The Role of HIV-1-Encoded microRNAs in Viral Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Bioinformatic Prediction of miRNAs
2.3. Preparation of HIV-1 Virus Stocks
2.4. The pLKO.1 Cloning Vector Lentivirus System
2.5. Tough Decoy (TuD) Lentivirus System
2.6. Microarrays
2.7. RT-PCR
2.8. Deep Sequencing
2.9. Transduction with Lentiviruses
3. Results
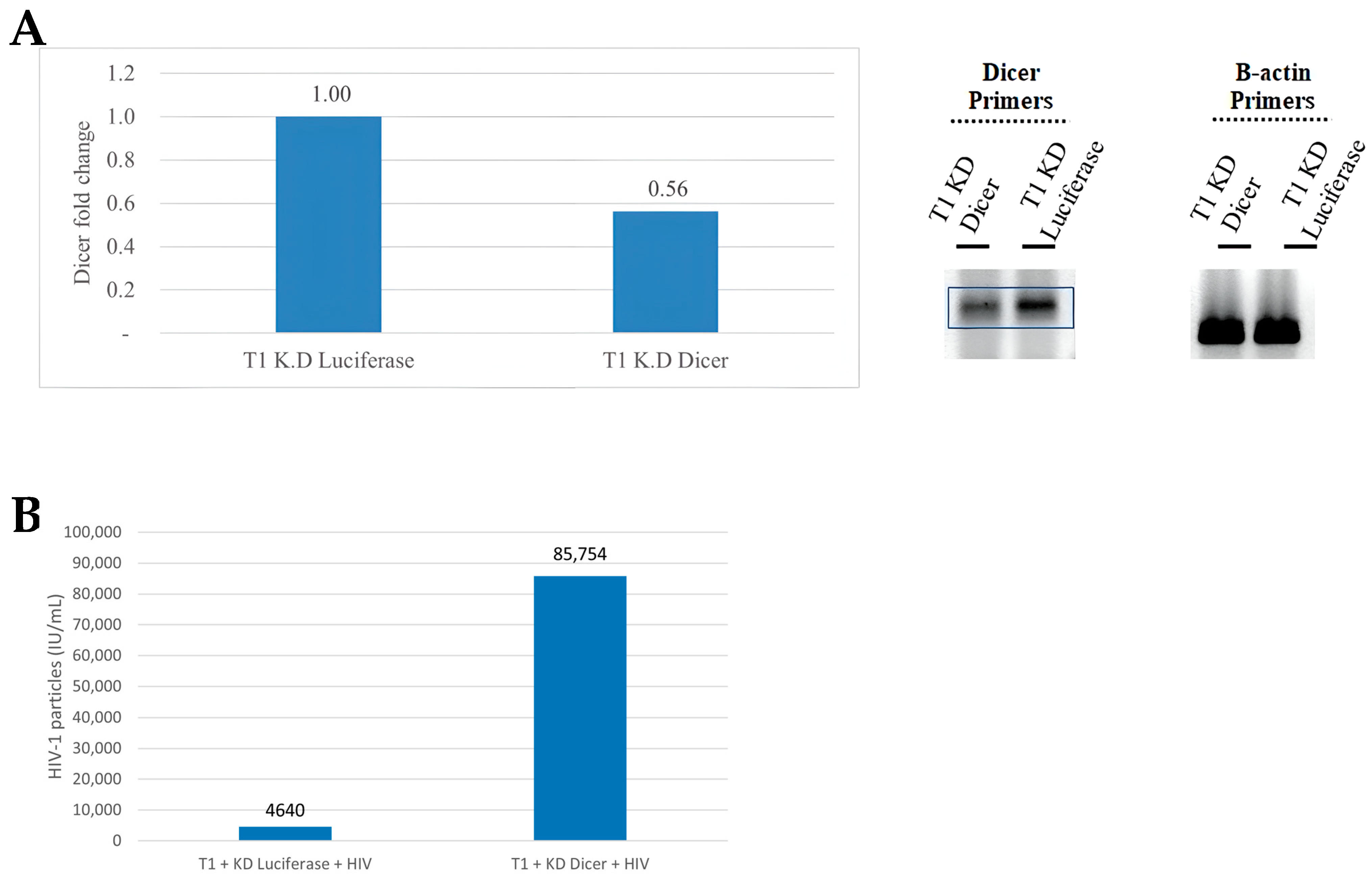
3.1. The Effect of Dicer Knockdown (KD) on HIV-1 Viral Replication
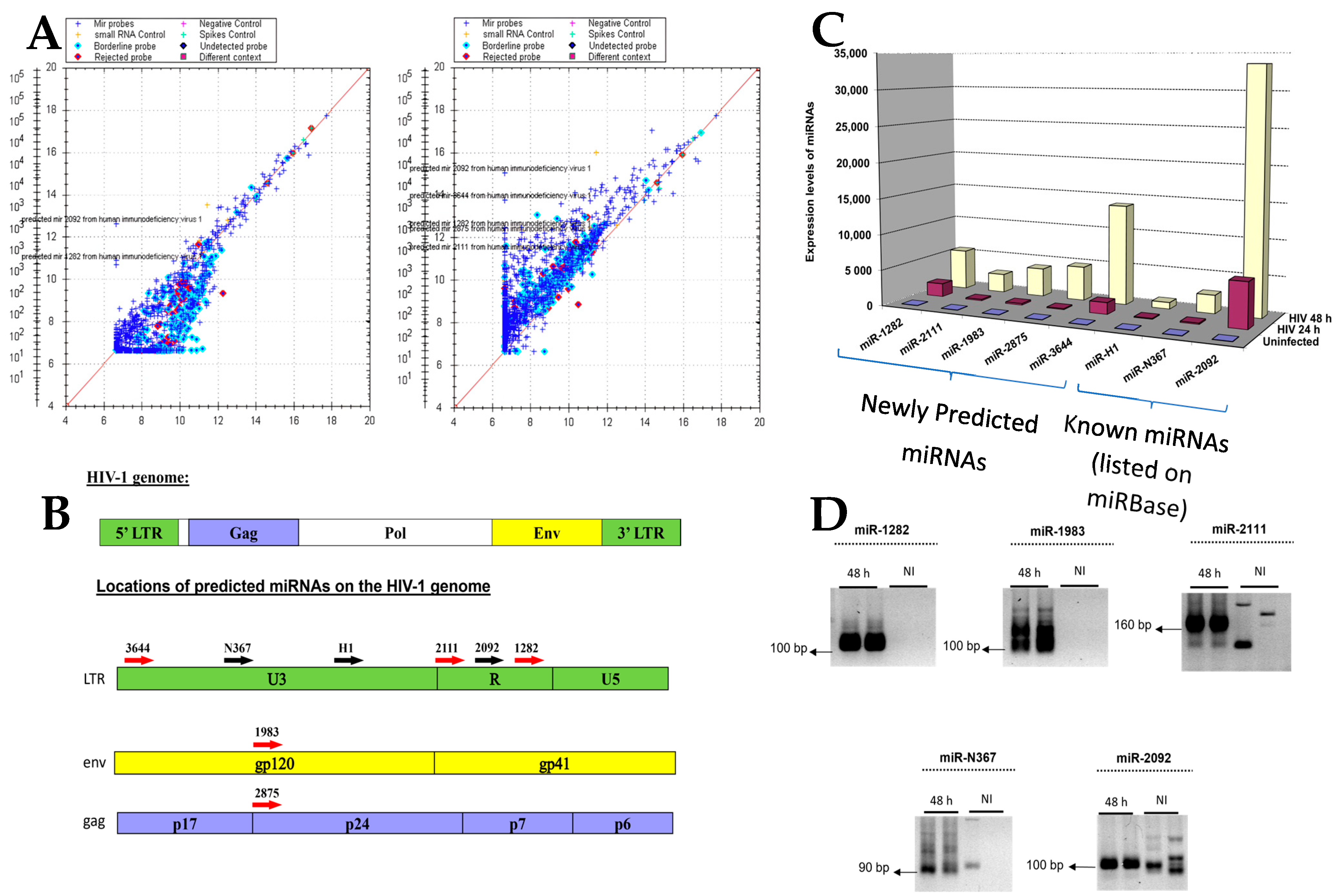
3.2. Validation of Bioinformatically-Predicted miRNA Sequences in the HIV-1 Genome
3.3. Identifying the Locations of miRNAs via Deep Sequencing
3.4. Confirmation of miRNA Expression in HIV-1-Infected Cells
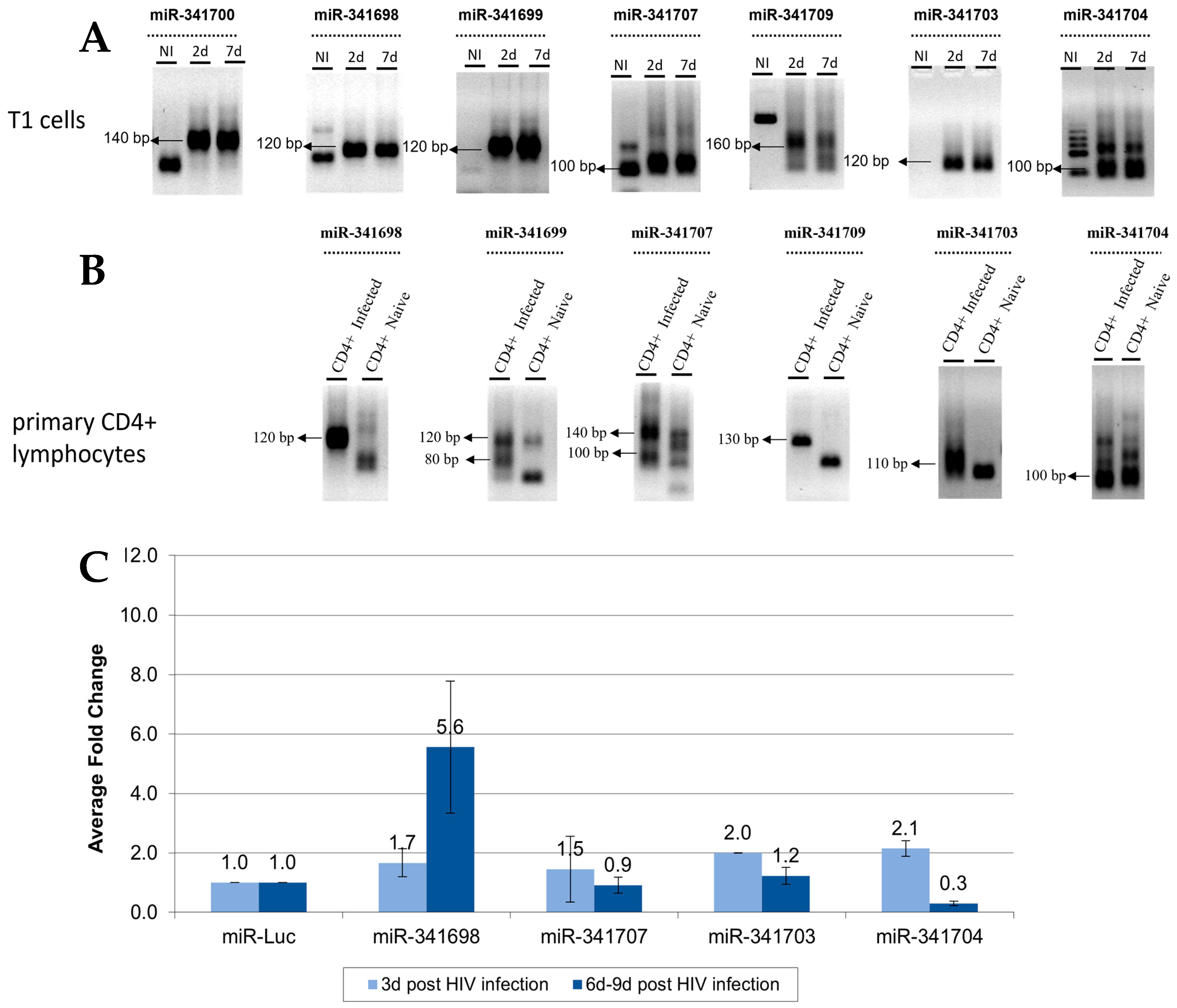
3.5. The Role of Candidate miRNAs in Viral Infection
3.6. Characterization of smRNA Sequences in HIV-1-Infected Cells
3.7. Effect of HIV-1-Associated smRNAs on the Suppression of Viral Replication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, M.; Pandhare, J.; Dash, C. Are microRNAs Important Players in HIV-1 Infection? An Update. Viruses 2018, 10, 110. [Google Scholar] [CrossRef]
- Bennasser, Y.; Le, S.Y.; Yeung, M.L.; Jeang, K.T. HIV-1 encoded candidate micro-RNAs and their cellular targets. Retrovirology 2004, 1, 43. [Google Scholar] [CrossRef]
- Klase, Z.; Kale, P.; Winograd, R.; Gupta, M.V.; Heydarian, M.; Berro, R.; McCaffrey, T.; Kashanchi, F. HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol. Biol. 2007, 8, 63. [Google Scholar] [CrossRef]
- Klase, Z.; Winograd, R.; Davis, J.; Carpio, L.; Hildreth, R.; Heydarian, M.; Fu, S.; McCaffrey, T.; Meiri, E.; Ayash-Rashkovsky, M.; et al. HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology 2009, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Ito, M.; Tsutsumi, Y.; Ichikawa, Y.; Okuyama, H.; Brisibe, E.A.; Saksena, N.K.; Fujii, Y.R. HIV-1 nef suppression by virally encoded microRNA. Retrovirology 2004, 1, 44. [Google Scholar] [CrossRef]
- Ouellet, D.L.; Plante, I.; Landry, P.; Barat, C.; Janelle, M.E.; Flamand, L.; Tremblay, M.J.; Provost, P. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic Acids Res. 2008, 36, 2353–2365. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, M.; Geng, G.; Liu, B.; Huang, Z.; Luo, H.; Zhou, J.; Guo, X.; Cai, W.; Zhang, H. A novel HIV-1-encoded microRNA enhances its viral replication by targeting the TATA box region. Retrovirology 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Zaporozhchenko, I.A. The Fundamentals of miRNA Biology: Structure, Biogenesis, and Regulatory Functions. Russ. J. Bioorg. Chem. 2019, 46, 1–13. [Google Scholar] [CrossRef]
- Girardi, E.; Lopez, P.; Pfeffer, S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef]
- Bellini, N.; Lodge, R.; Pham, T.N.Q.; Jain, J.; Murooka, T.T.; Herschhorn, A.; Bernard, N.F.; Routy, J.P.; Tremblay, C.L.; Cohen, E.A. MiRNA-103 downmodulates CCR5 expression reducing human immunodeficiency virus type-1 entry and impacting latency establishment in CD4(+) T cells. iScience 2022, 25, 105234. [Google Scholar] [CrossRef] [PubMed]
- Heinson, A.I.; Woo, J.; Mukim, A.; White, C.H.; Moesker, B.; Bosque, A.; Spina, C.A.; Woelk, C.H.; Macarthur, B.D.; Beliakova-Bethell, N. Micro RNA Targets in HIV Latency: Insights into Novel Layers of Latency Control. AIDS Res. Hum. Retroviruses 2021, 37, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Okoye, I.; Xu, L.; Oyegbami, O.; Shahbaz, S.; Pink, D.; Gao, P.; Sun, X.; Elahi, S. Plasma Extracellular Vesicles Enhance HIV-1 Infection of Activated CD4(+) T Cells and Promote the Activation of Latently Infected J-Lat10.6 Cells via miR-139-5p Transfer. Front. Immunol. 2021, 12, 697604. [Google Scholar] [CrossRef]
- Su, B.; Fu, Y.; Liu, Y.; Wu, H.; Ma, P.; Zeng, W.; Zhang, T.; Lian, S.; Wu, H. Potential Application of MicroRNA Profiling to the Diagnosis and Prognosis of HIV-1 Infection. Front. Microbiol. 2018, 9, 3185. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, J.; Wu, H.; Yang, Z.; Jin, C.; Wang, J.; Cheng, L.; Peng, X.; Liu, F.; Peng, X.; et al. High-throughput sequencing identifies HIV-1-replication- and latency-related miRNAs in CD4(+) T cell lines. Arch. Virol. 2017, 162, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Bazie, W.W.; Boucher, J.; Traore, I.T.; Kania, D.; Some, D.Y.; Alary, M.; Gilbert, C. Vesicular MicroRNA as Potential Biomarkers of Viral Rebound. Cells 2022, 11, 859. [Google Scholar] [CrossRef]
- Tarancon-Diez, L.; Consuegra, I.; Vazquez-Alejo, E.; Ramos-Ruiz, R.; Ramos, J.T.; Navarro, M.L.; Munoz-Fernandez, M.A. miRNA Profile Based on ART Delay in Vertically Infected HIV-1 Youths Is Associated with Inflammatory Biomarkers and Activation and Maturation Immune Levels. Front. Immunol. 2022, 13, 878630. [Google Scholar] [CrossRef]
- Kaul, D.; Hussain, A. Cellular AATF gene encodes a novel miRNA that can contribute to HIV-1 latency. Indian J. Biochem. Biophys. 2009, 46, 237–240. [Google Scholar]
- Narayanan, A.; Kehn-Hall, K.; Bailey, C.; Kashanchi, F. Analysis of the roles of HIV-derived microRNAs. Expert Opin. Biol. Ther. 2011, 11, 17–29. [Google Scholar] [CrossRef]
- Weinberg, M.S.; Morris, K.V. Are viral-encoded microRNAs mediating latent HIV-1 infection? DNA Cell Biol. 2006, 25, 223–231. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, T.; Chen, Q. Exploring the expanding universe of small RNAs. Nat. Cell Biol. 2022, 24, 415–423. [Google Scholar] [CrossRef] [PubMed]
- DuBridge, R.B.; Tang, P.; Hsia, H.C.; Leong, P.M.; Miller, J.H.; Calos, M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 1987, 7, 379–387. [Google Scholar]
- Kimpton, J.; Emerman, M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 1992, 66, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Salter, R.D.; Howell, D.N.; Cresswell, P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 1985, 21, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Bentwich, I. Prediction and validation of microRNAs and their targets. FEBS Lett. 2005, 579, 5904–5910. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef]
- Haraguchi, T.; Ozaki, Y.; Iba, H. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009, 37, e43. [Google Scholar] [CrossRef]
- Barad, O.; Meiri, E.; Avniel, A.; Aharonov, R.; Barzilai, A.; Bentwich, I.; Einav, U.; Gilad, S.; Hurban, P.; Karov, Y.; et al. MicroRNA expression detected by oligonucleotide microarrays: System establishment and expression profiling in human tissues. Genome Res. 2004, 14, 2486–2494. [Google Scholar] [CrossRef]
- Shibata, R.; Hoggan, M.D.; Broscius, C.; Englund, G.; Theodore, T.S.; Buckler-White, A.; Arthur, L.O.; Israel, Z.; Schultz, A.; Lane, H.C.; et al. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 1995, 69, 4453–4462. [Google Scholar] [CrossRef]
- Ronen, R.; Gan, I.; Modai, S.; Sukacheov, A.; Dror, G.; Halperin, E.; Shomron, N. miRNAkey: A software for microRNA deep sequencing analysis. Bioinformatics 2010, 26, 2615–2616. [Google Scholar] [CrossRef]
- Coley, W.; Van Duyne, R.; Carpio, L.; Guendel, I.; Kehn-Hall, K.; Chevalier, S.; Narayanan, A.; Luu, T.; Lee, N.; Klase, Z.; et al. Absence of DICER in monocytes and its regulation by HIV-1. J. Biol. Chem. 2010, 285, 31930–31943. [Google Scholar] [CrossRef] [PubMed]
- Triboulet, R.; Mari, B.; Lin, Y.L.; Chable-Bessia, C.; Bennasser, Y.; Lebrigand, K.; Cardinaud, B.; Maurin, T.; Barbry, P.; Baillat, V.; et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 2007, 315, 1579–1582. [Google Scholar] [CrossRef]
- Biton, M.; Levin, A.; Slyper, M.; Alkalay, I.; Horwitz, E.; Mor, H.; Kredo-Russo, S.; Avnit-Sagi, T.; Cojocaru, G.; Zreik, F.; et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat. Immunol. 2011, 12, 239–246. [Google Scholar] [CrossRef]
- Yoshida, T.; Asano, Y.; Ui-Tei, K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Noncoding RNA 2021, 7, 57. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2019, 10, 3079. [Google Scholar] [CrossRef]
- Bennasser, Y.; Le, S.Y.; Benkirane, M.; Jeang, K.T. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 2005, 22, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.; Khanna, A.; Suman. Evidence and nature of a novel miRNA encoded by HIV-1. Proc. Indian Natn. Sci. Acad. 2006, 72, 91–95. [Google Scholar]
- Bukhari, M.M.M.; Mir, I.; Idrees, M.; Afzal, S.; Shahid, M. Role of MicroRNAs in Establishing Latency of Human Immunodeficiency Virus. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cullen, B.R. Analysis of the interaction of primate retroviruses with the human RNA interference machinery. J. Virol. 2007, 81, 12218–12226. [Google Scholar] [CrossRef]
- Pfeffer, S.; Sewer, A.; Lagos-Quintana, M.; Sheridan, R.; Sander, C.; Grasser, F.A.; van Dyk, L.F.; Ho, C.K.; Shuman, S.; Chien, M.; et al. Identification of microRNAs of the herpesvirus family. Nat. Methods 2005, 2, 269–276. [Google Scholar] [CrossRef]
- Cullen, B.R. Is RNA interference involved in intrinsic antiviral immunity in mammals? Nat. Immunol. 2006, 7, 563–567. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence | Number of Reads | No. of bps |
|---|---|---|---|
| smRNA-944 | AAACATCAGAAGGCTGTAGACAAATACTGGGA | 1393 | 32 |
| smRNA-2643 | AAAGCATTAGTAGAAATTTGTACAGAGATGGA | 4122 | 32 |
| smRNA-1667 | TTAGAGACTATGTAGACCGGT | 2448 | 21 |
| smRNA-5033 | TAGGGATTATGGAAAACAGATGGC | 2164 | 24 |
| smRNA-6185 | TTAATTGATAGACTAATAGAAAGAGCAGA | 1381 | 29 |
| smRNA-6516 | AATGACATGGTAGAACAGATGCATGAGGAT | 657 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmi, O.; Gotlieb, Y.; Shemer-Avni, Y.; Bentwich, Z. The Role of HIV-1-Encoded microRNAs in Viral Replication. Microorganisms 2024, 12, 425. https://doi.org/10.3390/microorganisms12030425
Carmi O, Gotlieb Y, Shemer-Avni Y, Bentwich Z. The Role of HIV-1-Encoded microRNAs in Viral Replication. Microorganisms. 2024; 12(3):425. https://doi.org/10.3390/microorganisms12030425
Chicago/Turabian StyleCarmi, Ofira, Yosef Gotlieb, Yonat Shemer-Avni, and Zvi Bentwich. 2024. "The Role of HIV-1-Encoded microRNAs in Viral Replication" Microorganisms 12, no. 3: 425. https://doi.org/10.3390/microorganisms12030425






