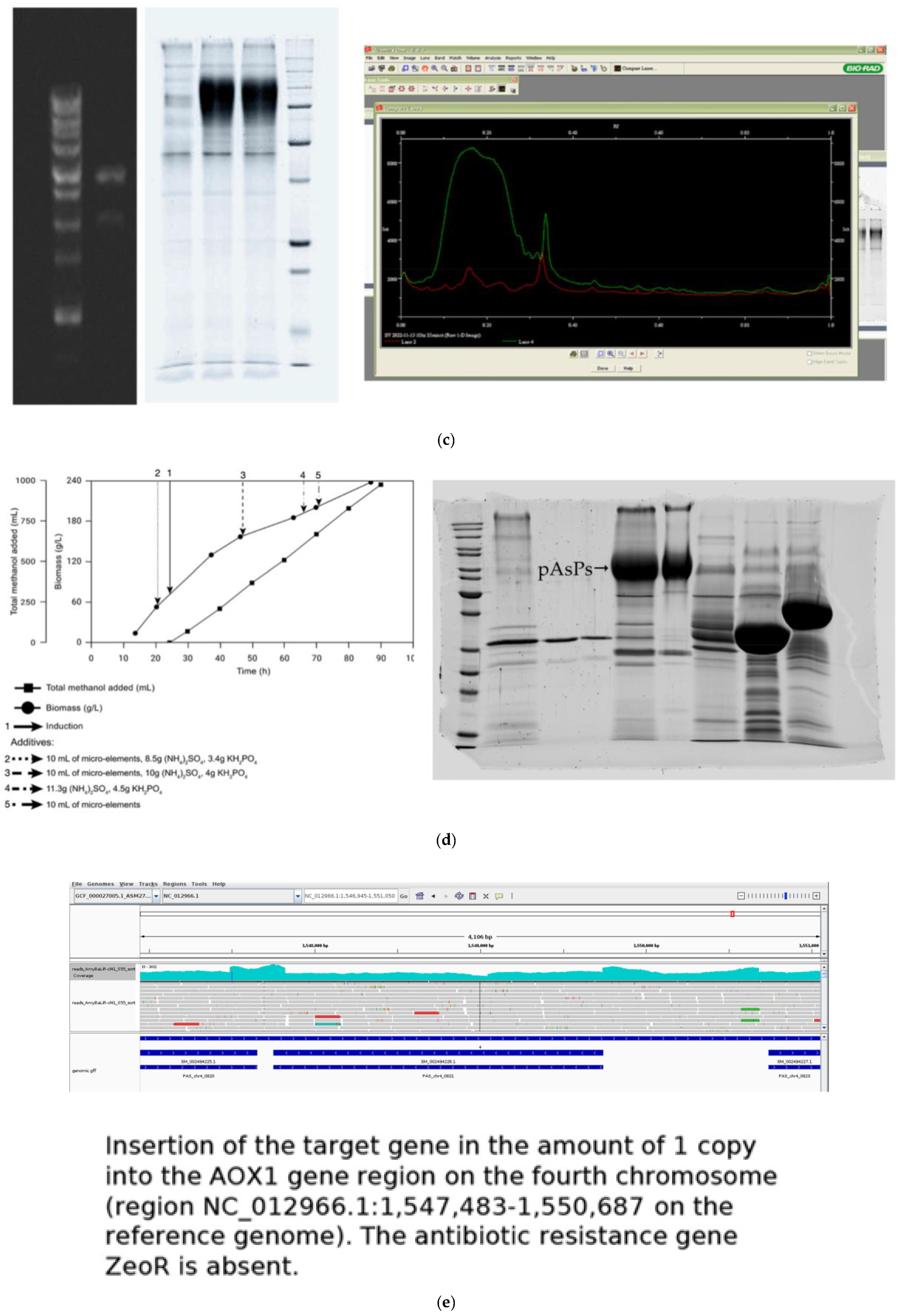
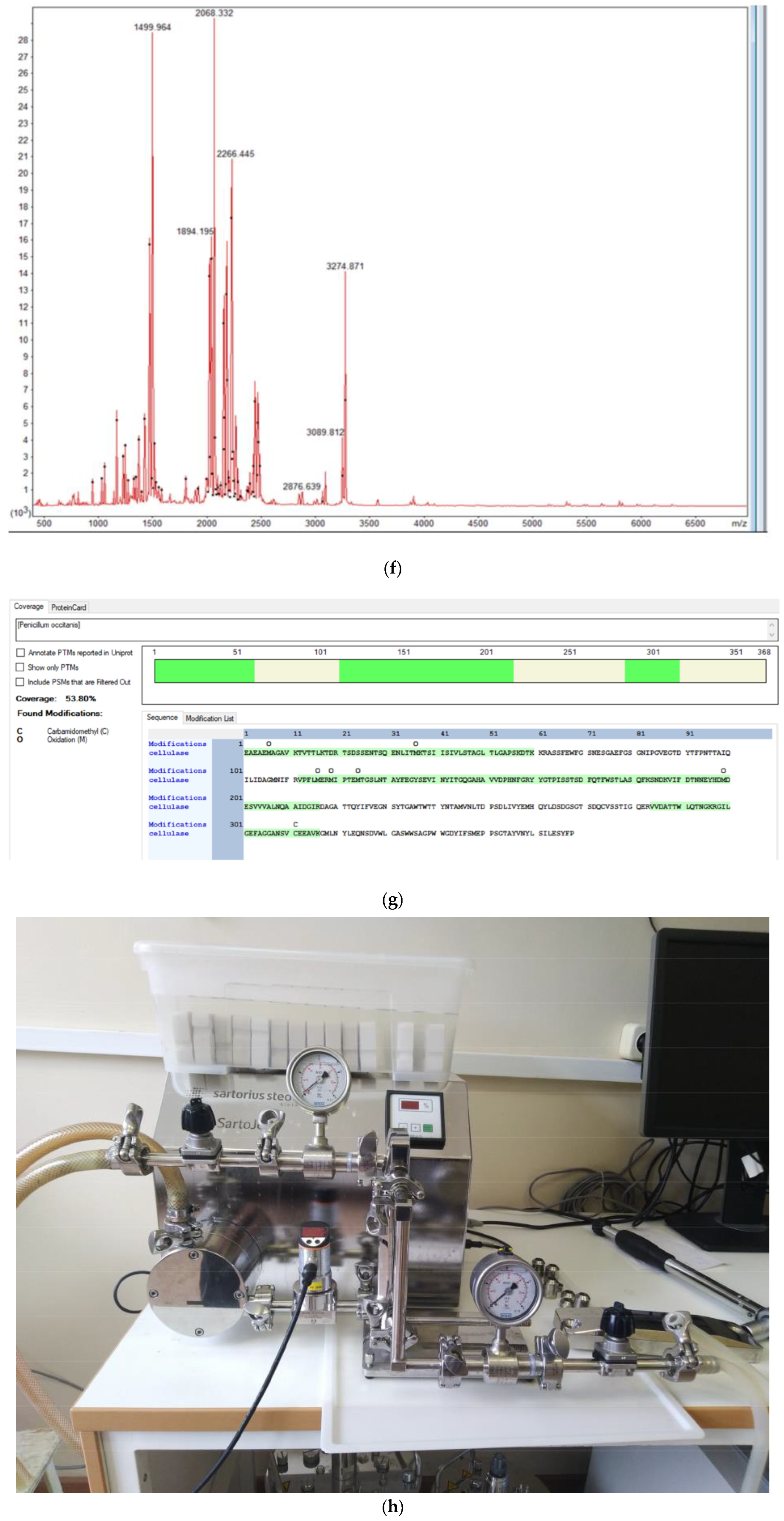
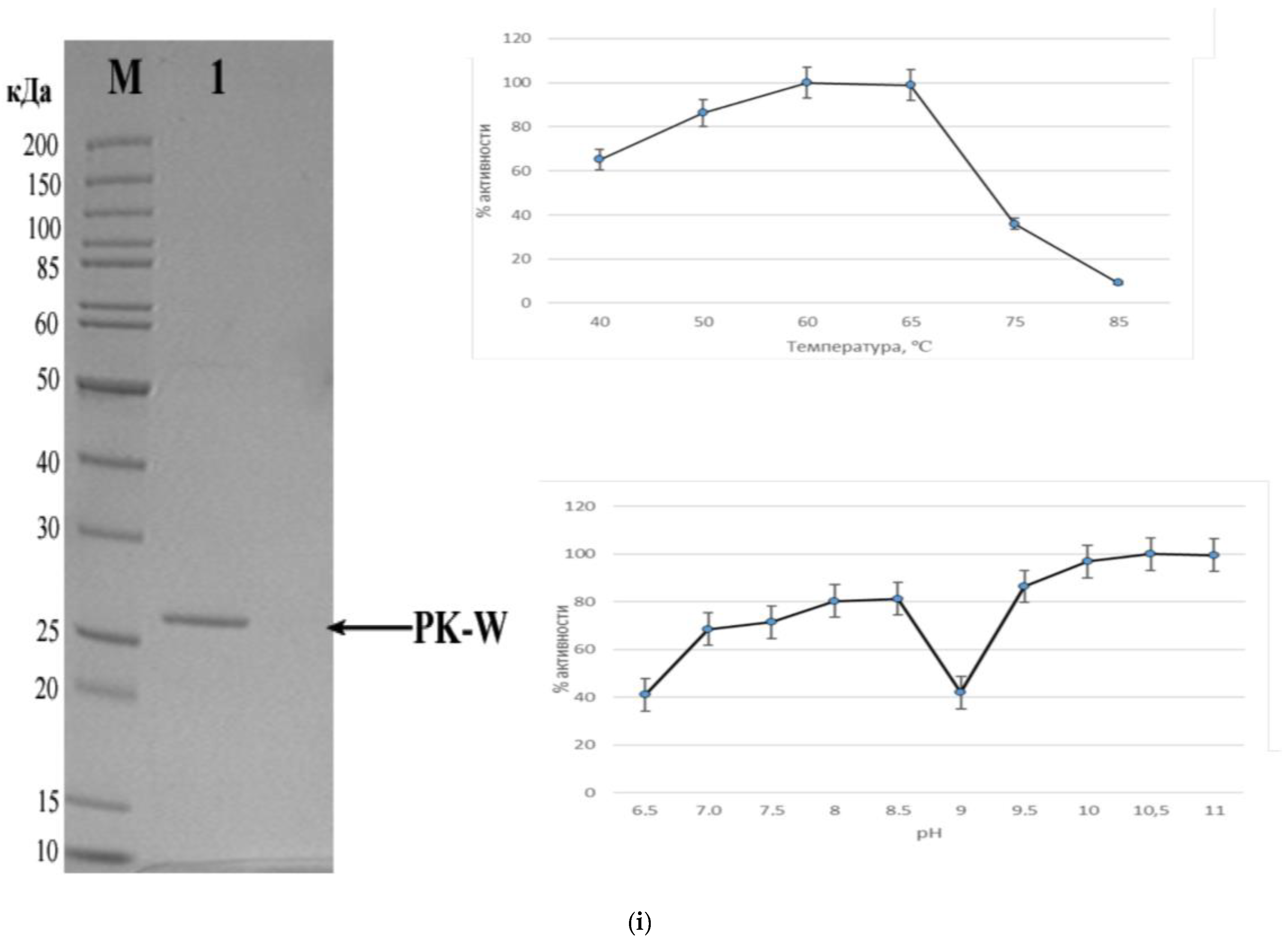
| Protein Name | Description | Effects of Protein Co-Overexpression on Target Protein Production in K. phaffii * |
|---|---|---|
| Bfr2 | Essential protein possibly involved in secretion; multicopy suppressor o1f sensitivity to brefeldin A [136] | The secretion of Fab antibody fragment showed a 1.4-fold increase [136] |
| Bmh2 | 14-3-3 protein isoform; binds proteins and DNA, involved in regulation of many processes, including exocytosis and vesicle transport, protein exit from the ER [136,137] | The secretion of Fab antibody fragment showed a 1.4-fold increase [136] |
| Cup5 | Vacuolar ATP synthase proteolipid subunit (EC 3.6.3.14) required for vacuolar acidification. Important for copper and iron metal ion homeostasis [131] | The secretion of Fab antibody fragment showed a 1.7-fold increase [136] |
| Ero1 | Pdi oxidase, protein-thiol disulfide exchange; required for oxidative protein folding in the ER [131,136] | The secretion of Fab antibody fragment showed a 1.4-fold increase [136]. The secretion of human albumin (HSA) fusion protein IL2-HSAa showed a 2.3-fold increase [23]. Phytase production was not improved by sole co-overexpression of Ero1, while the co-overexpression Pdi1 and Ero1 improved phytase production by 1.21 ± 0.06-fold [56] |
| Hac1 | bZIP transcription factor that regulates the unfolded protein response via UPRE binding and membrane biogenesis [136] | Increase, decrease, or have no effect on the production of the target protein [136] |
| Kar2 | Binding protein BiP, ATPase involved in protein import into the ER, acts as a chaperone to mediate protein folding in the ER; regulates the unfolded protein response [131] | The secretion of Fab antibody fragment showed a 1.5-fold increase [133]. The secretion of human albumin (HSA) fusion protein IL2-HSAa showed a 1.9-fold increase [23] No effect on the phytase AppA production [56] |
| Kin2 | Serine/threonine protein kinase involved in regulation of exocytosis; localizes to the cytoplasmic face of the plasma membrane [131] | The secretion of Fab antibody fragment showed a 1.5-fold increase [136] |
| Pdi1 | Protein disulfide isomerase, multifunctional protein resident in the ER lumen, essential for the formation of disulfide bonds in secretory and cell-surface proteins [131] | Increases the production of heterologous proteins when K. phaffii serves as a cell host [23,136,137,138] |
| Sbh1 | A subunit of the Sec61p ER translocation complex [139] | No effect on the IgG production [139] |
| Sec1 | Interacts with vesicle trafficking between the Golgi and cell membrane [56] | The secretion of human albumin (HSA) fusion protein IL2-HSAa 2.5-fold increase [23]. No effect on phytase AppA production [56] |
| Sly1 | Regulates ER-Golgi trafficking and Sec1 interacts with vesicle trafficking between the Golgi and cell membrane [56] | The secretion of human albumin (HSA) fusion protein IL2-HSAa showed a 1.9-fold increase [23]. No effect on the phytase AppA production [56] |
| Ssa4 | Cytoplasmic member of the HSP70 family; highly induced upon stress; plays a role in SRP-dependent cotranslational protein-membrane targeting and translocation [131] | The secretion of human albumin (HSA) fusion protein IL2-HSAa showed a 1.9-fold increase [23]. No effect on the phytase AppA production [56] |
| Sse1 | ATPase that is a component of the Hsp90 chaperone complex; binds unfolded proteins; member of the HSP70 family; localized to the cytoplasm [131] | The secretion of Fab antibody fragment showed a 1.4-fold increase [136] |
| Gene Origin | Specific Activity, U/mg | Temperature Optima, °C | pH Optima, (Range) | Thermostability | Productivity, mg/mL | Reference |
|---|---|---|---|---|---|---|
| Cronobacter turicensis | 1705 | 50 | 4.5 (2–8) | 65%, 5 min, 60 °C | – | [36] |
| Aspergillus niger | 142 | 60 | 2.5 and 5.5 | t1/2 10 min, 80 °C | 6.1 | [40] |
| E. coli | 19,880 | – | 5.5 | 30%, 99 °C, 60 min | 0.04 | [198] |
| Shigella sp. CD2 | 967 | 60 | 5.5 (3.5–6.5) | >60% 30 min, 70 °C | – | [203] |
| Citrobacter braakii | 3.5 × 106 | 55 | 4.5 (2–7) | – | 3.2 | [205] |
| C. amalonaticus | 3548 | 50 | 4.5 | – | 4.2 | [206] |
| C.gillenii | 1577 | 55 | 4.5 (3–6) | 50%, 65 °C, 5 min | – | [207]. |
| Yersinia intermedia | 3960 | 55 | 4.5 (2–6) | >50%, 80 °C, 15 min | – | [208] |
| Gene Origin | Activity | Temperature Optima, °C | pH Optima | Stability | Reference |
|---|---|---|---|---|---|
| Chaetomium sp. CQ31 | 10,017 U/mL 1208 U/mg | 85 | 6.5 (5.0–9.5) | stable up to 60 °C | [241] |
| Gloeophyllum trabeum | 1205 ± 28 U/mg | 75 | 4.5 | >60%, 70 °C, 30 min | [242] |
| Myceliophthora thermophila | 2010 U/mL | 60 | 6.0 | 60%, 70 °C, 30 min 70%, pH 2–12, 60 min | [243] |
| Aspergillus sulphureus | 1684 U/mL 218 U/mg | 55 | 3.0 (2–4) | t1/2 39.6 min, 60 °C t1/2 9.5 min, 70 °C | [244] |
| Aspergillus sulphureus | 180 U/mL | 70 | 5.0 (4.5–6.5) | 50% 30 min, 60 °C | [245] |
| Aspergillus oryzae | 258,240 U/mL | 60 | 7.5 (2.5–10) | stable up to 85 °C | [246] |
| Bispora sp. MEY–1 | 73,400 U/mL | 85 | 3 and 4.5–5.0 | 100%, 80 °C, 60 min >87%, 90 °C, 10 min t1/2 45 h, 80 °C t1/2 3 h, 85 °C 80%, pH 1.5–6.0, 1 h | [247] |
| Trichoderma asperellum | 393 ± 18 U/mg | 50 | 3 (2–5) | >60% 30 min, 55 °C | [248] |
| Paecilomyces thermophila | 52,940 U/mL 6536 U/mg | 75 | 7 (4.5–10) | stable up to 80 °C | [249] |
| Aspergillus niger | 52,940 U/mg | 50 | 5.0 (2.2–7.0) | >40%, 90 °C, 10 min | [250] |
| Thermobifida fusca | 515.8 U/mg | 80 | 5.0–9.0 | >60%, pH 6–9, 60 min >60%, 80 °C, 30 min | [251] |
| Streptomyces sp. FA1 | 3925 U/mL 289 U/mg | 55 | 5.0 | – | [252] |
| Thermotoga maritima | 40,020 U/mL 3962 U/mg | 100 | 5.5 | 90%, pH 4–11, 30 min >90%, 90 °C, 30 min | [253] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlebodarova, T.M.; Bogacheva, N.V.; Zadorozhny, A.V.; Bryanskaya, A.V.; Vasilieva, A.R.; Chesnokov, D.O.; Pavlova, E.I.; Peltek, S.E. Komagataella phaffii as a Platform for Heterologous Expression of Enzymes Used for Industry. Microorganisms 2024, 12, 346. https://doi.org/10.3390/microorganisms12020346
Khlebodarova TM, Bogacheva NV, Zadorozhny AV, Bryanskaya AV, Vasilieva AR, Chesnokov DO, Pavlova EI, Peltek SE. Komagataella phaffii as a Platform for Heterologous Expression of Enzymes Used for Industry. Microorganisms. 2024; 12(2):346. https://doi.org/10.3390/microorganisms12020346
Chicago/Turabian StyleKhlebodarova, Tamara M., Natalia V. Bogacheva, Andrey V. Zadorozhny, Alla V. Bryanskaya, Asya R. Vasilieva, Danil O. Chesnokov, Elena I. Pavlova, and Sergey E. Peltek. 2024. "Komagataella phaffii as a Platform for Heterologous Expression of Enzymes Used for Industry" Microorganisms 12, no. 2: 346. https://doi.org/10.3390/microorganisms12020346







