Utilization of Spent Coffee Grounds for Bioelectricity Generation in Sediment Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation of Caffeine Determination
2.2. Preparation of Spent Coffee Grounds
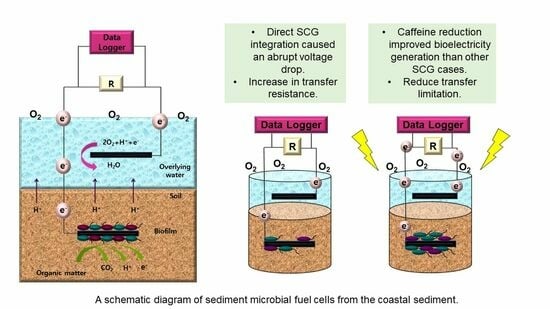
2.3. Establishment of SMFC Reactors
2.4. Electrochemical Measurements
3. Results and Discussion
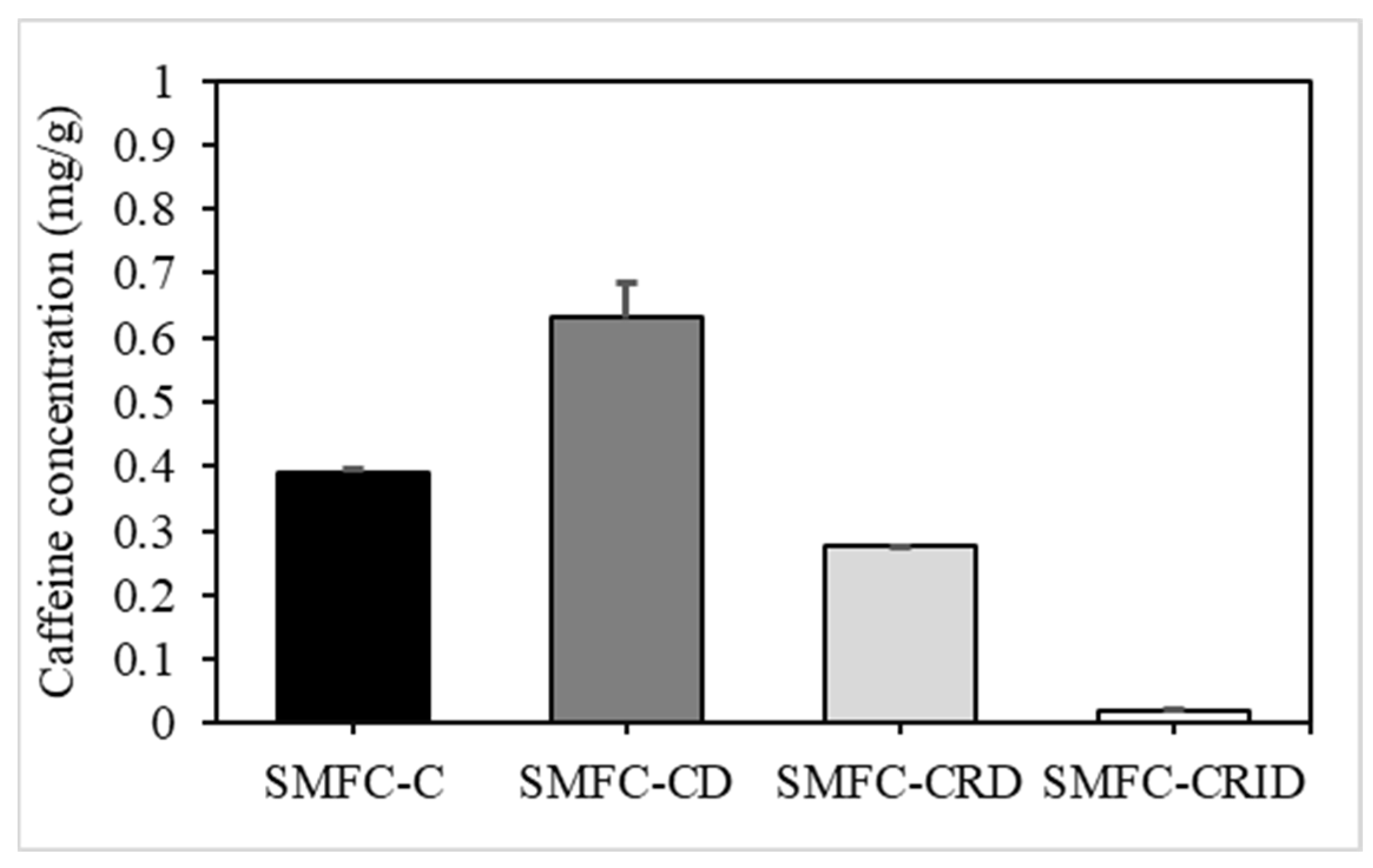
3.1. Caffeine Concentration after Simple Pretreatment
3.2. Performance of Open-Circuit Voltage in SMFC Cases
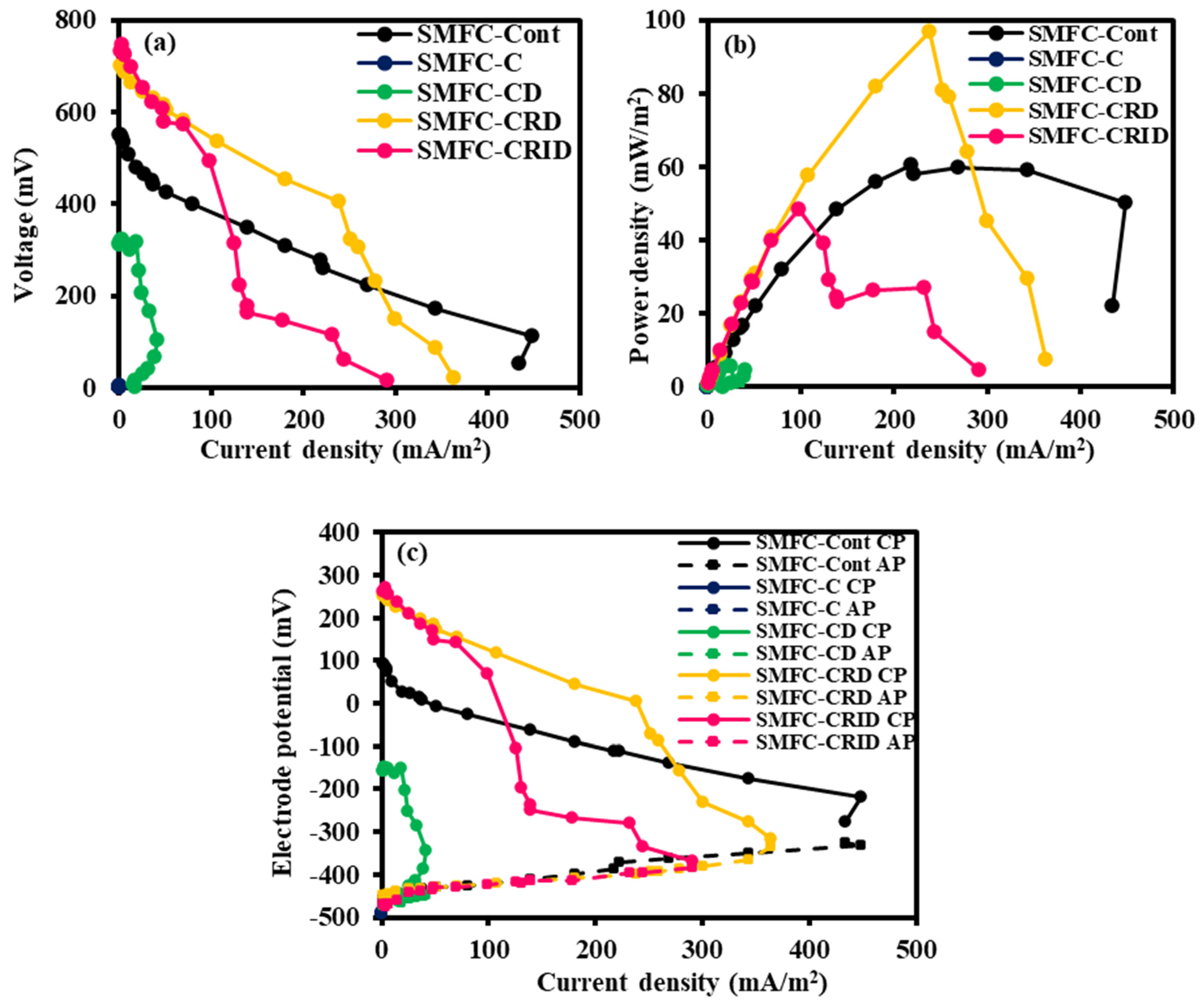
3.3. Bioelectricity Generation of SMFC
3.4. Polarization Behavior of SMFC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Kim, J. Green Tea, Coffee, and Caffeine Consumption Are Inversely Associated with Self-Report Lifetime Depression in the Korean Population. Nutrients 2018, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.L.; Yusoff, M.S.; Aziz, H.A.; Umar, M. Influence of Impregnation Ratio on Coffee Ground Activated Carbon as Landfill Leachate Adsorbent for Removal of Total Iron and Orthophosphate. Desalination 2011, 279, 225–234. [Google Scholar] [CrossRef]
- Johnson, L.S.; Poovathingal, K.R.; Kuttykandy, S. Evaluation of the Physio-Chemical Properties and Microbial Load of the Soil Polluted with Coffee Processing Wastes. Malays. J. Microbiol. 2013, 16, 132–142. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical Composition and Value-Adding Applications of Coffee Industry by-Products: A Review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-Situ Transesterification of Wet Spent Coffee Grounds for Sustainable Biodiesel Production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kampioti, A.; Komilis, D. Anaerobic Co-Digestion of Coffee Waste with Other Organic Substrates: A Mixture Experimental Design. Chemosphere 2022, 297, 134124. [Google Scholar] [CrossRef] [PubMed]
- Md Khudzari, J.; Tartakovsky, B.; Raghavan, G.S.V. Effect of C/N Ratio and Salinity on Power Generation in Compost Microbial Fuel Cells. Waste Manag. 2016, 48, 135–142. [Google Scholar] [CrossRef]
- Sales, A.L.; Depaula, J.; Mellinger Silva, C.; Cruz, A.; Lemos Miguel, M.A.; Farah, A. Effects of Regular and Decaffeinated Roasted Coffee (Coffea arabica and Coffea canephora) Extracts and Bioactive Compounds on in Vitro Probiotic Bacterial Growth. Food. Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef]
- Yap, K.L.; Ho, L.N.; Ong, S.A.; Guo, K.; Liew, Y.M.; Oon, Y.S.; Thor, S.H.; Tan, S.M.; Teoh, T.P. Microbial Fuel Cell for Simultaneous Caffeine Removal and Bioelectricity Generation under Various Operational Conditions in the Anodic and Cathodic Chambers. Environ. Technol. Innov. 2022, 25, 102158. [Google Scholar] [CrossRef]
- Souza, F.S.; Féris, L.A. Degradation of Caffeine by Advanced Oxidative Processes: O3 and O3/UV. Ozone Sci. Eng. 2015, 37, 379–384. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, B.; Jahng, D. Spent Coffee Ground as a New Bulking Agent for Accelerated Biodrying of Dewatered Sludge. Water. Res. 2018, 138, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Jiang, H.; Feng, J. Removal of Caffeine from Water by Combining Dielectric Barrier Discharge (DBD) Plasma with Goethite. J. Saudi Chem. Soc. 2017, 21, 545–557. [Google Scholar] [CrossRef]
- Oktavitri, N.I.; Nakashita, S.; Hibino, T.; Tran, T.V.; Jeong, I.; Oh, T.G.; Kim, K. Enhancing Pollutant Removal and Electricity Generation in Sediment Microbial Fuel Cell with Nano Zero-Valent Iron. Environ. Technol. Innov. 2021, 24, 101968. [Google Scholar] [CrossRef]
- Tran, T.V.; Lee, I.C.; Kim, K. Electricity Production Characterization of a Sediment Microbial Fuel Cell Using Different Thermo-Treated Flat Carbon Cloth Electrodes. Int. J. Hydrogen Energy 2019, 44, 32192–32200. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H. Real-Time Monitoring of Sediment Bulking through a Multi-Anode Sediment Microbial Fuel Cell as Reliable Biosensor. Sci. Total Environ. 2019, 697, 134009. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of Spent Coffee Grounds: A Review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Wang, C.T.; Lee, Y.C.; Liao, F.Y. Effect of Composting Parameters on the Power Performance of Solid Microbial Fuel Cells. Sustainability 2015, 7, 12634–12643. [Google Scholar] [CrossRef]
- Hung, Y.H.; Liu, T.Y.; Chen, H.Y. Renewable Coffee Waste-Derived Porous Carbons as Anode Materials for High-Performance Sustainable Microbial Fuel Cells. ACS. Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Yeo, J.; Yang, Y. Energy Harvesting from Beverage Residues Using a Microbial Fuel Cells. In Proceedings of the 2020 International Conference on Electronics, Information, and Communication, ICEIC 2020, Barcelona, Spain, 19–22 January 2020; pp. 1–3. [Google Scholar] [CrossRef]
- Beltrán, J.G.; Leask, R.L.; Brown, W.A. Activity and Stability of Caffeine Demethylases Found in Pseudomonas Putida IF-3. Biochem. Eng. J. 2006, 31, 8–13. [Google Scholar] [CrossRef]
- Nayak, S.; Harshitha, M.J.; Maithili, M.; Sampath, C.; Anilkumar, H.S.; Vaman Rao, C. Isolation and Characterization of Caffeine Degrading Bacteria from Coffee Pulp. Indian J. Biotechnol. 2012, 11, 86–91. [Google Scholar]
- Ibrahim, S.; Shukor, M.Y.; Syed, M.A.; Ab Rahman, N.; Abdul Khalil, K.; Khalid, A.; Ahmad, S. Bacterial Degradation of Caffeine: A Review. Asian J. Plant Biol. 2014, 2, 18–27. [Google Scholar] [CrossRef]
- Erazo, S.; Agudelo-Escobar, L.M. Determination of Electrogenic Potential and Removal of Organic Matter from Industrial Coffee Wastewater Using a Native Community in a Non-Conventional Microbial Fuel Cell. Processes 2023, 11, 373. [Google Scholar] [CrossRef]
- Kadi Allah, I.; Bekka, A.; Kameche, M.; Dinnebier, R.E.; Touati, W.; Alaoui, C.; Karmaoui, M. Utilisation of Py-ro-chlore-Typeoxideas Catalyst in Bioanode of Coffee Mac Microbial Fuel Cell: Boost of Electric Voltage and Reduction of Wastes. Int. J. Environ. Anal. Chem. 2021, 103, 4430–4449. [Google Scholar] [CrossRef]
- Jang, H.; Ocon, J.D.; Lee, S.; Lee, J.K.; Lee, J. Direct Power Generation from Waste Coffee Grounds in a Biomass Fuel Cell. J. Power Sources 2015, 296, 433–439. [Google Scholar] [CrossRef]
- Wang, C.T.; Liao, F.Y.; Liu, K.S. Electrical Analysis of Compost Solid Phase Microbial Fuel Cell. Int. J. Hydrogen Energy 2013, 38, 11124–11130. [Google Scholar] [CrossRef]
- Jeong, I.; Kim, K. Utilizing a Granulated Coal Bottom Ash and Oyster Shells for Nutrient Removal in Eutrophic Sediments. Mar. Pollut. Bull. 2022, 177, 113549. [Google Scholar] [CrossRef]
- Sallam, E.R.; Fetouh, H.A. Comparative Study for the Production of Sustainable Electricity from Marine Sediment Using Recyclable Low-Cost Solid Wastes Aluminum Foil and Graphite Anodes. ChemistrySelect 2022, 7, e202103972. [Google Scholar] [CrossRef]
- Flambeau, K.J.; Yoon, J. Characterization of Raw and Roasted Fully Washed Specialty Bourbon Cultivar of Coffea Arabica from Major Coffee Growing Areas in Rwanda. Food Eng. Prog. 2018, 22, 89–99. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Schnupf, U.; Mason, P.E.; Saboungi, M.L.; Cesàro, A.; Brady, J.W. Molecular Dynamics Simulation Studies of Caffeine Aggregation in Aqueous Solution. J. Phys. Chem. 2011, 115, 10957–10966. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Brown, K.; Ghoshdastidar, A.J.; Hanmore, J.; Frazee, J.; Tong, A.Z. Membrane Bioreactor Technology: A Novel Approach to the Treatment of Compost Leachate. Waste Manag. 2013, 33, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic Limitations in Microbial Fuel Cells: An Overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- Gunina, A.; Kuzyakov, Y. Sugars in Soil and Sweets for Microorganisms: Review of Origin, Content, Composition and Fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Utilization of Waste from Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of Spent Coffee Grounds as Food Ingredient in Bakery Products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Lee, H.S.; Torres, C.I.; Rittmann, B.E. Effects of Substrate Diffusion and Anode-Respiring, Parameters. Environ. Sci. Technol. 2009, 43, 7571–7577. [Google Scholar] [CrossRef]
- Mohd Noor, N.N.; Oktavitri, N.I.; Kim, K. Effect of Submerged and Floating Cathodes on Sustainable Bioelectricity Generation and Benthic Nutrient Removal in Sediment Microbial Fuel Cells. Fuel 2024, in press. [Google Scholar]

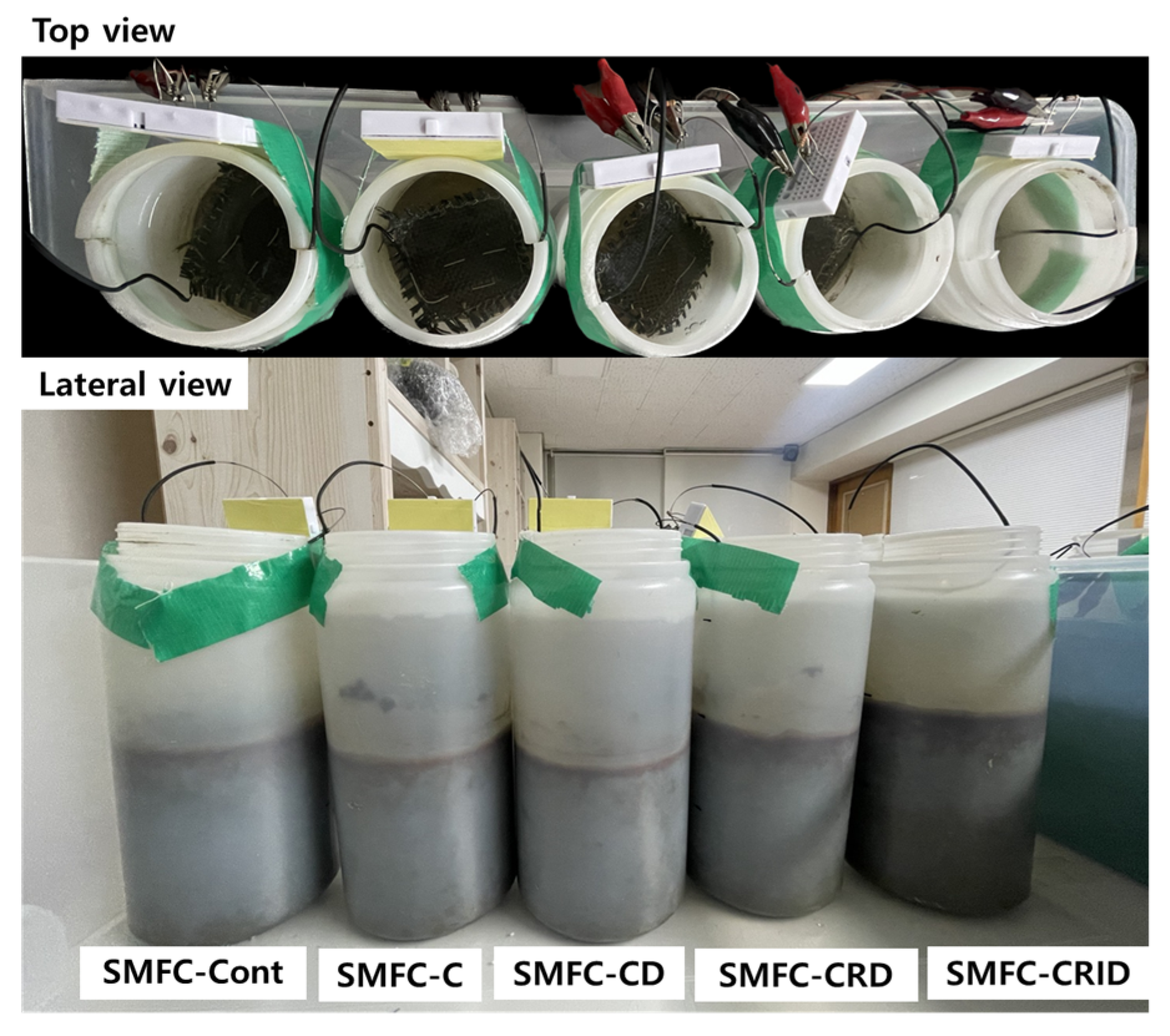


| Cases | SCG (C) | Rinsing (R) | Immerse (I) | Drying (D) |
|---|---|---|---|---|
| SMFC-Cont | - | - | - | - |
| SMFC-C | √ | - | - | - |
| SMFC-CD | √ | - | - | √ |
| SMFC-CRD | √ | √ | - | √ |
| SMFC-CRID | √ | √ | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Noor, N.N.; Jeong, I.; Yoon, S.; Kim, K. Utilization of Spent Coffee Grounds for Bioelectricity Generation in Sediment Microbial Fuel Cells. Microorganisms 2024, 12, 618. https://doi.org/10.3390/microorganisms12030618
Mohd Noor NN, Jeong I, Yoon S, Kim K. Utilization of Spent Coffee Grounds for Bioelectricity Generation in Sediment Microbial Fuel Cells. Microorganisms. 2024; 12(3):618. https://doi.org/10.3390/microorganisms12030618
Chicago/Turabian StyleMohd Noor, Nurfarhana Nabila, Ilwon Jeong, Seokjin Yoon, and Kyunghoi Kim. 2024. "Utilization of Spent Coffee Grounds for Bioelectricity Generation in Sediment Microbial Fuel Cells" Microorganisms 12, no. 3: 618. https://doi.org/10.3390/microorganisms12030618