How Organic Mulching Influences the Soil Bacterial Community Structure and Function in Urban Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Soil and Organic Mulches
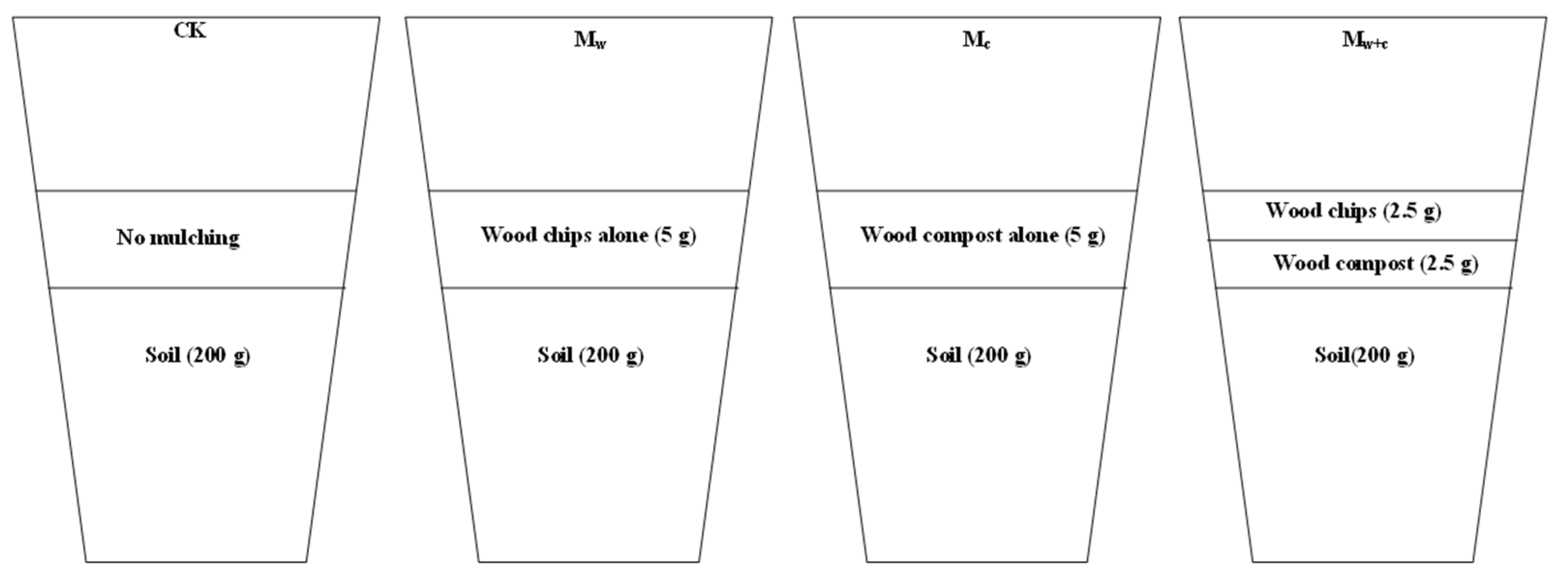
2.2. Experimental Design
2.3. Soil Physicochemical Properties Measurements
2.4. Soil DNA Extraction, Illumina Miseqsequencing
2.5. Statistical Analysis
3. Results
3.1. Effects of Organic Mulching on Soil Physicochemical Properties
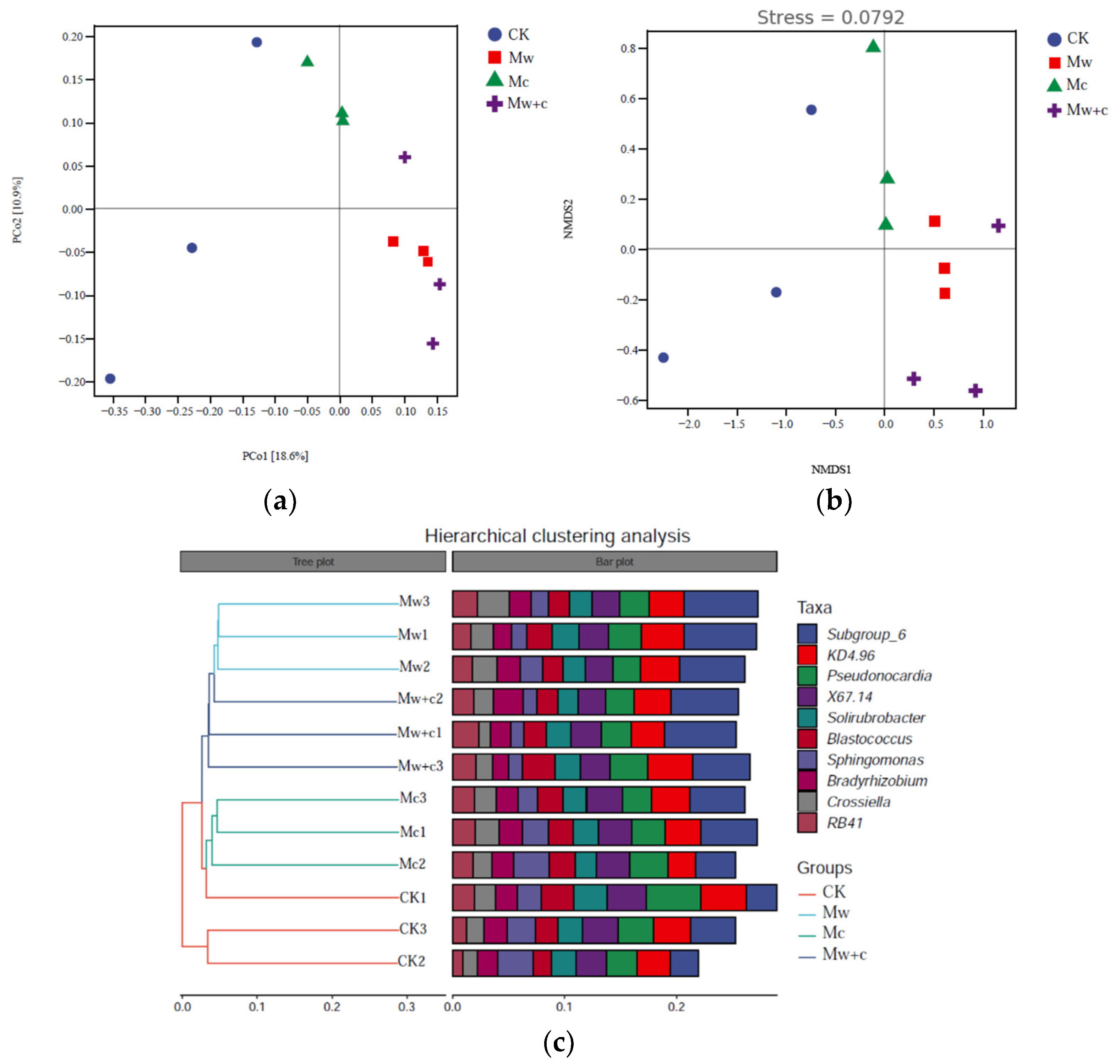
3.2. Effects of Organic Mulching on Soil Bacterial Community
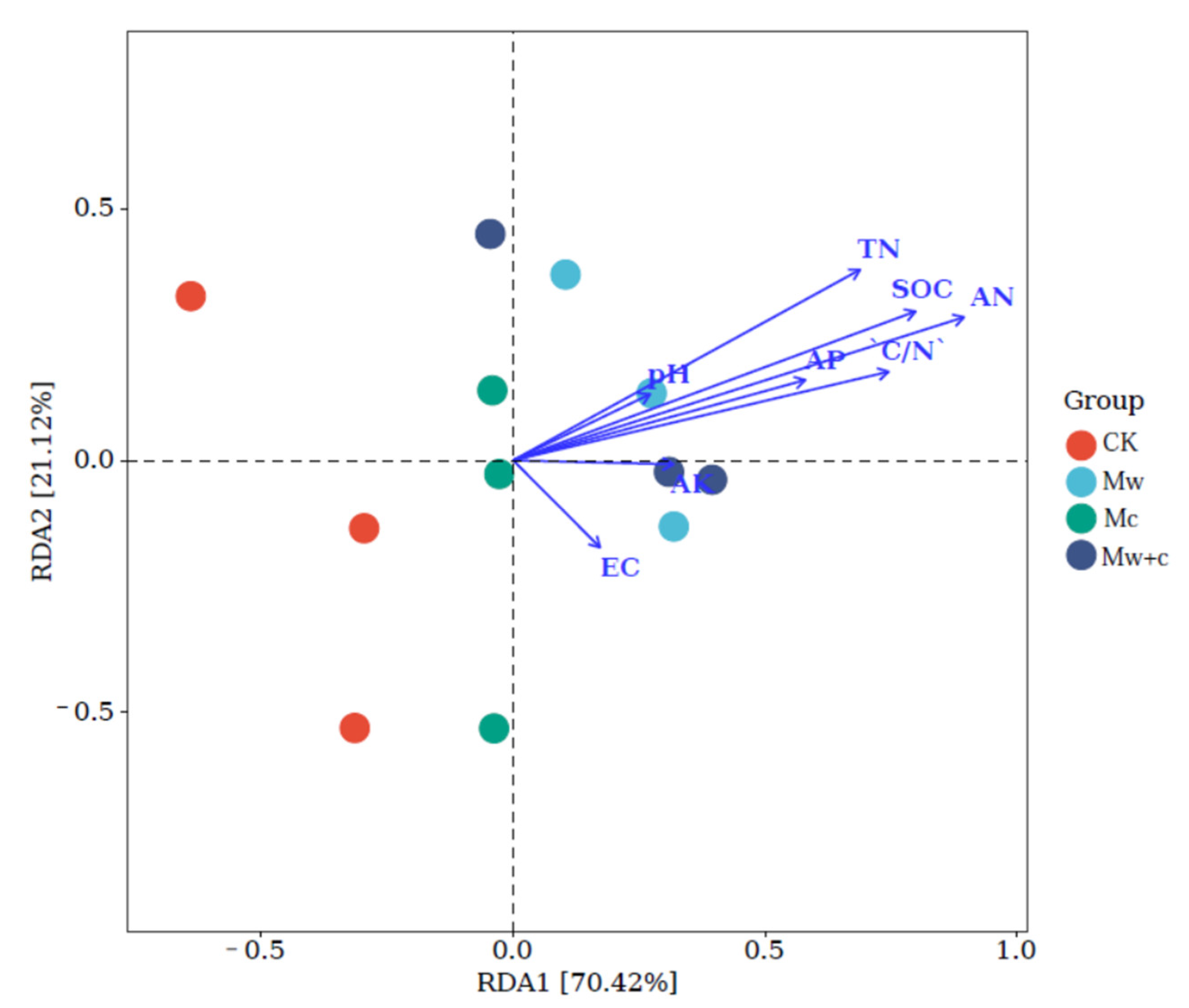
3.3. Relationship of Soil Physicochemicaly Properties and Bacterial Community
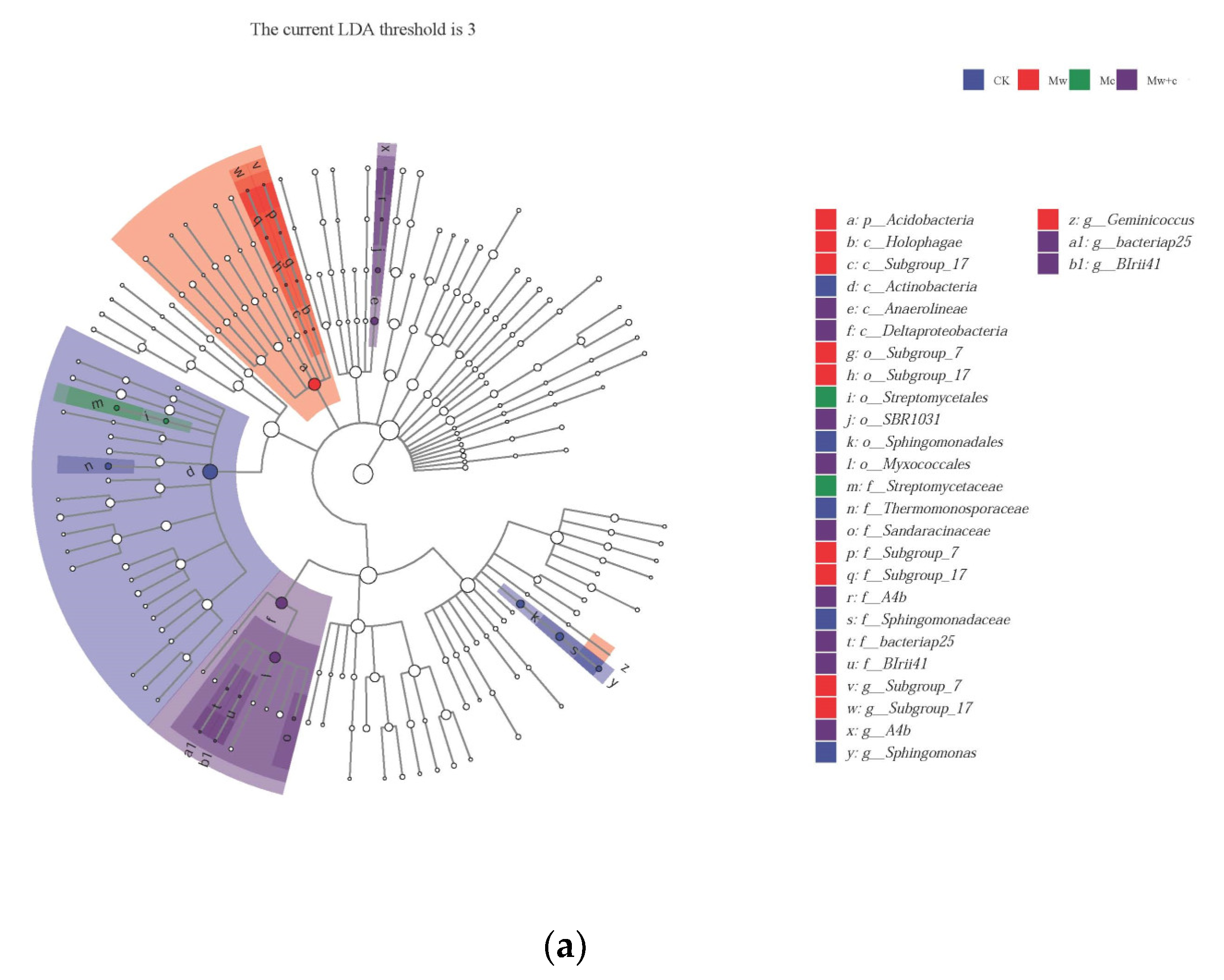
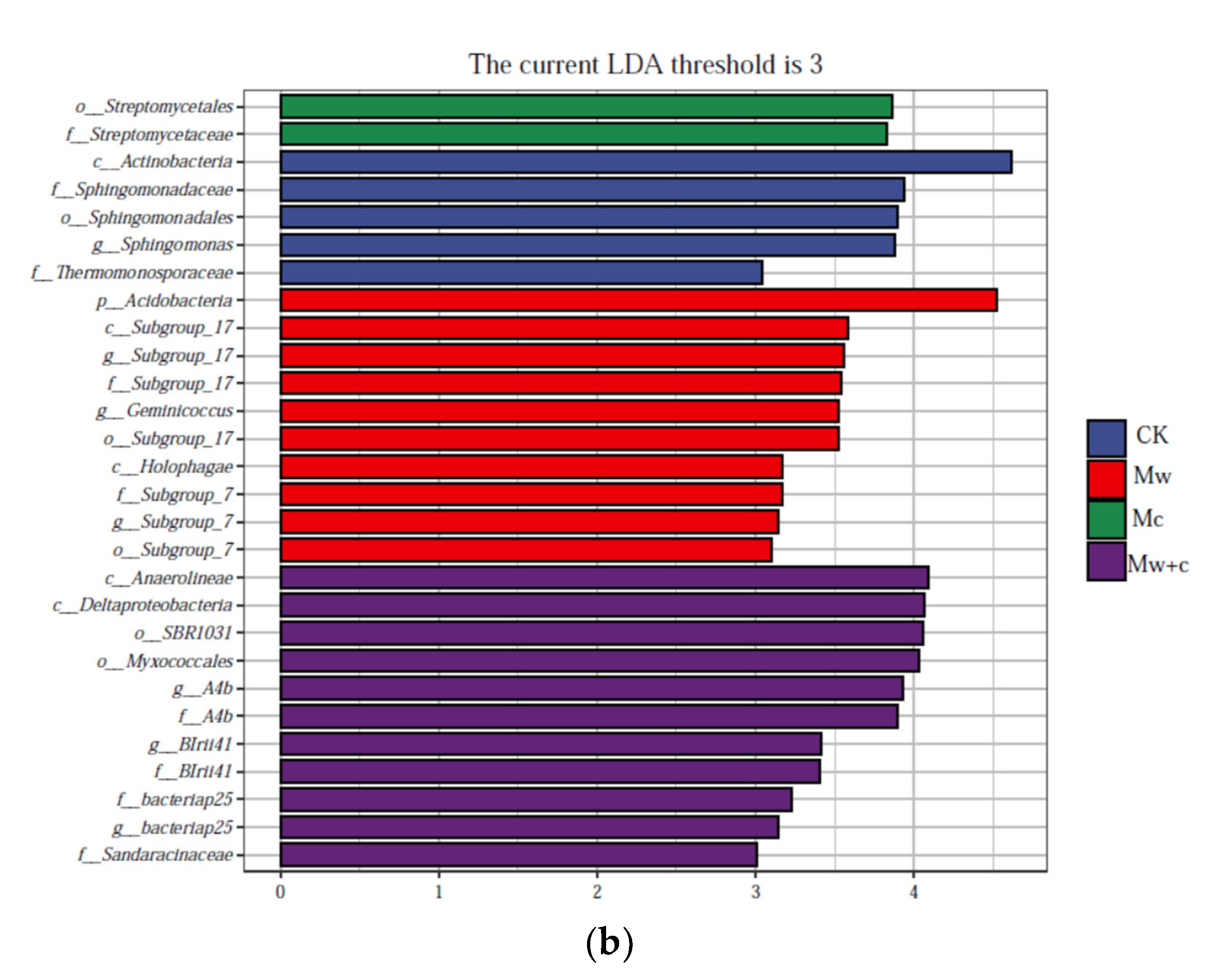
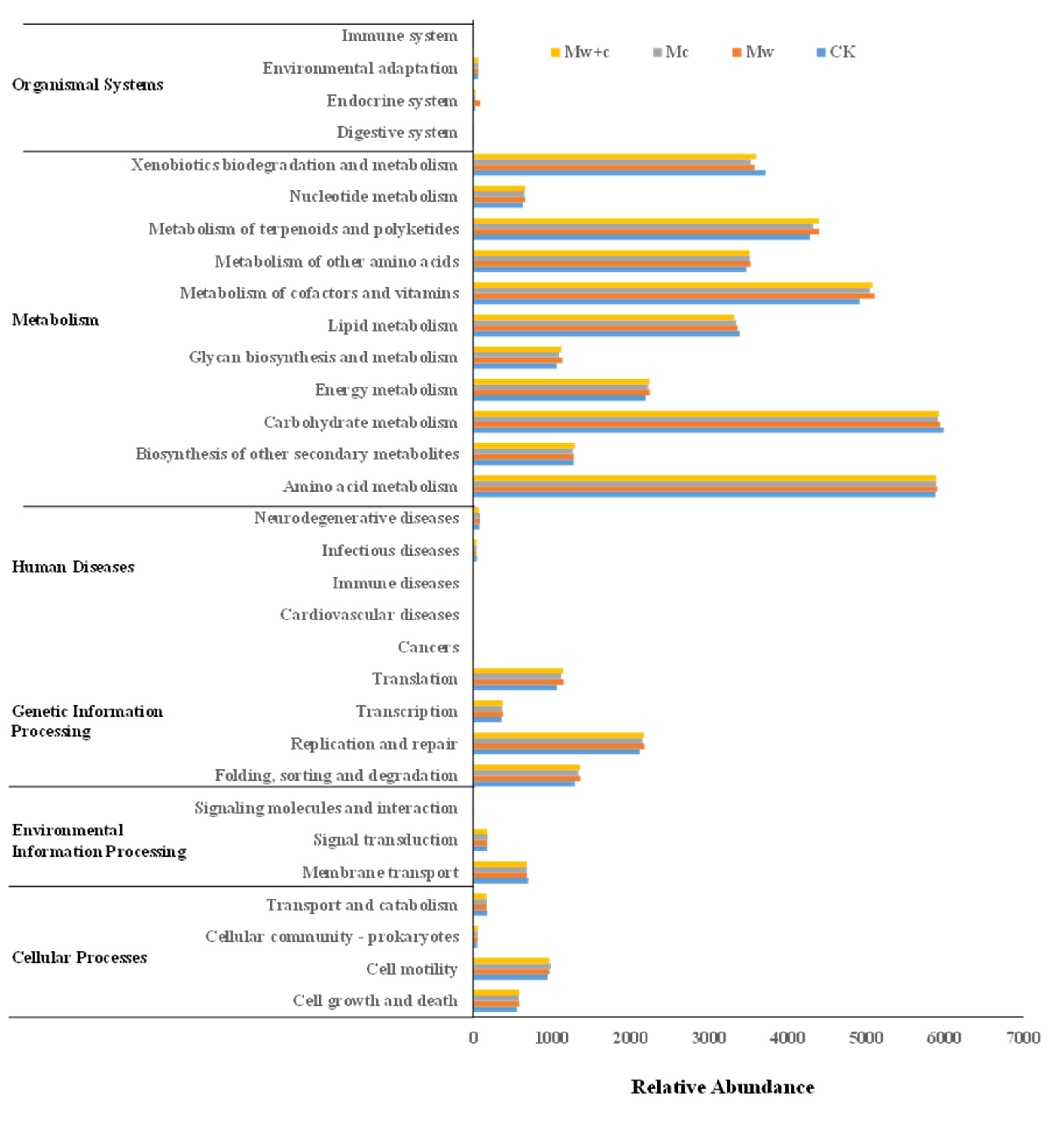
3.4. Effect of Organic Mulching on Soil Bacterial Community Function
4. Discussion
4.1. Organic Mulching Increased the Soil Nutrients
4.2. Organic Mulching Altered the Bacterial Community Structure and Composition
4.3. Soil Bacterial Community Was Closely Related to Soil Physicochemical Properties
4.4. Bacterial Function Was Altered under the Organic Mulching
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Wang, C.; Sun, Z.; Hao, Z.; Day, S. How do urban forests with different land use histories influence soil organic carbon? Urban For. Urban Green. 2023, 83, 127918. [Google Scholar] [CrossRef]
- Shen, F.; Fei, L.; Tuo, Y.; Peng, Y.; Yang, Q.; Zheng, R.; Wang, Q.; Liu, N.; Fan, Q. Effects of water and fertilizer regulation on soil physicochemical properties, bacterial diversity and community structure of Panax notoginseng. Sci. Hortic. 2024, 326, 112777. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, M.; Li, X.; Sun, G. Different Urban Forest Tree Species Affect the Assembly of the Soil Bacterial and Fungal Community. Microb. Ecol. 2022, 83, 447–458. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Xun, W.; Huang, X.; Huang, Q.; Ran, W.; Shen, B.; Zhang, R.; Shen, Q. Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl. Microbiol. Biotechnol. 2017, 101, 4761–4773. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, Q.; Wang, W.; Lin, X.; He, Z.; Li, G. Straw mulching decreased the contribution of Fe-bound organic carbon to soil organic carbon in a banana orchard. Appl. Soil Ecol. 2024, 194, 105177. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ye, Y.; Liao, J.; Tang, Y.; Wang, D.; Guan, Q. Organic mulching alters the composition, but not the diversity, of rhizosphere bacterial and fungal communities. Appl. Soil Ecol. 2021, 168, 104167. [Google Scholar] [CrossRef]
- Tibash, A.; Jourgholami, M.; Moghaddam Nia, A.; Latterini, F.; Venanzi, R.; Picchio, R. The Effects of Organic Mulches on Water Erosion Control for Skid Trails in the Hyrcanian Mixed Forests. Forests 2023, 14, 2198. [Google Scholar]
- Zhou, W.; Sun, X.; Li, S.; Du, T.; Zheng, Y.; Fan, Z. Effects of organic mulching on soil aggregate stability and aggregate binding agents in an urban forest in Beijing, China. J. For. Res. 2022, 33, 1083–1094. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Q.; Li, X.; Li, X.; Yu, Z.; Li, X.; Yang, T.; Su, Z.; Zhang, H.; Zhang, C. Effects of long-term no-tillage with different straw mulching frequencies on soil microbial community and the abundances of two soil-borne pathogens. Appl. Soil Ecol. 2020, 148, 103488. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; Liao, J.; Soromotin, A.V.; Smirnov, P.V.; Kuzyakov, Y. Organic Mulching Increases Microbial Activity in Urban Forest Soil. Forests 2022, 13, 1352. [Google Scholar] [CrossRef]
- Borja, M.; Zema, D. Short-term effects of post-fire mulching with straw or wood chips on soil properties of semi-arid forests. J. For. Res. 2023, 34, 1777–1790. [Google Scholar] [CrossRef]
- Wang, B.; Niu, J.; Berndtsson, R.; Zhang, L.; Chen, X.; Li, X.; Zhu, Z. Efficient organic mulch thickness for soil and water conservation in urban areas. Sci. Rep. 2021, 11, 6259. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Cui, M.; Zhou, Z.; Zhang, Q.; Li, Y.; Ha, W.; Pang, D.; Luo, J.; Zhou, J. Effects of secondary succession on soil fungal and bacterial compositions and diversities in a karst area. Plant Soil 2022, 475, 97–102. [Google Scholar] [CrossRef]
- de Souza, L.C.; Procópio, L. The adaptations of the microbial communities of the savanna soil over a period of wildfire, after the first rains, and during the rainy season. Environ. Sci. Pollut. Res. 2022, 29, 14070–14082. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Xiang, M.; Yang, S.; Wang, F.; Cao, W.; Yue, H.; Peng, S. Soil nutrients drive changes in the structure and functions of soil bacterial communities in a restored forest soil chronosequence. Appl. Soil Ecol. 2024, 195, 105247. [Google Scholar] [CrossRef]
- Lv, W.; Liu, Y.; Hai, X.; Liao, Y.; Li, J.; Dong, L.; Shangguan, Z.; Deng, L. Nitrogen Enrichment Regulates the Changes in Soil Aggregate-Associated Bacterial Community: Evidence from a Typical Temperate Forest. Forests 2024, 15, 77. [Google Scholar] [CrossRef]
- Cong, P.; Wang, J.; Li, Y.; Liu, N.; Dong, J.; Pang, H.; Zhang, L.; Gao, Z. Changes in soil organic carbon and microbial community under varying straw incorporation strategies. Soil Tillage Res. 2020, 204, 104735. [Google Scholar] [CrossRef]
- Yu, C.; Han, F.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Ma, Q.; Liao, J.; Wang, D.; Guan, Q.; Jones, D.L. Organic mulching promotes soil organic carbon accumulation to deep soil layer in an urban plantation forest. For. Ecosyst. 2021, 8, 2–11. [Google Scholar] [CrossRef]
- Liu, G.; Bai, Z.; Shah, F.; Cui, G.; Xiao, Z.; Gong, H.; Li, D.; Lin, Y.; Li, B.; Ji, G.; et al. Compositional and structural changes in soil microbial communities in response to straw mulching and plant revegetation in an abandoned artificial pasture in Northeast China. Glob. Ecol. Conserv. 2021, 31, e01871. [Google Scholar] [CrossRef]
- Li, L.; He, X.Z.; Wang, M.; Huang, L.; Wang, Z.; Zhang, X.; Hu, J.; Hou, F. Grazing-driven shifts in soil bacterial community structure and function in a typical steppe are mediated by additional N inputs. Sci. Total Environ. 2024, 912, 169488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Degré, A.; De Clerck, C.; Li, S.; Lian, J.; Peng, Y.; Sun, T.; Luo, L.; Yue, Y.; Li, G.; et al. Changes in bacterial community structure and carbon metabolism in sandy soil under the long-term application of chitin-rich organic material and attapulgite. Appl. Soil Ecol. 2024, 194, 105161. [Google Scholar] [CrossRef]
- Prats, S.A.; Serpa, D.; Santos, L.; Keizer, J.J. Effects of forest residue mulching on organic matter and nutrient exports after wildfire in North-Central Portugal. Sci. Total Environ. 2023, 885, 163825. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.; Zhang, Y.; Yang, S.; Zhang, Y.; Chen, Y.; Shao, Y.; Gao, D.; Yuan, Z.; Zhang, Y. Mulches assist degraded soil recovery via stimulating biogeochemical cycling: Metagenomic analysis. Appl. Microbiol. Biotechnol. 2024, 108, 20. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Adams, J.M.; Qiu, C.; Qin, W.; Chen, J.; Jin, L.; Xu, C.; Liu, J. Nutrient improvement and soil acidification inducing contrary effects on bacterial community structure following application of hairy vetch (Vicia villosa Roth L.) in Ultisol. Agric. Ecosyst. Environ. 2021, 312, 107348. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, P.; Chang, S.; Wu, J.; Lin, L. Organic mulch and fertilization affect soil carbon pools and forms under intensively managed bamboo (Phyllostachys praecox) forests in southeast China. J. Soils Sediments 2010, 10, 739–747. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Tian, M.; Shah Jahan, M.; Shu, S.; Sun, J.; Li, P.; Ahammed, G.J.; Guo, S. Mixing of biochar, vinegar and mushroom residues regulates soil microbial community and increases cucumber yield under continuous cropping regime. Appl. Soil Ecol. 2021, 161, 103883. [Google Scholar] [CrossRef]
- Wu, B.; Luo, H.; Wang, X.; Liu, H.; Peng, H.; Sheng, M.; Xu, F.; Xu, H. Effects of environmental factors on soil bacterial community structure and diversity in different contaminated districts of Southwest China mine tailings. Sci. Total Environ. 2022, 802, 149899. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Whitman, W.B.; Coleman, D.C.; Jien, S.; Wang, H.; Chiu, C. Soil bacterial communities at the treeline in subtropical alpine areas. Catena 2021, 201, 105205. [Google Scholar] [CrossRef]
- Nam, S.; Alday, J.G.; Kim, M.; Kim, H.; Kim, Y.; Park, T.; Lim, H.S.; Lee, B.Y.; Lee, Y.K.; Jung, J.Y. The relationships of present vegetation, bacteria, and soil properties with soil organic matter characteristics in moist acidic tundra in Alaska. Sci. Total Environ. 2021, 772, 145386. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ling, J.; Qiao, F.; Xi, P.; Zeng, Y.; Zhang, J.; Lan, C.; Jiang, Z.; Peng, A.; Li, P. Organic mulch can suppress litchi downy blight through modification of soil microbial community structure and functional potentials. BMC Microbiol. 2022, 22, 155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, W.; Wang, X.; Xie, Z.; Wang, Y. Long-term effects of gravel mulching and straw mulching on soil physicochemical properties and bacterial and fungal community composition in the Loess Plateau of China. Eur. J. Soil. Biol. 2020, 98, 103188. [Google Scholar] [CrossRef]
- Chi, Z.; Zhu, Y.; Li, H.; Wu, H.; Yan, B. Unraveling bacterial community structure and function and their links with natural salinity gradient in the Yellow River Delta. Sci. Total Environ. 2021, 773, 145673. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, H.; Wang, R.; Wang, H.; Li, Z.; Li, W.; Xiang, P.; Xu, W. Effects of Tobacco Stem-Derived Biochar on Soil Properties and Bacterial Community Structure under Continuous Cropping of Bletilla striata. J. Soil. Sci. Plant Nutr. 2021, 21, 1318–1328. [Google Scholar] [CrossRef]
- Chi, Z.; Ju, S.; Li, H.; Li, J.; Wu, H.; Yan, B. Deciphering edaphic bacterial community and function potential in a Chinese delta under exogenous nutrient input and salinity stress. Catena 2021, 201, 105212. [Google Scholar] [CrossRef]
- Gui, H.; Wang, L.; Yan, D.; Li, P.; Zhang, X.; Han, L.; Han, W. Organic management practices shape the structure and associations of soil bacterial communities in tea plantations. Appl. Soil Ecol. 2021, 163, 103975. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree Species Shape Soil Bacterial Community Structure and Function in Temperate Deciduous Forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, B.; Wei, M.; Wang, S.; Rong, X.; Du, D.; Wang, C. Changes in community structure and metabolic function of soil bacteria depending on the type restoration processing in the degraded alpine grassland ecosystems in Northern Tibet. Sci. Total Environ. 2021, 755, 142619. [Google Scholar] [CrossRef] [PubMed]
- Bei, S.; Zhang, Y.; Li, T.; Christie, P.; Li, X.; Zhang, J. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric. Ecosyst. Environ. 2018, 260, 58–69. [Google Scholar] [CrossRef]



| Treatments | Shannon | Simpson | Chao1 | PD | Coverage (%) |
|---|---|---|---|---|---|
| CK | 11.14 ± 0.21 b | 0.998 ± 0.08 a | 6273.48 ± 198.99 b | 274.45 ± 6.23 b | 98.02 ± 0.15 a |
| Mw | 11.46 ± 0.02 a | 0.999 ± 0.003 a | 7080.63 ± 89.95 a | 319.84 ± 9.95 a | 97.59 ± 0.04 a |
| Mc | 11.29 ± 0.23 ab | 0.999 ± 0.05 a | 6525.27 ± 628.96 a | 286.99 ± 27.61 ab | 97.78 ± 0.31 a |
| Mw+c | 11.47 ± 0.12 a | 0.999 ± 0.01 a | 6814.58 ± 434.18 a | 315.52 ± 14.10 ab | 97.89 ± 0.41 a |
| Phylum | Relative Abundances (%) | |||
|---|---|---|---|---|
| CK | Mw | Mc | Mw+c | |
| Actinobacteria | 43.52 ± 3.32 a | 34.53 ± 1.79 b | 38.09 ± 0.14 ab | 34.69 ± 3.48 b |
| Proteobacteria | 29.90 ± 3.80 a | 29.62 ± 2.33 a | 31.00 ± 2.68 a | 29.69 ± 2.70 a |
| Acidobacteria | 7.94 ± 1.32 c | 14.03 ± 0.74 a | 11.12 ± 0.63 b | 13.63 ± 1.31 ab |
| Chloroflexi | 7.14 ± 0.19 a | 9.10 ± 0.64 a | 7.38 ± 1.75 a | 9.80 ± 0.86 a |
| Gemmatimonadetes | 5.58 ± 0.71 a | 6.35 ± 0.14 a | 6.58 ± 0.52 a | 5.79 ± 0.30 a |
| Bacteroidetes | 1.37 ± 0.75 a | 1.31 ± 0.22 a | 1.24 ± 0.19 a | 1.47 ± 0.22 a |
| Rokubacteria | 0.73 ± 0.10 b | 0.98 ± 0.14 a | 0.96 ± 0.02 ab | 0.91 ± 0.08 ab |
| Patescibacteria | 0.75 ± 0.07 a | 0.94 ± 0.34 a | 0.82 ± 0.25 a | 0.91 ± 0.20 a |
| Firmicutes | 0.96 ± 0.51 a | 0.36 ± 0.40 a | 0.67 ± 0.08 a | 0.61 ± 0.13 a |
| Nitrospirae | 0.54 ± 0.10 a | 0.49 ± 0.06 a | 0.65 ± 0.11 a | 0.59 ± 0.06 a |
| Others | 1.57 ± 0.227 a | 2.30 ± 0.29 a | 1.48 ± 0.36 a | 1.90 ± 0.73 a |
| Class | ||||
| Actinobacteria | 27.32 ± 1.16 a | 19.30 ± 1.27 b | 22.18 ± 1.49 b | 19.88 ± 3.09 b |
| Alphaproteobacteria | 20.21 ± 2.22 a | 17.87 ± 2.50 a | 19.27 ± 2.44 a | 16.99 ± 2.58 a |
| Thermoleophilia | 12.23 ± 1.90 a | 10.93 ± 0.55 a | 11.45 ± 1.17 a | 10.16 ± 0.41 a |
| Gammaproteobacteria | 6.98 ± 1.99 a | 7.40 ± 0.42 a | 7.74 ± 0.55 a | 7.69 ± 0.34 a |
| Gemmatimonadetes | 5.35 ± 0.72 a | 6.28 ± 0.12 a | 6.43 ± 0.52 a | 5.70 ± 0.29 a |
| Subgroup-6 | 3.30 ± 0.88 c | 6.618 ± 0.39 a | 4.70 ± 0.82 bc | 6.09 ± 0.74 ab |
| Deltaproteobacteria | 2.68 ± 0.55 b | 4.32 ± 0.84 a | 3.96 ± 0.36 ab | 4.97 ± 0.61 a |
| KD4-96 | 3.43 ± 0.57 a | 3.47 ± 0.35 a | 3.01 ± 0.48 a | 3.44 ± 0.55 a |
| Blastocatellia-(Subgroup-4) | 2.11 ± 0.54 b | 2.81 ± 0.41 ab | 2.85 ± 0.10 ab | 3.11 ± 0.25 a |
| Acidobacteriia | 1.91 ± 0.13 b | 2.81 ± 0.24 ab | 2.47 ± 0.45 ab | 2.98 ± 0.54 a |
| Others | 14.46 ± 0.94 c | 18.19 ± 0.61 ab | 15.94 ± 1.7 bc | 18.99 ± 0.24 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Sun, X.; Li, S.; Qu, B.; Zhang, J. How Organic Mulching Influences the Soil Bacterial Community Structure and Function in Urban Forests. Microorganisms 2024, 12, 520. https://doi.org/10.3390/microorganisms12030520
Zhou W, Sun X, Li S, Qu B, Zhang J. How Organic Mulching Influences the Soil Bacterial Community Structure and Function in Urban Forests. Microorganisms. 2024; 12(3):520. https://doi.org/10.3390/microorganisms12030520
Chicago/Turabian StyleZhou, Wei, Xiangyang Sun, Suyan Li, Bingpeng Qu, and Jianbing Zhang. 2024. "How Organic Mulching Influences the Soil Bacterial Community Structure and Function in Urban Forests" Microorganisms 12, no. 3: 520. https://doi.org/10.3390/microorganisms12030520




